A geographical paradox: microbiological profile and antibiotic resistance of diabetic foot infection in North West England
Abstract
Background: The microbiological composition of diabetic foot infection (DFI) and its antimicrobial resistance exhibit variations in different parts of the world.
Aims: This study aimed to shed light on the microbial load associated with DFI and the patterns of antibiotic resistance in Northwest England.
Methods: This was a retrospective descriptive study that included 67 patients (55 male [78.6%]). The mean age at diagnosis was 45.6 years (standard deviation, 15.8). The culture of deep tissue samples was analysed together with antibiotic resistance.
Results: A total of 114 causative pathogens were identified. Of note, 40 patients (60.00%) had polymicrobial infections. Moreover, 58.77% of the microbial cohort was composed of gram-positive bacteria. Staphylococcus spp. were found in 32 patients (47.76%) and were the most prevalent pathogen in our cohort. Anaerobic bacteria were found in 17 patients (25.37%) and were the second most common pathogen in our cohort. Corynebacterium spp., Streptococcus spp. and Enterococcus spp. were identified in 11 (16.42%), 10 (14.93%) and 9 (13.43%) patients, respectively. Among the gram-negative bacteria, Escherichia spp. were found in 7 patients (10.45%), Enterobacter spp. were found in 6 patients (8.96%), Klebsiella spp. were found in 4 patients (5.97%), Proteus spp. were found in 4 patients (5.97%) and Alcaligenes spp. were found in 2 patients (2.99%). The remaining less common organisms collectively accounted for 1.49% prevalence. Regarding antibiotic therapy, the highest resistance was observed for ciprofloxacin (12 [17.91%]), followed by amoxicillin (11 [16.42%]), penicillin (10 [14.93%]), clarithromycin (7 [10.45%]), trimethoprim (7 [10.45%]), doxycycline (6 [8.96%]) and piperacillin/tazobactam (5 [7.46%]).
Conclusions: In contrast to the predominant aerobic gram-negative bacteria in Asia, the Middle East and Africa, our study found a paradoxically higher prevalence of gram-positive and anaerobic bacteria in North West England. Moreover, our study found a high incidence of resistance to ciprofloxacin and amoxicillin.
Introduction
Diabetes mellitus poses a significant worldwide health concern, affecting approximately 382 million individuals worldwide. The prevalence of diabetes on a global scale is on the ascent, and projections indicate that approximately 592 million people will be affected by the year 2035.1 Approximately 7% of the UK population now have diabetes; moreover, approximately 1 million people have undiagnosed type 2 diabetes and more than 3000 children are being diagnosed with diabetes mellitus every year. The National Health Service UK spends at least £10 billion a year on diabetes mellitus, equivalent to 10% of its budget. Of note, 80% of the budget is spent on treating complications. Moreover, one in six hospital inpatients has diabetes mellitus.2
Inadequately managed diabetes mellitus can increase the risk of developing diabetic foot ulcer (DFU) or diabetic foot infection (DFI). Statistics suggest that 15% to 25% of individuals with diabetes mellitus will experience DFU during their lifetime.3-5 The financial implications associated with DFU care are considerable. For instance, in the years 2014–2015, the annual expenses associated with diabetic foot care in England were estimated to amount to approximately £1 billion.6 DFI is multifactorial, and three factors specifically predispose to tissue damage, namely neuropathy, peripheral vascular disease and susceptibility to infection of the foot at risk.7-9 Although initially appearing as superficial issues, DFI has the potential to evolve into complications, such as osteomyelitis. Addressing DFI involves a limited range of approaches, including wound care, administration of antibiotics and, in more severe cases, amputation. These infections can be difficult to manage, and despite the administration of multiple antibiotic regimens, the likelihood of clinical resolution is low. Moreover, the repeated use of antibiotics raises concerns about the emergence of antimicrobial resistance. A recent study conducted in the UK revealed that, even a year after diagnosis, 55% of individuals with DFI still had ongoing infections and that nearly 15% of individuals had undergone amputation procedures.10 DFI can be categorised as either monomicrobial or polymicrobial, with the latter being prevalent in cases of chronic infections that have previously been subjected to antibiotic treatments.11
The start of antibiotic treatment for patients with a DFI typically involves empirical measures that aim to identify potential pathogens while avoiding overly broad-spectrum regimens. Subsequent therapeutic decisions should be fine-tuned according to the patient's clinical response to the initial approach and the outcomes yielded by meticulously collected specimens. Historically, research (largely concentrated in the regions of North America and Europe) has exhibited consistent findings – chiefly centred around aerobic gram-positive cocci, notably Staphylococcus aureus and, to a lesser extent, Streptococcus spp. and coagulase-negative staphylococci,12 as the predominant causative agents of DFI.
In contrast, studies from countries in Asia, the Middle East and Africa show that aerobic gram-negative organisms, including Pseudomonas spp., Escherichia coli, Klebsiella spp., Enterobacter cloacae and Proteus mirabilis have been observed predominantly.13
Deeper DFI has been shown in some studies to be associated with the presence of anaerobic organisms.14, 15 Bacteria frequently form biofilms that resist immune clearance and promote antimicrobial resistance.16 In one study, 78.2% of chronic wounds showed biofilm production.17 Pathogens in biofilms are more difficult to treat, but some antibiotics (eg rifampicin, daptomycin and fosfomycin) seem to be more effective against biofilm than others.18, 19
Effective treatment of DFI presents a substantial clinical hurdle. Furthermore, the escalation of antimicrobial resistance presents an exacerbating issue linked to the ineffectiveness of treatment and unfavourable outcomes across the spectrum of DFI. To effectively address this clinical challenge, it is crucial to have a thorough understanding of the microbiology associated with DFI. A considerable amount of research has been conducted on the microbiology of DFI via both prospective and retrospective studies conducted beyond the confines of the UK. Although several investigations of small to moderate scale have been conducted, it seems that there is a lack of available literature on the microbial composition of DFI within the UK. Our study aimed to shed light on the microbial load associated with DFI and the patterns of antibiotic resistance observed in Northwest England, which may provide useful data for future empirical treatment of DFI.
Methods
This retrospective descriptive study was conducted at the Orthopaedic Department of Wythenshawe Hospital, Manchester, UK. Medical records from 1 October 2019 to 31 March 2022, (2.5 years) were reviewed. All patients aged >18 years with DFIs who underwent surgical interventions were included. For patients with a history of multiple surgical procedures, only the first operation was considered. Patients with incomplete data were excluded from the study. Demographic data, such as age, gender, type of infection and type of surgical intervention, were extracted. In addition, the microbiological profile of DFI was retrieved from medical records, including the type of specimen, the causative pathogens and the sensitivity of the pathogens to the antibiotics tested. Only deep tissue or bone samples obtained during surgery were included. The samples were tested using the VITEK 2 system (bioMérieux), a new automated bacterial identification and susceptibility testing system that uses fluorescence-based technology.
All statistical analyses were performed using R statistical software (version 4.3.1; R Core Team 2021).20 Venn diagrams were generated using the library ggvenn.21
Results
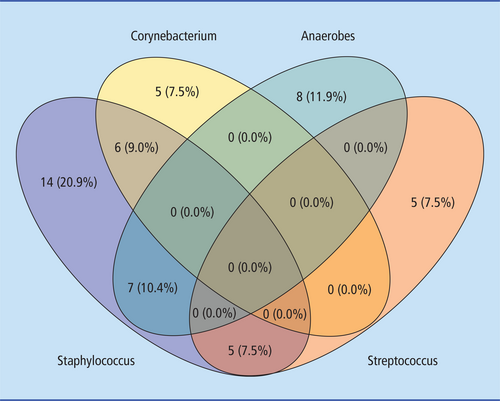
There were 67 patients included in our study who underwent surgery for DFI during the aforementioned period. Most of the patients were men (55 [78.6%]). The mean age at diagnosis was 45.6 years (standard deviation [SD], 15.8), and the mean age at first surgery was 61.5 years (SD, 11.4). Overall, 114 causative pathogens were identified. Of note, 40 patients (60.00%) had polymicrobial infections. Moreover, 58.77% of the microbial cohort was composed of gram-positive bacteria (Table 1). Staphylococcus spp. were found in 32 patients (47.76%) and were the most prevalent pathogen (Figure 1). Anaerobic organisms were found in 17 patients (25.37%) and were the second most common pathogen. Corynebacterium spp., Streptococcus spp. and Enterococcus spp. were identified in 11 (16.42%), 10 (14.93%) and 9 (13.43%) patients, respectively. Among the gram-negative bacteria, Escherichia spp. were found in 7 patients (10.45%), Enterobacter spp. were found in 6 patients (8.96%), Klebsiella spp. were found in 4 patients (5.97%), Proteus spp. were found in 4 patients (5.97%) and Alcaligenes spp. were found in 2 patients (2.99%). The remaining less common organisms collectively accounted for 1.49% prevalence.
| Variable | Number of patients (n) | Percentages (%) |
|---|---|---|
| Sex | ||
|
Male Female |
52 15 |
77.61 22.38 |
| Microbiology | ||
|
G+ G− Staphylococcus Corynebacterium Klebsiella Anaerobes Escherichia Proteus Enterococcus Pseudomonas Brevibacterium Candida Alcaligenes Streptococcus Haemophilus Anaerococcus Weeksella Oligella Pseudoglutamicibacter Serratia Enterobacter Dermabacter Prevotella timonensis Finegoldia Citrobacter |
67 30 32 11 4 17 7 4 9 1 1 5 2 10 1 1 1 1 2 1 6 1 1 1 1 |
58.77 26.32 47.76 16.42 5.97 25.37 10.45 5.97 13.43 1.49 1.49 7.46 2.99 14.93 1.49 1.49 1.49 1.49 2.99 1.49 8.96 1.49 1.49 1.49 1.49 |
| Antibiotic resistance | ||
|
Ciprofloxacin Clarithromycin Amoxicillin Piperacillin and tazobactam Trimethoprim Doxycycline Penicillin Tigecycline Clindamycin Gentamicin |
12 7 11 5 7 6 10 3 3 3 |
17.91 10.45 16.42 7.46 10.45 8.96 14.93 4.48 4.48 4.48 |
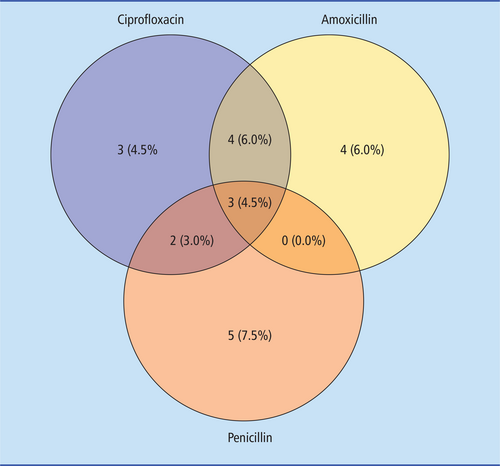
Regarding antibiotic therapy, the highest resistance was observed for ciprofloxacin (12 [17.91%]), followed by amoxicillin (11 [16.42%]), penicillin (10 [14.93%]), clarithromycin (7 [10.45%]), trimethoprim (7 [10.45%]), doxycycline (6 [8.96%]) and piperacillin/tazobactam (5 [7.46%]). Resistance to tigecycline, gentamicin and clindamycin was found in 3 patients (4.48% each) (Figure 2).
Discussion
The notable prevalence of polymicrobial infections in DFU is widely acknowledged. The rate of polymicrobial infection in a few studies from the Middle East ranged from 38% to 84%.22, 23 Almost similar findings were observed in an Indian study that revealed the predominant polymicrobial nature of infections (55.7% of cases). Monomicrobial infections were observed in 44.3% of cases.24 Other studies have revealed contradicting results, with monomicrobial infections being more prevalent. A study by Tiwari et al.25 showed that monomicrobial infections (27/62 [43.5%]) were more prevalent than polymicrobial infections (22/62 [35.5%]). In contrast, an equal number of polymicrobial and monomicrobial infections was reported (44.4% of cases).26 Our study documented polymicrobial infection in 60% of patients. This is comparable with most numbers in the literature, as mentioned above. The polymicrobial nature of DFI has always been associated with disease severity. Hence, prompt diagnosis and the selection of the appropriate empirical antibiotic are paramount while waiting for the specific culture results.
All samples in this study were obtained via deep tissue culture during the surgical procedures, which is a more reliable method for the identification of microbial flora than superficial swabs.27 The International Working Group on the Diabetic Foot (IWGDF) guidelines strongly advocate for deep tissue sampling in their recommendations. Two systematic reviews,28, 29 one small prospective study30 and one well-designed prospective study31 have generally shown that the sensitivity and specificity of deep tissue specimens for culture results are higher than those for swabs.
In developed countries, most DFIs are caused by gram-positive cocci, mainly S. aureus. Gram-positive aerobes are reported in 66% to 84% of DFIs in these settings.32, 33 In contrast, studies from Asian and African countries showed a higher prevalence of gram-negative organisms in DFI: aerobic gram-negative organisms were found in 51.2% of DFIs in Kuwait and in 65.1% of DFIs in India.34 A study by authors in Malaysia further enhances this claim.35 In patients with DFI, 129 cases (29.7%) were caused by gram-negative bacteria, and 84 cases (19.4%) were caused by gram-positive bacteria. Interestingly, mixed growth infection had a substantial rate among patients with DFI, occurring in 80 patients (18.4%). The data show that Pseudomonas aeruginosa (9.4%) was the most common causative gram-negative microorganism, followed by Proteus spp. (4.6%), P. mirabilis (2.5%) and E. coli (2.5%). S. aureus (6.0%) was the most prevalent gram-positive bacteria, followed by Streptococcus agalactiae (4.1%). Fungal pathogens and their role in DFI are rarely studied. A report by Chellan et al.36 revealed a high prevalence (27.9%) of fungal infection in the deep tissues of lower extremity wounds of patients with diabetes, with Candida parapsilosis topping the list. Fungal infections accounted for 9% of all isolates in a study by Bansal et al.37
In contrast, we observed a lower prevalence of fungal infections in our cohort. The most frequently isolated pathogens in our study were aerobic gram-positive bacteria and anaerobes. This result is in line with previous findings in North America and other regions of Europe. In contrast, chronic limb ischaemia that often coexists with DFI provides an ideal environment for anaerobes to thrive.38 The pronounced prevalence of gram-negative organisms in developing countries could primarily be attributed to the increased severity of infection upon presentation. In addition, several other investigators reported a higher prevalence of gram-negative bacteria in higher-grade ulcers than milder ones.39, 40 The severity of infection is often directly related to delayed presentation of patients in developing countries. This is mostly due to a lack of awareness and limited access to proper health care in those regions. In some socioeconomically deprived areas, poor sanitation and inadequate access to clean water can contribute to the spread of infections. Gram-negative bacteria are often found in environments with contaminated water and inadequate hygiene practices.41
Regarding methicillin-resistant S. aureus (MRSA), varying findings have been reported. In a study conducted in an underprivileged area in India, the prevalence of MRSA was alarmingly high, reaching up to 66%; however, another study focussed on Southeast Asia discovered an MRSA incidence rate of 2.8%42. These disparities underscore the complex nature of MRSA epidemiology and its potential sensitivity to regional factors. International guidelines recommend MRSA coverage if the local prevalence of MRSA exceeds 50% of all isolates in mild infection and 30% in severe infection.27 Our study revealed no evidence of MRSA infection. This could stem from various contributing factors. First, having stringent infection control practice in a well-established health care system helps. These measures include proper hand hygiene, isolation precautions and strict adherence to protocols that prevent the spread of MRSA in health care settings. Apart from that, a comprehensive surveillance system that tracks the occurrence of MRSA infections allows for the early detection and containment of outbreaks, thus reducing the overall spread of the bacteria. Most importantly, antibiotic stewardship programmes promote the responsible use of antibiotics, reducing the development of antibiotic-resistant strains, such as MRSA.
In addition to the aforementioned issue of MRSA, antibiotic resistance remains a pressing concern for treating DFIs. Bacteria constantly evolve and adapt to various antibiotics, leading to resistance and making the effective management of DFIs increasingly difficult. The literature reports varying degrees of antibiotic resistance,43 and a study by Boschetti et al.44 identified high levels of resistance to fluoroquinolones, piperacillin and carbapenems. No one antibiotic class or agent has been shown to be superior to others, but tigecycline was found to be clinically inferior to ertapenem (with or without added vancomycin) for treating soft tissue (and, in a small subset, bone) infections in a well-designed clinical trial of more than 1000 patients.45 In addition, this study showed that the rates of adverse events were significantly higher in tigecycline-treated patients.
A landmark paper published in Naples, Italy, showed increased antibiotic resistance during the COVID-19 pandemic, with specific mentions of vancomycin, oxacillin, carbapenem, colistin and third-/fourth-generation cephalosporin resistance.46
Other trials conducted in Brazil47 and Pakistan48 revealed ciprofloxacin and trimethoprim resistance, respectively.
Our study showed a high prevalence of ciprofloxacin and amoxicillin resistance. This can be attributed to several factors. Among others, the extensive and inappropriate use of these antibiotics, owing to their ready accessibility in both inpatient and outpatient settings, undoubtedly contributes significantly to antibiotic resistance. In particular, ciprofloxacin stands out as it exhibits effectiveness against both gram-positive and gram-negative bacteria. It is commonly used not only for treating bone and joint infections but also for treating urinary tract, respiratory tract and intra-abdominal infections.49 In addition, the risk of developing resistance can significantly increase because of suboptimal dosages and inadequate treatment durations for ciprofloxacin and amoxicillin.
Our paper provides insights into the microbial profiles of DFIs in Northwest England. In contrast to the predominant aerobic gram-negative bacteria found in Asia, the Middle East and Africa, our study found a paradoxically higher prevalence of gram-positive and anaerobic bacteria. The presence of Staphylococcus spp., Corynebacterium spp. and Streptococcus spp. underscores their significance as primary causative agents. In addition, the prevalence of anaerobic bacteria highlights the complex nature of DFUs, particularly in cases of chronicity and tissue ischaemia. Our data concerning antibiotic resistance to commonly used ciprofloxacin and amoxicillin further add to the growing concern about antimicrobial resistance in the management of DFIs. The emergence of this situation necessitates a re-evaluation of antibiotic selection and prescribing practices. The findings from this study emphasise the obligation of a multidisciplinary approach in diabetic foot care, involving health care providers, microbiologists and infectious disease specialists. Individualised treatment plans that consider the specific microbial composition and resistance profiles are crucial for achieving better clinical outcomes and preventing the escalation of antibiotic resistance. Moreover, this study contributes to the ongoing surveillance and research efforts to monitor evolving new resistance patterns and can guide the development of novel treatment guidelines to combat this growing public health concern.
Declaration of interests
The authors report no conflict of interest.
KEY POINTS
- The microbial profile of diabetic foot infections (DFIs) in Northwest England revealed a higher prevalence of gram-positive and anaerobic bacteria. The presence of Staphylococcus spp., Corynebacterium spp. and Streptococcus spp. underscores their significance as primary causative agents.
- There was a low incidence of fungal infection and no evidence of methicillin-resistant Staphylococcus aureus infection in our cohort.
- Antibiotic resistance to commonly used ciprofloxacin and amoxicillin adds to the growing concern of antimicrobial resistance in the management of DFIs in Northwest England.