Impact of Regulatory Action on Dose Maximalization for Vitamin B6 Dietary Supplements on the Reporting Pattern for Neuropathy
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Vitamin B6 deficiency is linked to neurological disorders. However, supplementation with high doses of vitamin B6 has also been linked to neuropathy as an adverse drug reaction. Review of cases from the Dutch Spontaneous Reporting System (SRS) and other data led to a regulatory action to lower the maximum daily dose (DD) of vitamin B6 in supplements to 21 mg/day from October 1, 2018.
Purpose
The aim of this study was to investigate if there was an effect of the regulatory action in 2018 on maximum daily dosage for vitamin B6 on the reporting pattern of neuropathy to the SRS in the Netherlands.
Methods
We investigated trends in the number of reports received until December 31, 2023, DD mentioned in the reports, and the correlation between DD and plasma vitamin B6 levels. A change point analysis was used to get insight into the pattern of reports over time.
Results
Two hundred and twenty-four reports were included. After the regulatory action for dose maximization from October 2018, only one report mentions a dosage which is much higher than the recommended 21 mg DD. Only 15% of the variability in plasma levels mentioned in reports can be explained by the DD. Twelve statistical change points were noted, especially around some peaks in the reporting pattern for instance in 2018. However, from the second half of 2019, the number of reports on vitamin B6 and neuropathy per time period is lower and no change points were detected.
Conclusions
Although our study has limitations, we clearly see an effect of regulatory action on the doses of vitamin B6 used in neuropathy reports. However, some cases describing neuropathy related to vitamin B6 supplementation with lower doses are still reported after the regulatory action in 2018. Therefore, the association between low-dose vitamin B6 products and neuropathy should be studied further.
Summary
- The use of dietary supplements, including vitamins, is wide spread.
- Vitamin B6 supplementation has been linked to neuropathy and therefore a restriction for maximum daily dosage was given in 2018 in the Netherlands.
- Studying the effects of regulatory actions for dietary products is challenging but important.
- We studied the change in the reporting pattern of neuropathy associated with vitamin B6 products in a spontaneous reporting system over time.
- An effect of regulatory action on the doses of vitamin B6 in neuropathy cases is seen after 2018.
1 Background
The use of dietary supplements, including vitamins, is wide spread. The Food Consumption Survey (RIVM, 2019–2021) showed that 57% of Dutch adults had used a food supplement in the past 12 months. This use was higher among women than among men: 69% versus 44% [1]. Vitamin B6 is a water-soluble vitamin that naturally exists in six forms, so-called B6 vitamers, which are pyridoxine, pyridoxal, pyridoxamine, and their phosphorylated derivatives pyridoxine 5′-phosphate, pyridoxal 5′-phosphate, and pyridoxamine 5-phosphate [2]. The biologically active pyridoxal 5′ phosphate (PLP) functions as a coenzyme in many physiological reactions. Vitamin B6 deficiency is linked to neurological disorders. Paradoxically, supplementation with vitamin B6 has also been linked to polyneuropathy as an adverse drug reaction (ADR) [3]. Studies have investigated the mechanism behind this seemingly paradoxical effect. It has been proposed that high levels of the biologically inactive pyridoxine may inhibit PLP-dependent enzymes by competing with the active vitamer PLP. Consequently, a high level of vitamin B6 in the form of pyridoxine may thus inhibit the physiological function of the active form of vitamin B6, that is, PLP [4]. This would explain how a high dose of pyridoxine may lead to similar symptoms as a vitamin B6 deficiency [2].
Various organizations in the Netherlands work together in collaboration to monitor adverse reactions to non-registered products, such as vitamins, and can signal about these products for enforcement actions. According to a study by de Boer et al. “Both EU and Dutch food law address food safety and stipulate that public health must be protected, but there is no legal requirement for a post-marketing system to monitor for safety unlike the legal requirements that are in place for registered drugs” [5]. The Netherlands Pharmacovigilance Centre Lareb is the reporting and knowledge centre for ADRs of medicines, vaccines and non-registered health-enhancing products. For the latter, Lareb reports signals on ADRs to the Netherlands Consumer and Product Safety Authority (NVWA), which is the Dutch inspection and enforcement authority for food and consumer products [5].
Both healthcare professionals (HCPs) and patients can report to Lareb. In 2023, Lareb received a total of 17 241 reports directly and of these 13 709 were from consumers/patients and 3534 from HCPs. In addition, 14 682 reports were received indirectly through marketing authorization holders. For non-registered health enhancing products, the reporting is much lower than for registered drugs and vaccines. In 2023, there were 146 reports in total from consumers (n = 130), followed by general practitioners (n = 11), pharmacists (n = 3), and other HCPs (n = 2) [6].
One of the most commonly reported associations for non-registered health-enhancing products to Lareb is on vitamin B6 products and the occurrence of neuropathy [6]. In the past, Lareb had signaled the association between high-dosed vitamin B6 products and the occurrence of neuropathy, especially after long-term usage of vitamin B6, to the NVWA [7]. Review of this signal and other data by the NVWA led to advice to lower the maximum doses of vitamin B6 in supplements to 21 mg/day in the Netherlands. This advice was adopted by the Dutch Ministry of Health, Welfare, and Sport. From October 1, 2018, the maximum daily dose of vitamin B6 in dietary supplements may not exceed 21 mg [8]. Previously, the European Food Safety Authority (EFSA) established a tolerable upper intake level (UL) of 25 mg/day, while the US-based Food and Nutrition Board recommends an UL of 100 mg/day based on their assessment of the evidence [9]. In 2023, a new EFSA scientific opinion on the tolerable upper intake level (UL) for vitamin B6 was given based on systematic reviews of the literature. An UL of 12 mg/day was established for vitamin B6 for adults [10].
Assessment of the impact of pharmacovigilance activities, including regulatory action, on population level is important [11]. However, for non-registered food supplements, it can be even more challenging to ascertain the impact of regulatory measures than for licensed medicines because there are no data on expenditure or usage of the products readily available.
2 Purpose
The aim of this study was to investigate if there was an effect of the regulatory action in 2018 on maximum daily dosage for vitamin B6 on the reporting pattern of neuropathy due to supplementation to the spontaneous reporting system (SRS) in the Netherlands.
3 Methods
We used a Standard Query Language query on the database of the Netherlands Pharmacovigilance Centre Lareb (“PVReport”, restricted access) to obtain a dataset of ADR reports on (symptoms of) neuropathy associated with supplements containing vitamin B6 from the date of the first report (August 2007) until December 31, 2023. ADRs were coded using the Medical Dictionary for Regulatory Activities (MedDRA). Reports were selected if a product contained vitamin B6 as coded in a custom-made coding system in the Lareb database [12] and ADRs based on the standardized MedDRA query (SMQ) (Broad) “peripheral neuropathy.” For this dataset, we describe the number of reports over time, age, and sex of the patient, daily dosage (DD) of vitamin B6 used, and vitamin B6 plasma levels as described in the reports. We calculated the coefficient of determination (R2) between DD and plasma levels [13]. To detect changes in reporting pattern, we performed a changepoint analysis (CPA) [14] based on the mean in the number of received reports per week and allowing for multiple changepoints (PELT method). The Akaike Information Component was used to compensate for overfitting. In addition, we plotted DD in the reports over time and added trend lines for two periods: before and after December 31, 2018, respectively. Data analysis was performed in R version 4.3.2. For the changepoint analysis, the function cpt.mean from the package changepoint (version 2.2.4) was used.
4 Results
In total, 224 reports on vitamin B6 and (symptoms of) neuropathy were included; 55 (24.6%) reports about men and 169 (75.4%) about women. Mean age was 52 years (median 53 years, standard deviation 15.5 years) with a range from 3 to 92 years. Figure 1 shows (A) the cumulative number of ADR reports related to Vitamin B6 exposure per year. (B) Scatterplot of the daily vitamin B6 dose in relation to the time of reporting, in which the blue lines are linear trend lines. (C) Scatterplot of plasma vitamin B6 levels in relation to the daily dose. (D) Changepoint analysis for ADR reports related to Vitamin B6 exposure. Changes in the red lines indicate changepoints.
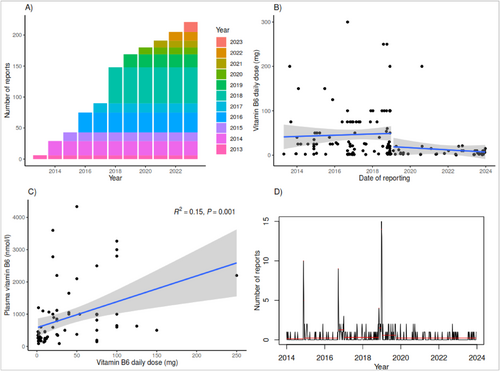
Figure 1B shows that since the regulatory action for dose maximization from October 2018, very few reports are about higher dosages. One case from the year 2020 reports on the usage of a product with 50 mg of vitamin B6, used four times daily, leading to a DD of 200 mg. Figure 1C shows the extent to which the reported DD can explain the reported plasma levels; in this case, 15% of the variability in plasma levels can be explained by the DD, which is a weak correlation. Figure 1D reveals 12 statistical change points, especially around some peaks in the reporting pattern, for instance, in 2018. However, from the second half of 2019, the number of reports per time period is lower, and no change points were detected.
5 Conclusions
This analysis shows that the regulatory action was associated with a reduction in the dosage of vitamin B6 in reported cases to the Dutch SRS on vitamin B6 and (symptoms of) neuropathy. This is in line with a recent EFSA report from 2023 on the availability of vitamin B6 supplements on the market in Europe that states that the labeled recommended dose per serving of 2145 products ranged from 0.02 up to 90 mg of vitamin B6 with an average of 3 mg per dose (median 1.4 mg per dose) [10]. However, despite the lower DD of products, Figure 1D shows cases describing neuropathy related to vitamin B6 supplementation are still reported after the regulatory action in 2018. In November 2024, Lareb brought this to the attention of NVWA.
It should be noted that SRS are prone to underreporting and various types of bias. For instance, we noted a clear effect of reporting based on media attention around signals of vitamin B6 and neuropathy and the regulatory action in 2018, so-called “notoriety bias” [15]. A clear limitation of our study, inherent to the SRS, is the lack of denominator data; we do not know how many consumers used vitamin B6-containing products and based on an SRS, we cannot calculate incidence rates for neuropathy after usage of these products. It should be noted that for the trend analysis with CPA, we did not take into account that the overall amount of consumer reports in the Dutch SRS is steadily increasing [16]. Previous studies have shown that not just the dosage of vitamin B6 used but also the vitamer of B6 present in the dietary supplements plays a critical role in the development of neuropathy [8]. Unfortunately, this information is not available for many reports in the Dutch SRS. Without information on the vitamer in the product, the dosage information is difficult to interpret. Also, declared dosages on dietary supplements might not be accurate, and it is also not always known how many doses of the product patients took or what their dietary intake of vitamin B6 was. For the blood test results that were available, we do not always know when testing took place and what vitamer was measured. The duration of use is not always known and has not been taken into account in this analysis. In our analysis, only 15% of the variance in blood levels can be explained by the DD. Our findings align with the possibility of significant interindividual differences in metabolism and sensitivity, also with the use of lower dosages, within vulnerable individuals that could explain the variance in blood levels [17]. In some reports a potential additional risk factor was mentioned. In over 90 cases, patients reported the use of concomitant medication. In some of these cases, either neuropathy or symptoms like paraesthesia are mentioned as ADRs in the Summary of Product Characteristics (SmPC) of the concomitant drug. For instance, seven patients used metoprolol for which the Dutch SmPC mentions “Paraesthesia” as a potential ADR [18]. Besides concomitant medication, underlying illness can also play a role in the occurrence of neuropathy. Potential other causes mentioned in the reports were, for instance, diabetes mellitus (n = 2), alcohol abuse (n = 1), Lyme disease (n = 1), radiotherapy (n = 1), or pre-existing small fiber neuropathy (n = 1). Information on genetic predisposition is unfortunately very rarely reported. It should be noted that for the current analysis it was out of scope to perform a full causality analysis at the individual case level for all reports. In a previous study on the first 90 cases, we have done a comprehensive case-series assessment [7]. Although our study has limitations, we clearly see an effect of regulatory action on the doses of vitamin B6 used in neuropathy reports. However, some cases describing neuropathy related to vitamin B6 supplementation with lower doses are still reported after the regulatory action in 2018. Therefore, the association between low dose vitamin B6 products and neuropathy should be studied further.
5.1 Plain Language Summary
The use of dietary supplements, including vitamins, is wide spread. Vitamin B6 is present in many foods, and therefore it is rare for persons to have a deficiency from inadequate intake, except for instance in persons with malabsorption disorders, alcohol abuse, or with the use of drugs that deplete vitamin B6 stored in the body. Vitamin B6 deficiency is linked to neurological disorders. Paradoxically, supplementation with vitamin B6 has also been linked to neuropathy as an adverse drug reaction (ADR). Review of cases from the Dutch Spontaneous Reporting System (SRS) and other data led to a regulatory action to lower the maximum daily dose (DD) of vitamin B6 in supplements to 21 mg/day from October 1, 2018. The aim of this study was to investigate if there was an effect of the regulatory action on maximum DD for vitamin B6 on the reporting pattern of neuropathy due to supplementation to the SRS in the Netherlands. We used a dataset of ADR reports on (symptoms of) neuropathy associated with supplements containing vitamin B6. In total, 224 reports were included. This analysis shows that there has been an effect of the regulatory actions on vitamin B6 dose maximization on the products Lareb receives reports about. However, some cases describing neuropathy related to vitamin B6 supplementation with lower doses are still reported after the regulatory action in 2018. In our analysis, only 15% of the variance in blood levels can be explained by the DD. The association between low-dose vitamin B6 products and neuropathy should be studied further.
Ethics Statement
The authors have nothing to report.
Consent
Data are based on reports the Dutch Spontaneous Reporting System, where patients give consent for their data to be used in the reporting form. No additional consent was needed for this study.
Conflicts of Interest
The authors declare no conflicts of interest.




