Recent advances in crystallization and self-assembly of polypeptoid polymers
Funding information: Guangdong Province Introduction of Innovative R&D Team, Grant/Award Number: 2016ZT06C322; National Natural Science Foundation of China, Grant/Award Number: 21803020
ABSTRACT
Poly(N-substituted glycine)s, or polypeptoids, have attracted great interest from researchers due to their structural similarity to polypeptides. By taking advantage of relative ease of synthesis and good solubility in common solvents, polypeptoid polymers provide a versatile platform to generate peptide mimics with well-defined chemical structures and architectures. As a class of peptidomimetic polymers, a deep understanding on the impact of side-chain structures, main-chain topologies, and backbone conformations with respect to the aggregation behaviors is the prerequisite for developing peptidomimetic polymers toward macromolecular and supramolecular engineering. This mini-review highlights recent developments in the crystallization and self-assembly of homopolymers, block copolymers, and macrocycles of polypeptoids.
1 INTRODUCTION
Polypeptoids, or poly(N-alkyl glycine)s, represent a class of peptidomimetic polymers, in which the substituent position is on the nitrogen atom, instead of the α-carbon (Scheme 1).1-6 This structural feature eliminates the inter- and intra-chain hydrogen bonding compared to their structural isomers of natural and synthetic polypeptides. It gives rise to a highly enhanced solubility of polypeptoids in common organic solvents, and apparently conformational flexibility of main chains. The fruitful toolbox of polypeptoids allows designing biologically inspired polymers with tunable side-chain structures and the physical properties. In particular, precisely controlled sequence and programmable side-chain chemistry of polypeptoids are accessible by solid phase submonomer synthesis (SPSS).7, 8 So far, more than 250 diverse residues, including congenial species of all the canonical amino acids, have been demonstrated.9, 10 For solid-phase approach, the available degree of polymerization (DP) is limited to around 50, whereas polypeptoids with higher molar mass are feasible to achieve by living ring-opening polymerization (ROP) of N-substituted glycine N-carboxyanhydrides (NNCAs) or N-substituted N-thiocarboxyanhydrides (NNTAs).3, 4, 11, 12
These synthetic approaches offer the feasibility to synthesize polypeptoids with rationally designed molecular mass, side-chain structures, and monomer sequence. Indeed, polypeptoids provide a versatile toolkit to exploit the aggregation behavior of peptidomimetic polymers, such as crystallization and self-assembly.5, 13, 14 In particular, by taking the advantage of precise monomer sequence via solid-phase synthesis, a deep understanding on the relations between main-chain and side-chain structures, as well as the defect effect of semi-crystalline biopolymers, can be achieved by rational molecular designing.15 In addition, the almost isoenergetic cis- and trans- conformations of tertiary amide bonds in polypeptoids are of importance for the formation of secondary structures. Due to the absence of hydrogen bonds, the backbones of polypeptoid polymers have a higher flexibility than polypeptides. The fact is that, in self-assembled nanostructures, polypeptoids could exhibit a novel secondary-structure motif that does not exist in natural biomolecules.16 In addition, to control the conformation, different methods are usually used to enhance the rigidity of polypeptoid chains, for example, backbone cyclization.17 Cyclic polypeptoids not only provide a unique building block for assembly of solid-state supramolecular architectures, but also can be employed to construct novel metal-organic framework (MOF) and ion channels.18 Acting as highly modular protein mimics, polypeptoids have shown their potential for bio-applications, such as drug delivery and therapeutics (use as pharmacological agents).5, 6, 19, 20
As several reviews on the synthesis and application of polypeptoids have been published in recent years, the present mini-review is not meant to be a comprehensive review.3, 4, 6, 14, 15, 21, 22 Instead, in this mini-review, we will discuss recent progress on the crystallization and self-assembly of polypeptoids, including homopolymers, block copolymers, and macrocycles. In the first part, we will focus on the crystallization behaviors of polypeptoid polymers, such as melting and recrystallization. Then, our attention will turn to the recent studies on the self-assembly of polypeptoid homopolymers and block copolymers. Finally, the self-assembly of cyclic polypeptoids will be introduced.
2 CRYSTALLIZATION AND SELF-ASSEMBLY OF POLYPEPTOID POLYMERS
Owing to the absence of hydrogen bond, the aggregation behavior of polypeptoids is mainly determined by the chemical structures of side chains and the molecular architectures. The sequence of polypeptoids can be precisely controlled by solid-phase synthesis method, which enables to acquire a deep understanding on the effects of sequence-specific defects with respect to the crystallization of polypeptoid oligomers and polymers, and other biologically inspired polymers.
2.1 Crystallization and melting
Luxenhofer and coworkers studied the thermal properties and crystalline behavior of a series of polypeptoid homopolymers bearing short (C1-C5) side chains.23 In their studies, the value of DP of main chains can be precisely tuned between 10 and 100. All the synthesized polypeptoids, except poly(sarcosine) (PSar) with DP of 10, were thermally stable up to at least 200°C. The high thermal stability of polypeptoids should be attributed to the absence of active proton. Its noteworthy that the crystallization behavior of polypeptoids is not only dependent on the structures of main chains, but also the nature of side chains. Among the investigated polymers, PSar, poly(N-ethyl glycine) (poly[N-EtGly]) and poly(N-propyl glycine) (poly[N-PrGly]) with DP of 10 do not exhibit an apparent melting peak in differential scanning calorimetry (DSC) measurement, whereas the crystalline state of the ones with butyl, and pentyl substituents were confirmed. The XRD results are consistent with the DSC measurement. PSar and poly(N-EtGly) show no sharp signals, whereas poly(N-PrGly) with DP of 25, poly(N-butyl glycine) (poly[N-BuGly]), poly(N-pentyl glycine) (poly[N-PenGly]) exhibit sharp diffraction peak with 2θ of 20°, which resulted from the formed crystals of the polymers. These results are in agreement with that reported by Rosales24, 25 and Zhang.26 In addition, the values of melting temperature (Tm) of the polypeptoids increased with the chain length toward a plateau (Figure 1A). However, the highest Tm was found in poly(N-BuGly) with the largest Mn, rather than the one with the longest side chain, poly(N-PenGly). It demonstrated the nonlinear relations between Tm and the length of side chains.
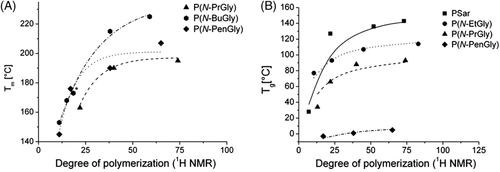
Moreover, the glass transition temperature (Tg) of polypeptoids also shown a function of the length of side chains and main chains (Figure 1B). Among the investigated samples, the increasing length of side chains gave rise to the decay of Tg. It is expectable because the elongation of side chains can enhance the mobility of polypeptoid chains. At the same time, Tg increased with DP to a constant value.
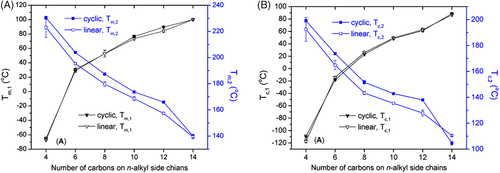
Zhang and coworkers systematically studied the crystallization and melting behaviors of the linear and cyclic polypeptoids with various alkyl side chains (C2-C14) (Figure 2).27 Interestingly, except the one with C2 side chains, all the polypeptoids exhibited double melting peaks during second heating. The lower melting temperature (Tm,1) was attributed to the side-chain crystallization, whereas the higher melting temperatures (Tm,2) associated with the main-chain crystallization (Figure 2A). It was found that the Tm,1 increased with the increase of a number of carbons on n-alky side chains. At the same time, the Tm,2 decreased with the length of side chains. These results are in line with the previous reports. A similar trend could be observed in the crystallization process during DSC cooling scanning. Namely, with the increasing of side-chain length, the first crystallization peak (Tc,1) increased whereas the second crystallization peak (Tc,2) decreased (Figure 2B). In addition, it confirmed that the crystallization of polypeptoids could be strongly suppressed by the presence of short linear alkyl (eg, two carbons) or asymmetrically branched alkyl side chains. Compared with linear polypeptoids, the cyclic architecture mainly enhanced the main-chain crystallization and mildly affected the side-chain crystallization, which was evidenced by the increased melting temperature of the main chains and the preserved melting temperature of the side chains. As the final crystalline structures of polypeptoids are dominated by both of the main-chain and the side-chain structures, the annealing temperature could be selected to tune the crystallization of polypeptoids. If the annealing temperature is higher than Tm,2, the simultaneous movement of the main chains and side chains could give rise to a highly ordered crystalline texture. However, if the sample was annealed at a temperature between Tm,1 and Tm,2, only the crystallization of side chains was allowed, resulting in a lower degree of order.
By taking the advantage of solid-phase polymerization, Segalman and coworkers designed and synthesized a series of polypeptoids with the exactly identical number of monomers, including the ones with the side-chain substitutions different to others.24 In this case, the influence of the defects on the crystallization of polypeptoids can be investigated with respect to the density and location of the defects. It was found that, in some cases, crystallization could be suppressed completely by the presence of defects. Intriguingly, for polypeptoid copolymers with two defects at the exact location, the sequence of monomers merely slightly affected the melting temperature but strongly influenced enthalpies of melting.
Ling and coworkers synthesized homopolymers and corresponding random copolymers of polypeptoids containing both of hydrophilic and hydrophobic monomers by using rare earth catalyst.28 The homopolymer of poly(N-BuGly) exhibited two melting temperatures at 60°C and 196°C, respectively. However, the corresponding melting enthalpies are quite different from the value measurement by Zhang and coworkers.27 This probably resulted from the difference in the dispersity of synthesized polypeptoids.
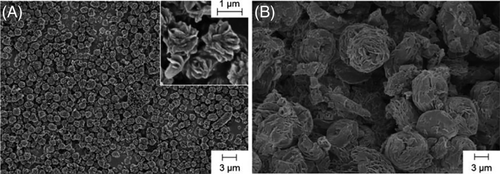
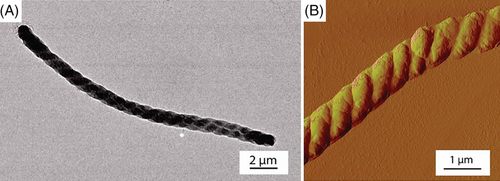
Schlaad and coworkers synthesized a series of polypeptoids with various C3 side chains, such as n-propyl, allyl, propargyl, and isopropyl, by ROP of NNCAs.29 It was found that the prepared polymers could be dissolved in water to a quite high concentration, whereas poly(N-propargyl glycine), was non-soluble. The cloud point temperatures are determined by the structures and electronic properties of the side chains. Interestingly, crystalline particles of poly(N-PrGly) and poly(N-allyl glycine) could be achieved from the aqueous solution after long-time annealing above the cloud point temperature. The crystals formed by polypeptoids exhibited rose-bud-like morphology (Figure 3). However, the detailed mechanism on the formation of such structures was not provided.
Sun and coworkers reported a microphase-separation-induced crystallization in monodisperse peptoid diblock copolymers.30 They synthesized poly(N-decylglycine)-block-poly(N-2-[2-(2-methoxyethoxy)ethoxy]ethylglycine) (pNdc-b-pNte) with a different volume fraction of pNte. These two monomers have nearly equal molecular volumes, but the pNte block is amorphous whereas the pNdc block is crystalline. Interestingly, two distinct crystalline phases were observed in pNdc-b-pNte diblock copolymers at low temperature. Namely, besides the semi-crystalline block of pNdc, the pNte block, which is intrinsically non-crystalline, also crystallized under the inducement of connected pNdc segments. The melting behavior of pNdc and pNte was determined by the chain length of pNdc segment. However, poly(N-isoamyl glycine), which is also a crystalline block, was not able to induce the crystallization of pNte. Thus, it was speculated that the induced crystallization of pNte was dependent on the degree of molecular similarity. This is the first report of crystallization of one block induced by the other block. The ionic conductivity of semi-crystallized polypeptoid block copolymers was also studied by the same authors.31 Because the lithium salt is incorporated into the pNte crystalline phase, the conductivities of block copolymers were determined by the second blocks, namely, the nonconducting blocks. It provides a new platform for studying the dependence of ion conductivity on the solid structures of polypeptoids.
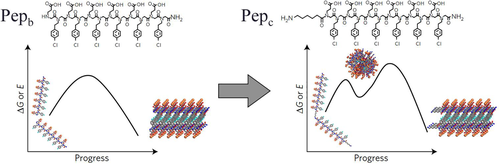
Ma and coworkers studied the crystallization of polypeptoids with different end groups.32 Pepc and Pepb had the same backbone structures containing alternated carboxyl (hydrophilic) and chlorophenethyl (hydrophobic) side chains, but the former one owns an additional hydrophobic group (Figure 4). Crystallization of polypeptoids can be induced by mixing with CaCl2 solutions. By in situ observing with atomic force microscopy (AFM), it was found that the crystallization pathways are sequence dependent. The crystallization of Pepc initiated with the generation of disordered clusters and then transformed into crystalline particles (two-step), however, only crystalline particles can be found for Pepb. It indicated that a metastable phase was created by the addition of the extra hydrophobic region at the chain end, and the pathway of crystallization (one-step or two-step) can be controlled by the chemical structures of chain ends. In this case, the crystallization of polypeptoids is a kinetic-controlled process.
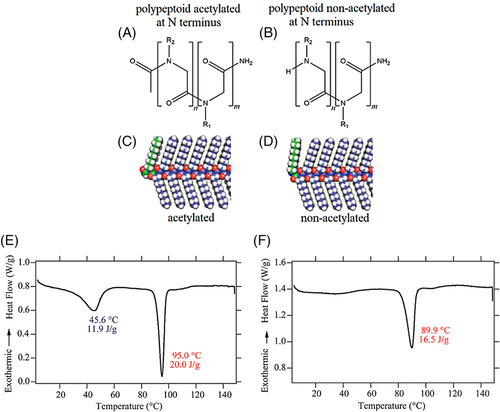
In addition, Greer and coworkers recently studied the crystallization of polypeptoid diblock copolymers with distinct end groups (Figure 5A,B).33 These polypeptoids containing hydrophobic alkyl side chains and hydrophilic ethylene oxide side chains. The N-terminal nitrogen atoms in the acetylated polymer and the non-acetylated polymer are sp2-hybridized (trigonal planar) and sp3-hybridized (tetrahedral) (Figure 5C,D), respectively. As a result, the angle between the N-terminal side chains and the backbones in the block copolymers is determined by the chain ends. It is interesting to find that the block copolymers with an acetylated N-terminus (Ac-Ndc9-Nte9) exhibited two thermal transitions (Figure 5E), whereas the one with a non-acetylated N-terminus (H-Ndc9-Nte9) shown only one thermal transition (Figure 5F). Such crystallization of polypeptoid diblock copolymers was almost unaffected by neither the main- and side-chain length nor the composition. For acetylated block copolymers, the high-temperature peak in the DSC measurement was ascribed into the melting of a liquid crystalline phase, whereas the low temperature peak was related to a transition from a crystalline phase to a sanidic liquid crystalline mesophase. This study provided an example that the chemical structure of end group could impact the order of molecule packing, which determines the final crystallization and melting behaviors of polypeptoids.
Very recently, the same authors systematically studied a series of crystalline polypeptoid copolymers with varied side-chain length (S) and main-chain length (N) using X-ray scattering and molecular dynamics (MD) simulations.34 It was found that the crystal dimensions exhibited linear dependence of N and S: a = 4.55 ± 0.02 (Å), b = [2.98 ± 0.08]N + [0.35 ± 1.70] (Å), and c = [1.86 ± 0.10]S + [5.5 ± 0.8] (Å). These expressions indicated that the polypeptoid molecules prefer to have extended and planar conformations in both crystals and self-assembled nanosheets. In addition, the simulation results demonstrated that the backbones of peptoids exhibited all-cis conformation in the crystals, rather than trans-conformation which is energetically preferred.24, 30, 35
2.2 Ordered self-assembly
2.2.1 Polypeptoid-containing block copolymers
Block copolymers are typical amphiphiles that can self-assemble into various morphologies at the length scales from a few to hundreds of nanometers.36 Microphase separation is driven by the enthalpy of demixing the constituent components of the block copolymers, while phase separation at macro-scale is prevented by the chemical connectivity of the blocks. As the properties of polypeptoids could be tuned by side-chain structures, it provides an outstanding platform for the preparation of copolymers with different chain topologies.37 There are several approaches could be used to the synthesis of polypeptoid-containing block copolymers, such as sequential ROP,38, 39 macroinitiator method,12, 21 bifunctional-initiator method,40 and so forth. As the intra- and inter-molecular interactions of polypeptoids can be finely tuned, it enables the rational design of block copolymers for the studies of microphase separation. The copolymers can be classified according to the polymer segments attached with polypeptoids. The recent studies on PSar-containing block copolymers have been reviewed by Barz and coworkers.37 In addition, by taking advantage of the synthesis methods, the dispersity and side-chain structures of polypeptoids could be well controlled. As a result, polypeptoid homopolymers and copolymers provide an ideal system for achieving deep understanding on self-assembly of biomaterials at a wide length scale.
Zuckermann and coworkers synthesized poly(N-2-phenethyl glycine)-block-poly(N-2-carboxyethyl glycine) (pNpe-pNce) block copolymers and it was found that helical nanostructures could be formed by self-assembly of partially charged amphiphilic block copolymers in solution (Figure 6).35 The results of X-ray diffraction and TEM/AFM observation indicated that the formation of such chiral nanostructures was initiated from the crystalline nanosheets of polypeptoids. Under the driving force of hydrophobic effect and electrostatic forces, these nanosheets gradually transferred into the helical nanostructure. Interestingly, it was found that all the helices having an identical handedness (left-handed), suggesting the presence of homochiral evolution during the hierarchical self-assembly.41-43
The same authors also synthesized polystyrene-b-polypeptoid block copolymers by using “click chemistry,” that is, azide-alkyne coupling.44 The Flory-Huggins interaction parameter (χ) of the block copolymers was tuned through the ratio of two monomers, N-(2-methoxyethyl)glycine (Nme) and N-(2-phenylethyl)glycine (Npe), and lamellar and cylinder nanostructures could be formed by microphase separation (Figure 7). The revisable order-disorder transition (ODT) of self-assembled nanostructures revealed that these ordered morphologies were equilibrium structures. The combination of polypeptoids and nondegradable synthetic polymers is an ideal strategy to design and synthesize amphiphilic block copolymers, which are promising in their use as biological materials, such as biosensors, biomimetic scaffolds, biomimetic membranes.45
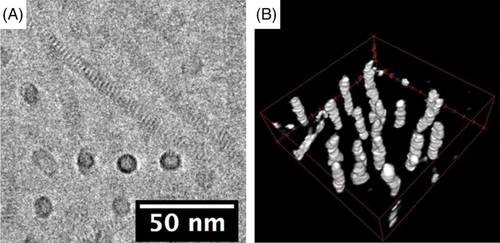
Zuckermann and coworkers synthesized block copolymers containing two types of polypeptoids with different side-chain structures.46 Interestingly, the self-assembled nanostructures of the block copolymers were independent on the volume fraction of the segments, and only lamellar or disordered morphologies could be observed. These results are not in accord with classic theories of microphase separation in block copolymers. By contrast, the monodisperse amphiphilic diblock copolypeptoids, pNdc-b-pNte, can form half-overlapped brick-like patterns, and these patterns can interlock together to form nanotubes with a uniform diameter (Figure 8).47 The polypeptoid chains in the nanotubes should have extended conformation, and their backbones were perpendicular to the tube axis. It is noteworthy that both blocks are crystalline, as evidenced by DSC and X-ray diffraction, but it still maintained a stable structure with large curvature under the driving force of van der Waals interactions between side chains.
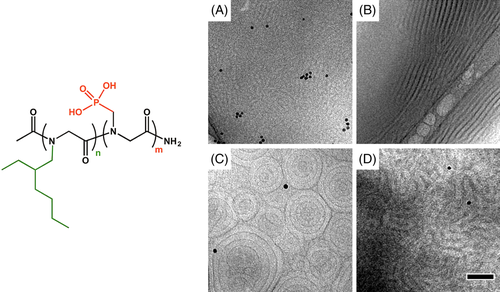
In addition, varied morphologies could be achieved by self-assembly of polypeptoid block copolymers with negatively charged side chains, that is, phosphonate groups.48 The synthesized block copolymers, poly(N-[2-ethyl]hexyl glycine)-block-poly(N-phosphonomethyl glycine) (pNeh-b-pNpm), were almost monodispersed and the volume fractions of pNpm values ranged from 0.13 to 0.44. Due to the strong segregation strength between two segments, self-assembled nanostructures driven by microphase separation could be achieved in both of the dry and hydrated state. Various morphologies of pNeh-b-pNpm can be observed by tuning the chain length and volume fraction (Figure 9). In particular, the order-order phase transition (OOT) from lamellar to cylinder was found in certain block copolymer upon hydration. The authors measured the conductivity of block copolymers and found that the ordered nanostructures of polypeptoid block copolymers were critical to the conductivity of polypeptoids. The disordered morphology of polypeptoids with low molecular weight gave rise to an extremely low conductivity (<10−7 S/cm), showing insulator-like behavior. By contrast, the one with high molecular weight exhibited a high conductivity, and the conductivity linearly increased with the DP of the pNpm block.
Taking advantage of the nature of living polymerization, multiple block copolymers of polypeptoid can be prepared by sequence ROP of NNCA. Zhang and coworkers synthesized ABC triblock copolypeptoids, that is, poly(N-allyl glycine)-block-poly(N-methyl glycine)-block-poly(N-decyl glycine), and studied their hydrogel properties, as well as the potential application in tissue engineering materials.49 All the block copolymers own a relatively narrow dispersity (<1.10). In aqueous solutions, micelle structure can be observed due to the self-assembly of these amphiphilic block copolymers and rapid thermo-reversible sol-gel transitions can occur by alternating temperature. The authors investigated the rheological properties and injectability of polypeptoid hydrogels, indicating minimal cytotoxicity toward human adipose-derived stem cells (hASCs). This study suggested the potential application of polypeptoids in tissue engineering and protein storage applications.
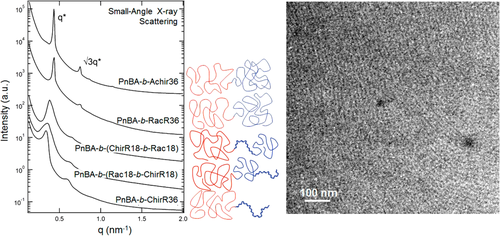
Segalman and coworkers studied self-assembly of a series of poly(n-butyl acrylate) block copolymers containing chiral polypeptoids.50 By tuning the chirality of side chains, that is, racemic or enantiomeric, the stiffness of polypeptoids can be controlled along the whole chains or part of chains. Thus, it provided a model system to study the effects of rod/coil interactions and conformational asymmetry in the microphase separation of block copolymers. It was found that, as the block copolymers with polypeptoid having more than 36 monomers and a volume fraction larger than 25%, hexagonally packed cylinders could be achieved. The block copolymers with chiral side chains exhibited larger domain spacing than that with racemic side chains at the same DP, due to the more extended structure of the all-cis backbone conformation (Figure 10). In addition, the location of chiral blocks could also contribute to domain size, namely, mid-racemic segment resulted in a larger d-spacing. However, the presence of chiral blocks in the polypeptoids did not lead to the formation of chiral self-assembly.41-43 Very recently, the same authors studied the sequence effects on the microphase separation of polystyrene-b-polypeptoid block copolymers.51 It was found that the distribution of the compatibilizing groups in polypeptoid segment could impact both of domain spacing and order-disorder transition temperature. Again, these results confirmed that the morphological and thermal properties of polypeptoid block copolymers could be tuned at the molecular scale.
Zhang and coworkers reported that the formation of micellar structures of sequence-defined polypeptoid could be tuned by the position and number of ionic monomers along the chains.52 It was found that the increasing amount of ionic monomers closer to the junction of hydrophilic and hydrophobic segments could reduce the micellar radius, which could minimize electrostatic repulsive interaction. This study demonstrated that the self-assembly of polypeptoid block copolymers could be controlled by their chain topologies. In addition, it provides a new pathway to access very small micelles in aqueous solution.
Li and coworkers reported the self-assembly of triblock copolymers containing polypeptides, poly(ethyl glycol)-block-poly(L-glutamic acid)-block-poly(N-octyl glycine) (PEG-b-PGA-b-PNOG).53 It was found that these amphiphilic block copolymers could form two-dimensional nanodisks and nanosheet-like structures in aqueous solution, and the final morphologies were determined by the pH and molar fraction. Crystalline nanosheets from PEG-b-PNOG diblock copolymer were firstly reported by the same authors recently.54 Both the segments are crystalline. During solvent annealing, the self-assembled nanostructures of the block copolymers gradually transferred from sphere to cylinders and final to nanosheets, which mimicked the multi-step crystallization of protein. It was found that such transition is sensitive to the solvent selected. Very recently, they successfully prepared ultrathin nanosheets with thickness of 4-5 nm via self-assembly of PEG-PNpe.55 According to the results of grazing incidence wide-angle X-ray scattering (GIWAXS), the formation of nanosheets could be attributed to the driving force of the crystallization of the PNpe segments.
Dowing and coworkers studied crystalline nanosheet of polypeptoids (pNdc9-pNte9) and analyzed the crystals by high-resolution TEM.56 By applying crystallographic and single-particle methods, the authors were able to observe polypeptoids crystals at resolution of about 0.2 nm and individual polymer chains with extended conformation can be clearly recognized. Combination of experimental results and molecular simulations, it was revealed that polypeptoid chains could be trapped in periodic motifs at nonequilibrium state.
Hamley and coworkers studied the self-assembly of “lipopeptoids,” namely, polypeptide amphiphiles.57 The high geometric and hydrophobicity head-to-tail asymmetries of the lipopeptoids enable the formation of micellar structures. With appropriate peptoid sequence, stable spherical micelles with extremely small size (ca. 5 nm diameters) could be formed by self-assembly of lipopeptoids, due to the conformational flexibility of the polypeptoid backbone. These ultra-small nanoparticles, which could be explored as globular protein mimics, are potential for drug delivery and biosensing applications.
2.2.2 Polypeptoid nanosheets
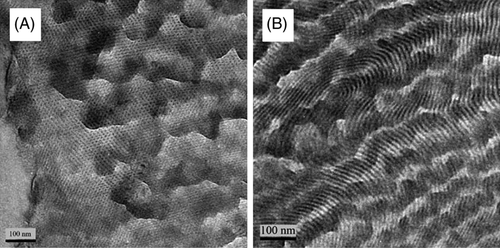
In 2010, Zuckermann and coworkers synthesized several sequence-specific amphiphilic polypeptoids consisting of monomers with either polar or non-polar substitutions.25 Interestingly, two polypeptoids, (N-[2-carboxyethyl] glycine-N-[2-phenethyl] glycine)18 (Nce-Npe)18 and (N-[2-aminoethyl] glycine-N-[2-phenethyl] glycine)18 (Nae-Npe)18, composed of alternating polar and nonpolar residues assembled into sheet-like nanostructures when they were mixed (Figure 11A). High-resolution TEM image revealed that the fully extended chains of polypeptoids oriented along the edge of nanosheet (Figure 11B, C). The authors speculated that the driving force of ordered nanosheets was the synergy of aromatic π-π interactions, electrostatic attraction and the exposure of hydrophobic groups to water. In addition, it is demonstrated that such sheet-like structures could be formed by polypeptoids which was end-modified by streptavidin, a biologically active ligand, and showing the potential application for well-defined hydrophilic scaffolds.
Such polypeptoid nanosheets can also be successfully prepared at the air-water interface, through an approach, which is similar to the Langmuir-Blodgett (LB) deposition process.58 It indicated that the formation of nanosheets was controlled by compression strength, which was related to the free energy of the system. Once sufficient energy has been applied, free-floating nanosheet can be constructed stably. Not only the air-water interface, oil-water interface can also be applied to generate such ordered nanosheets.59
Besides air-water and oil-water interface, namely, “soft interface,” polypeptoid nanosheets can be induced by “hard interface.” Chen and coworkers studied the formation of polypeptoid nanosheets on mica surface using in situ AFM.60 It was observed that polypeptoids first self-assembled into particle-like nanostructures and then transformed into hexagonally patterned nanosheets due to the inducement of the surface structure of mica, that is, epitaxial growth. Such oriented nanostructures can be found in both of pure polypeptoids and polypeptoid/ Ca2+ cations complex.
As it is demonstrated that the combination of polar and nonpolar residues is necessary to the formation of polypeptoid nanosheets, the single polypeptoid system containing polar and non-polar motifs, for example, (Nae-Npe-Nce-Npe)9, was thus designed and synthesized.61 Nanosheets of polypeptoids were successfully obtained from the multiblock copolymers, via inter-chain charge-charge interactions. For polypeptoids containing monomers with opposite charges, the attractive interactions could promote tightly packing and alignment. However, for polypeptoids with different monomers having same charges, the repulsive interactions inhibit the formation of nanosheets by preventing the ordered packing of chains.59
According to these studies mentioned above, the formation of nanosheets is mainly determined by the chemical structures of polypeptoids, namely, the inter-chain interactions and intra-chain interactions. To clarify the connection between chemical structures and self-assembly behaviors of polypeptoids, a quantitative metric for predicting the capability of forming nanosheets could be achieved by surface dilation rheology measurements.62 For polypeptoid can self-assembled to nanosheets, the residence time of the polypeptoid within the monolayer (τD) should be larger than 5000 seconds, suggesting a slow rate of peptoid desorption and re-adsorption. By contrast, if τD value is shorter than 500 seconds, the polypeptoids cannot form nanosheets due to the insufficient peptoid residence time. It revealed the fact that weak inter-chain interactions restrict the formation of nanosheets. The viscoelastic properties of polypeptoids reflect the interchain interactions of polypeptoids that determining the final morphology of self-assembly.
In order to further clarify, the relationship between the chemical structures of polypeptoids and their ability to form nanosheets, Zuckermann and coworkers synthesized a series of polypeptoids with different side-chain substitutions.63 It was found that the position of a single methyl-substituted unit played a significant role in the aggregation behavior. In addition, the side-chain structures could also determine the chain conformation of polypeptoids, which is also critical to the formation of nanosheets, in addition to the residence time of the peptoid chains.
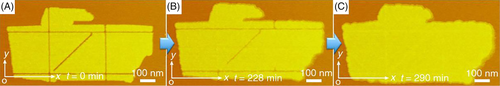
Chen and coworkers synthesized a series of lipid-like polypeptoid block copolymers, and they successfully prepared membrane-mimetic lamellar crystals in both solution and solid surface.64 The X-ray diffraction results and MD simulation indicated an anisotropic packing of polypeptoid chains in the membrane due to the anisotropic inter-chain interactions during crystallization. Interestingly, the polypeptoid membrane can self-repair at room temperature within serval hours (Figure 12). It was noted that the rate of healing was also anisotropic, that is, the most rapid repair occurred along the x-direction.
The conformation polypeptoids is of importance for the crystallization and self-assembly of polypeptoids. The amide bonds of polypeptoid backbones could exhibit both cis- and trans-conformation.65 Blackwell and coworkers reported that polypeptoids containing chiral side chains could adopt a novel secondary structure, namely, “peptoid ribbon.”66 Such secondary structure has resulted from alternating cis- and trans- geometry of the main-chain amides and the preclusion of hydrogen bonds within the polypeptoid backbone.
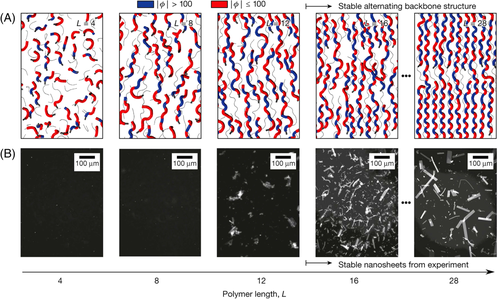
Whitelam and coworkers studied the structures of polypeptoid nanosheets and indicated that nanosheets are structurally and dynamically heterogeneous.16 They found that the polypeptoids shorter than 12 monomers do not form stable nanosheets. (Figure 13). It is because that, for short polypeptoids, the nanosheets is relatively unstable compared with disordered aggregates. Also, it was found that a zigzag secondary structure adopted by polypeptoids, which is called Σ(“sigma”)-strand, was vital to the formation of nanosheets. Such zigzag conformation probably results from the ability of adjacent monomers to adopt opposed orientation, which could keep the linear and untwisted backbone conformation. This is a unique secondary structure that is not seen in any natural peptide or protein.
Very recent, Paravastu and Whitelam, respectively, studied the chain conformation in the nanosheets of polypeptoids by using solid NMR and simulation.67, 68 It indicated that, again, the conformation of polypeptoids in nanosheets was determined by the interplay of inter-chain interactions and the conformational free energy of the backbones. Polypeptoids are linear and untwisted in nanosheets, resulting in the flat and extended conformation. In order to maintain the linearity of backbones, polypeptoids prefer a predominantly cis-conformation which is energetically favorable. By contrast, the trans-conformation is favored in backbone amide bonds within an amorphous state of polypeptoids. It is noteworthy that the conformation of polypeptoids is also sensitive to temperature and solvent.69
As a mimic of peptides, polypeptoid nanosheets are highly potential for the application for novel 2D biomaterials.22, 70 For example, polypeptoid nanosheets provide a robust, high-surface area scaffold, and the functionalized nanosheets can serve as substrates for enzymes, or as templates for the growth of defined inorganic materials.71 Recently, glycosylated peptoid nanosheets were applied as a multivalent scaffold for protein recognition.72 The polypeptoid nanosheets are simple mimetics of the cell surface, in which the lectins Con A and WGA, as well as the clinically relevant Shiga toxin subunit B can be decorated. It provides a platform, which can be applied for therapeutics and bioremediation of threat agents. In addition, the polypeptoids can be used as a 2D template for calcium carbonate mineralization, which developed an approach to prepare artificial nacre materials.73
2.2.3 Cyclic polypeptoid polymers
As introduced above, polypeptoids with well-defined side-chain structures and programmable sequence exhibit novel and unique crystallization and self-assembly behaviors. Also, the research of folding principles in polypeptoids indicates that stable secondary structures could be formed in synthetic biomimics, which shows promise for applications in biomaterials. The fact is that the conformation of polypeptoids (cis- and trans-) is determined by the interactions between proximal backbone amide groups and side chains, which is critical to the construction of secondary structures. In order to establish conformational homogeneity of polypeptoids, one effective strategy is the inclusion of covalent macrocyclic constraints.74, 75 After the first synthesis of cyclic PSar by Dale and Titlestad in 1969, crystallization structures of cyclic peptoids in bulk state had been reported in the early 1970s.76 Groth systematically examined the crystal structures of a series cyclopeptoids with ring numbers from 2 to 12 at high and low temperatures.77-81 With the development of methods for the synthesis of head-to-tail macrocycles, the crystallization, conformation and the reaction to metal ions of cyclic polypeptoids have been widely studied.
In 2007, Kirshenbaum and coworkers developed an effective head-to-tail cyclization to prepared polypeptoids with well-defined sequences and chain lengths.82 The crystal structures of cyclic polypeptoids were determined by single crystal X-ray diffraction. For the first time, it revealed that the polypeptoid backbone could accommodate distorted cis- and trans-amide conformers in the solid state. In this case, almost all amide bonds exhibited nonplanar twisted configurations. It suggests that the cyclization of polypeptoids provides a pathway for the synthesis of stable mimics of peptide reverse-turn structures. As polypeptoids bearing bulky N-alkyl side chains are important biomimetic “foldamer” compounds, which could exhibit helical structures via repeating cis-amide bonds.83 The same authors studied the side-chain effect on the backbone conformation in both of linear and cyclic polypeptoids. It indicated that the presence of N-aryl glycine monomer could always direct the trans-amide bonds within a polypeptoid sequence. In particular, the incorporation of N-alkyl and N-aryl glycine monomers led to a combination of both cis- and trans-amide bonds throughout the backbone, and the location of N-aryl glycines could specify the position of trans-amide bonds. It gives the means to control overall backbone structure. By using “click chemistry,” they also synthesized bicyclic polypeptoids.84 By introducing azide and alkyne groups into side chains at a certain position, secondary cyclization can be performed by copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), after the cyclization of backbone. For both of macrocyclic and bicyclic polypeptoids, cis- and trans-amide bonds are coexisted. It confirmed that the N-aryl side chains promoted a trans-amide geometry, whereas the other N-alkyl side chains led to cis-amide bonds.
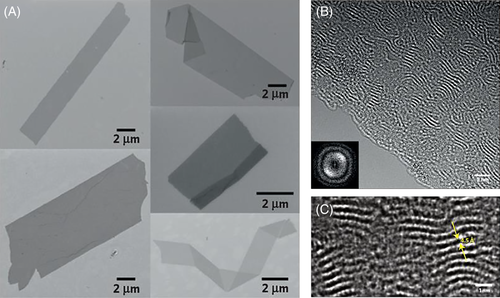
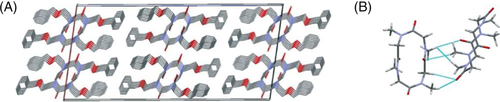
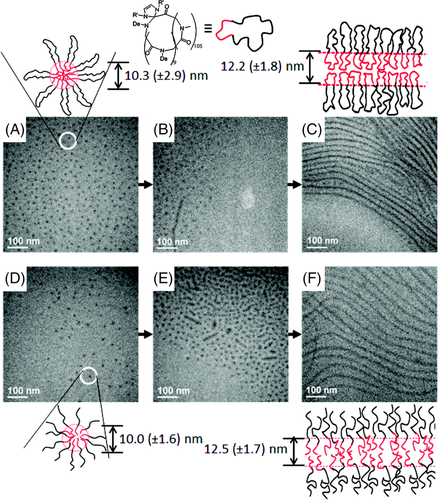
De Riccardis and coworkers synthesized 9-, 12-, and 18-membered N-benzyloxyethyl cyclic polypeptoids and studied their conformational features.85 The results of single crystal X-ray analysis demonstrated that the conformation of the 12-membered cyclic polypeptoids was ctct “chair” tetralactam geometry, whereas the 18-membered cyclic polypeptoids had an all-trans conformation in its complex with strontium picrate. To clarify the dependence of the side-chain structures (inter-chain interactions) and the size of the macrocycle on the conformation, the same authors systematically studied crystal structures of a series of cyclic polypeptoids with varied monomers and ring numbers.17 The results indicated that the side-chain structures have a significant impact on the solid self-assembly of polypeptoid macrocycles. For example, benzyloxyethyl side chains enhance the face-to-face packing of macrocycles and lead to tubular arrangement (Figure 14A). While methoxyethyl and propargyl side groups also induce the tubular arrangement, methyl and benzyl side chains often give rise to a T-shaped arrangement of macrocycles (Figure 14B). Also, the increasing on the size of macrocycles promotes tubular arrangement of macrocycles.
Very rencently, Izzo and coworkers demonstrated that the presence of distal propargyl side chains with methoxyethyl groups can effectively enhance the stability in a fully propargylated cyclic octamer, which gave rise to permanent 1D porosity.86 This study demonstrated that the inter-chain interactions are not only important for self-assembly of linear polypeptoid, like nanosheet, but also vital for crystallization of cyclic polypeptoids.
Ikeda and coworkers studied the influence of the number of backbone monomers and the chemical structures of side chains.87 It was found that there were two conformations, that is, all-cis (cccc) and cis-trans alternating (ctct), coexisting in the solid state of polypeptoids. The conformation of macrocycles is sensitive to the polarity of solvent, namely, polar solvent could lead to the cis-trans alternating conformation. Voelz and coworkers developed a method could predict the conformation of linear and cyclic polypeptoids by using replica exchange molecular dynamics (REMD) simulations and quantum mechanical (QM) methods.88 The backbone amide pattern of a macrocyclic nonamer was successfully predicated and the accuracy could reach 1.0 Å.
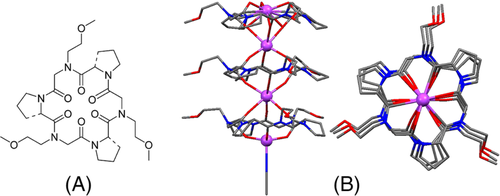
The cyclic topology of polypeptide macrocycles provides a rigid backbone as a host cavity with binding sites, which is suitable for constructing new coordination networks. Katsu and coworkers synthesized N,N′,N″-trisubstituted-cyclo-triglycine and demonstrated its high affinity for Ca2+.89 De Riccardis and coworkers reported that cyclic hexameric polypeptoids consisting of L-Proline and N-methoxyethyl glycine were effective ligands for hosting Na+ (Figure 15).18 This is the first report on metal-organic framework (MOF) structure based on peptoids as linkers.
Kirshenbaum and coworkers synthesized cyclic polypeptoid octamer and achieved its crystal with a tubular packing of molecules.90 Interestingly, the nanotube formed by cyclic octamer can be reversibly occupied by water molecules. According to X-ray results, the backbone of polypeptoid undergoes very tiny re-arrangement during the association and dissociation process. This is the first report with respect to the single-crystal-to-single-crystal (SCSC) transformation observed in cyclic polypeptoid. Shortly after, Tedesco and coworkers prepared cyclo-(N-[methoxyethyl] glycine-N-[prozzpargyl] glycine2)2 (cyclo-[Nme-Npa2]2) (Figure 16A), which could crystallize from hot acetonitrile.91 When these crystals were heated above 70°C, the guest molecule, that is, acetonitrile, will be released from the void of cyclic polypeptoid. During this process, the change on the transparency of crystals can be observed (Figure 16B). Different from that reported by Kirshenbaum and coworkers, the conformation of cyclic polypeptoid significantly changed after release of their guest molecules for efficiently occupying the space. The reversibility of such transformation is evidenced by X-ray diffraction and microbalance. The intercolumnar CH-π interactions between methylene H atoms and propargyl triple bonds of adjacent columns act as a CH-π zipper, the zipper will open and close with the uptake and release of guest molecules.

Izzo and coworkers studied the crystal structures of hexameric and octameric propargylated cyclic polypeptoids, that is, cyclo-(Npa)6 and cyclo-(Npa)8, and the impact of ring size on the crystallization of cyclic polypeptoids with guest molecules.92 For cyclo-(Npa)6, the guest molecules such as acetonitrile, water and DMSO molecules intercalate between layered crystal structure. By contrast, for the one with a larger ring, cyclo-(Npa)8, the guest molecules are able to occupy the void inside of the peptoid nanotube.
Zhang and coworkers studied the self-assembled nanostructures of amphiphilic cyclic diblock copolypeptoids, poly(N-methyl glycine)-block-poly(N-decyl glycine) (c-PNMG-b-PNDG).26 Figure 17 shows the cryo-TEM images of self-assembled nanostructures of c-PNMG-b-PNDG formed in methanol solution for different times, and it was found that the spherical aggregation of copolypeptoids could transfer to cylindrical micelles in 15 days. The linear counterpart of copolypeptoids shown a similar aggregation-induced morphological transition, but with a faster transition kinetics. Although the linear and cyclic polypeptoids exhibit distinct visco-elasticities, the self-assembled structures of copolypeptoids were independent on the topology of backbones.93, 94 This may be due to the driving force of the crystallization of the solvophobic block (PNDG). The same authors also found that cyclic polypeptoids could be prepared by zwitterionic ring-opening polymerization (ZROP) with 1,8-diazabicycloundec-7-ene (DBU) as an initiator.95 Beside cyclic homopolymer, that is, poly(N-BuGly), random copolypeptoids, poly([N-propargyl glycine]-r-[N-butyl glycine])s, could also be synthesized by ZROP. It provided an effective route toward cyclic polypeptoids with controlled molecular weight and narrow weight distribution.
Recently, De Riccardis and coworkers studied conformational and stereochemical properties of several hexameric polypeptoids decorated with N-isopropyl, N-isobutyl, and N-benzyl substituents, respectively.96 It demonstrated that the assembly of cyclic polypeptoids can complex with sodium ion via host and guest interactions, which provided excellent scaffolds for the mimicry of natural mycotoxins, enniatins, and beauvericins. In addition, the authors found the first example of highly cytotoxic cyclic polypeptoid, which exhibits a correlation between sodium transportability and cytotoxic activities on human cancer cell lines. This study suggested the powerful biological activities of cyclic polypeptoids as structural mimics of natural products.
3 CONCLUSIONS AND PERSPECTIVES
The studies reviewed show recent advances in the crystallization and self-assembly of polypeptoids homopolymers, block copolymers and macrocycles with variable chemical structures, chain topologies, and backbone conformation. Compared to polypeptides, the absence of strong inter- and intra-interactions in polypeptoid polymers enable clarification of the effects of side-chain structures and monomer sequence on the self-assembled nanostructures and their physical properties. In particular, by taking the advantage of solid-phase synthesis, the molecular mass and the chemical structures of side chains of polypeptoids can be precisely tuned, which provides an ideal system to achieve deep understanding on the crystallization and melting behavior of sequence-specific polymers. Also, many novel nanostructures, such as low-dimensional nanosheets with high aspect ratio and nanosheets, are able to be gained from the self-assembly of amphiphilic block copolymers containing polypeptoids, giving technological potential in the development of nanodevices. Such an ability to efficiently build functionalized nanostructures will give rise to many relative applications in membrane mimetics, imaging, separations, and so forth. Moreover, the combination of polypeptoid polymers and synthetic polymers will enable the development of novel protein mimics with highly specific functionality. Although the properties of polypeptoid polymers are still far from completely explored, the well-defined chemistry and controlled ordered structures provide tremendous flexibility to design and synthesize polypeptoid-based biomaterials for various applications.
ACKNOWLEDGMENTS
We acknowledge financial support from National Natural Science Foundation of China (No. 21803020), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (No. 2016ZT06C322), and the startup support from the South China University of Technology.
Biographies

Guangjian Zeng was born in Jiangxi, China, in 1992. He received his BS and MS in Chemistry from Nanchang University in Jiangxi, China. Under the guidance of Prof. Xiaoyong Zhang, he completed his thesis at the construction and application of polymeric fluorescent nanoprobe. Currently, he is a PhD candidate at South China Advanced Institute for Soft Matter Science and Technology, South China University of Technology (SCUT) with Prof. Tao Wen. His research interests focus mainly on the aggregation induced emission-based materials and self-assembly of block copolymers.

Lu Qiu received her BS in metallurgical engineering from Jiangxi University of Science and Technology, China. She joined the team of Prof. Tao Wen at the South China Advanced Institute for Soft Matter Science and Technology, South China University of Technology (SCUT). Currently, she is the MS candidate in Material Science and Engineering. Her current research focuses on the synthesis and characterization of advanced hybrid materials.

Tao Wen received his PhD degree from the Institute of Chemistry, Chinese Academy of Sciences (China) in 2013. After 1-year postdoctoral fellowship with Prof. Günter Reiter at the University of Freiburg, he moved to National Tsing Hua University and worked as postdoctoral fellowship with Prof. Rong-Ming Ho from 2014 to 2017. He joined South China University of Technology (SCUT) in 2018 as a professor. His current research is focused on the construction and application of hierarchical materials.




