Long-Term Outcomes and Quality of Life of Children With Intracranial Ependymoma Treated With Pencil Beam Scanning Proton Therapy
Eymeric Le Reun and Ilya Kotov contributed equally to this work.
ABSTRACT
Background
Ependymoma is a common brain tumor in children and adolescents. Adjuvant radiation therapy improves prognosis but carries potential toxicity risks, particularly for young patients. Proton therapy (PT) offers better conformal treatments and reduces dose exposure compared to traditional photon radiotherapy.
Procedure
This study retrospectively analyzed long-term outcomes of children treated with pencil beam scanning (PBS) PT for intracranial ependymomas (EPs) at the Paul Scherrer Institute (PSI) between 2004 and 2022.
Results
We identified 119 children, with most having infra-tentorial tumors (70.6%) and anaplastic ependymomas (82.4%). The median PT dose was 59.4 GyRBE delivered in 1.8 GyRBE/fraction. Follow-up at 5 years showed 70.4% local control, 63.5% progression-free survival (PFS), and 82.2% overall survival (OS). OS was better with upfront than relapse treatment (83% vs. 69.8%; p = 0.024), and complete resection improved both LC (74% vs. 65.1%; p = 0.033) and PFS (67.5% vs. 57.1%; p = 0.049) compared to subtotal resection. No hearing loss was observed with cochlea Dmax not exceeding 48 GyRBE (10.5% vs. 0%; p = 0.0097), whereas the risk of hormone deficiency was significantly increased with pituitary Dmean above 38 GyRBE (33.3% vs. 6.0%; p = 0.00007). Most patients (72.3%) had no late toxicity. Four secondary brain malignancies (3.4%) occurred within a median of 9.3 years after PT (range: 3.7–15). Quality of life 5 years after PT was good in older (>4 years) patients, though proxy-rated social functioning was poorer than the norm group.
Conclusion
Intracranial PBS-PT offers excellent tumor control and low late toxicity, and revealed good overall quality of life in children with ependymoma, both by proxy- and self-assessment.
Abbreviations
-
- C
-
- cognition regarding school and work performance domain
-
- CI
-
- confidence interval
-
- COG
-
- Children's Oncology Group
-
- CSI
-
- cranio-spinal irradiation
-
- CTCAE
-
- Common Terminology Criteria for Adverse Events
-
- CTV
-
- clinical target volume
-
- Dmax
-
- maximum dose
-
- Dmean
-
- mean dose
-
- E1
-
- timepoint before the start of proton therapy
-
- E2
-
- timepoint 2 months after proton therapy
-
- E3
-
- timepoint 1 year after proton therapy
-
- E4
-
- timepoint 2 years after proton therapy
-
- E5
-
- timepoint 3 years after proton therapy
-
- E6
-
- timepoint 4 years after proton therapy
-
- E7
-
- timepoint 5 years after proton therapy
-
- EV
-
- emotionnal functioning domain
-
- Fam
-
- social functioning with family domain
-
- Fr
-
- social functioning with peers domain
-
- Global
-
- subjective well-being domain
-
- GTV
-
- gross tumor volume
-
- Gy
-
- Gray
-
- KB
-
- body image domain
-
- KV
-
- physical functioning in terms of activity, energy, and pain domain
-
- LC
-
- local control
-
- OS
-
- overall survival
-
- PBS
-
- pencil beam scanning
-
- PedQoL
-
- Pediatric Quality of Life Questionnaire
-
- PedsQL
-
- Pediatric Quality of Life Inventory
-
- PFS
-
- progression-free survival
-
- PRV
-
- planning organ-at-risk volume
-
- PSI
-
- Paul Scherrer Institute
-
- PT
-
- proton therapy
-
- PTV
-
- planning target volume
-
- QoL
-
- quality of life
-
- RBE
-
- relative biological effectiveness
-
- RT
-
- radiation therapy
-
- SE
-
- autonomy domain
-
- SIOP
-
- International Society of Paediatric Oncology (Société Internationale d'Oncologie Pédiatrique)
-
- TFS
-
- toxicity-free survival
-
- TSH
-
- thyroid-stimulating hormone
-
- WHO
-
- World Health Organization
1 Introduction
Ependymoma represents a common brain tumor (10%) in children and adolescents, mainly located intracranially, and most of these tumors being in the posterior fossa [1]. The standard of care includes surgery, chemotherapy, and adjuvant radiation therapy (RT) [2]. Prognosis remains poor, with an overall survival (OS) of about 50% at 5 years, dropping down to 40% at 10 years [2-4]. The quality of resection is an important prognostic factor for disease control, as the 5-year progression-free survival (PFS) ranges from 0% to 26% in case of residual tumor, but can be increased to 50%–80% for completely resected tumors [4]. In order to preserve long-term patient quality of life (QoL), proton therapy (PT) offers—compared with photon RT—similar oncological efficacy while reducing the integral dose delivered to non-target tissues [5]. Therefore, PT is particularly indicated for children with a long life expectancy [6]. In addition, some authors showed that patients with intracranial ependymoma have better survival with proton than with photon irradiation [7]. In terms of the PT technique, pencil beam scanning (PBS) offers more conformal dose distributions and is now more used than passive scattering [8, 9].
For almost 20 years now, several studies have described the good results of PT in children with intracranial ependymoma [10-13]. However, most of these studies described small cohorts and/or older PT delivery techniques (passive scattering) [7, 11–16]. Therefore, the aim of the present study was to describe the efficacy and safety of PBS-PT in the pediatric population of interest, to establish a correlation between radiation-induced toxicity and report the post-treatment QoL.
2 Materials and Methods
2.1 Patients
All children with histologically confirmed intracranial ependymoma and treated with PBS-PT between January 1, 2004 and December 31, 2022 were included in this analysis. Patients were excluded if they were adults (>18 years), if they underwent re-irradiation, and if part of the treatment was performed with photons.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki. This non-interventional retrospective analysis was approved by the local ethics committee (EKNZ 2024-00488).
2.2 Multimodal Curative Approach
All patient cases were discussed by the institutional multidisciplinary tumor board. All patients were treated with primary surgery, followed by adjuvant PT, and optional chemotherapy, within the framework of pediatric oncology protocols [17-19]. For each patient, the parents signed an informed consent form and authorized PT.
2.3 Proton Therapy Planning and Treatment
Approximately 8 weeks after surgical resection, patients underwent a planning CT in the supine position. Based on the pre- and postoperative magnetic resonance imaging (MRI) images (particularly in T1, T2 FLAIR, and CISS) and the planning CT, the gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV), and planning organ-at-risk volume (PRV) were defined, at least since 2015, according to the International Society of Paediatric Oncology (SIOP [Société Internationale d'Oncologie Pédiatrique]) recommendations [19]. The GTVhigh_dose included the surgical bed and the potential residual tumor after surgery, adapted to the initial tumor extension. The CTVhigh_dose corresponded to the GTVhigh_dose extended by 5 mm margin (for possible microscopic spread of the disease), anatomically adapted, but allowing 2 mm inside the brainstem. The CTVboost was equivalent to the CTVhigh_dose excluding the whole brainstem, a 3 mm PRV around the C1 spinal canal, and a 3 mm PRV around the chiasm. Moreover, a 5 mm margin around CTVboost and CTVhigh_dose determined, respectively, the PTVboost (excluding the latter PRV) and the PTVhigh_dose, anatomically adapted. If the PTVhigh_dose encountered the axis or other cervical vertebrae, a PTVlow_dose was defined as the addition of the PTVhigh_dose and the vertebral bodies concerned.
In terms of proton dose prescription, fractionation of 1.8 Gyrelative biological effectiveness(RBE)/fraction was applied in a sequential scheme: 11 fractions for the vertebral low-dose coverage (19.8 GyRBE), 30 fractions for the high dose level (54.0 GyRBE), and 33 fractions for the boost (59.4 GyRBE), using a sequential boost mode.
To spare the brainstem and the cervical spinal cord, the dose was preferentially delivered with two or three proton fields, in a horizontal plane.
To ensure proper immobilization, all planning and treatment procedures were performed using either a bite-block (in this case, the patient is also intubated during planning exams), or else a face mask. For those requiring general anesthesia, midazolam and propofol were administered.
2.4 Follow-up
Clinical and radiological follow-up, including surveillance MRIs, was carried out regularly by the treating physician. Reports and images are actively requested by the study office at our institution. Follow-up questionnaires on the patient's well-being and quality of life were regularly sent to the patients and their parents. Toxicities were defined according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
2.5 Data Collection
Clinical data, such as patient gender, age at diagnosis and at first PT session, tumor histology (classic World Health Organization [WHO] Grade 2, anaplastic WHO Grade 3, ependymoblastoma WHO Grade 4), primary tumor location (infra- or supra-tentorial), extent of surgery, chemotherapy protocol, dosimetry, vital status, local and distant relapses, visual, hearing, and hormonal toxicities, as well as second cancers, were recorded from our institutional database. Radiological progression was defined on the basis of follow-up MRIs, including but not limited to sequences such as intravenous gadolinium-based contrast administration T1-weighted, T2-weighted (± contrast-enhanced fluid-attenuated inversion recovery, FLAIR), and diffusion-weighted imaging [20].
The consultation and follow-up imaging reports were collected as well as the follow-up questionnaires.
All data are retrieved from the clinical systems and stored in a separate study-specific database in coded form.
2.6 Statistical Analysis
Data processing and survival analyses were performed with R using the tidyverse, survival, survminer, and ggsurvfit packages. OS, PFS, and local control (LC), and late severe toxicity-free survival (TFS) at 5 years were estimated by Kaplan–Meier method. OS was defined as the time from start of the PT until death from any cause (event) or date of last known follow-up (censored). PFS was defined as the time from start of the PT until progression (i.e., local or distant failure) or death from any cause (both are events) or else date of last known follow-up (censored). LC was defined as the time from start of the proton therapy until local recurrence or else date of last known follow-up (censored). TFS was defined as the time from start of the PT until occurrence of a late toxicity with Grade ≥3 (event) or else date of last known follow-up (censored).
Several anamnestic and treatment-related parameters (including gender, age at PT, pathology grade, supra/infratentorial localization, resection extent, concomitant chemotherapy, total dose applied) were used to test for differences in OS, LC, PFS, hearing, and hormonal toxicities by performing univariate log-rank tests. The optimal pituitary and cochlea threshold doses for toxicity risk stratification were defined as values corresponding to the lowest p-values of the log-rank and likelihood ratio tests between the resulting two groups for the whole follow-up. If not specified otherwise, the provided p-values are the result of a log-rank test for the whole available follow-up and were considered significant for p < 0.05.
Health-related quality of life (QoL) was assessed by the validated Paediatric Quality of Life Questionnaire (PedQoL) [21], available in a self and proxy version. The instrument was developed over 20 years ago at the University of Münster in Germany, specifically for pediatric oncology patients. It can be used for patients over the age of 4 years and has been validated in different languages. The questionnaire covers eight domains: autonomy (SE), emotional functioning (EV), body image (KB), cognition regarding school and work performances (C), physical functioning in terms of activity, energy, and pain (KV), social functioning withs peers (Fr), social functioning with family (Fam), and subjective well-being (Global). For smaller children (≤4 years), the proxy Pediatric Quality of Life Inventory (PedsQL) was used for quality-of-life evaluation [22]. It covers four scales (physical, emotional, social, and school). The physical, emotional, and social scales are combined into the “psychosocial” scale and all four scales are combined into a “total” summary scale. The questionnaires were distributed before the start of PT (E1), 2 months after PT (E2), and yearly thereafter (E3 at 1 year, E4 at 2 years, E5 at 3 years, E6 at 4 years, E7 at 5 years). The calculated QoL score per domain/scale ranges from 0 to 100, with a higher score suggesting a better QoL. To compare the QoL values of the study cohort, a norm population with healthy children (self-assessment) and caregivers of healthy children (proxy assessment) were included in the analyses. For the PedQoL tool, medians and percentiles were presented, and differences between the study cohort and the norm population were tested with the Mann–Whitney U test. For the PedsQL tool, mean and standard deviation were used, and differences to the norm population were tested with t-test.
3 Results
3.1 Patient and Tumor Characteristics
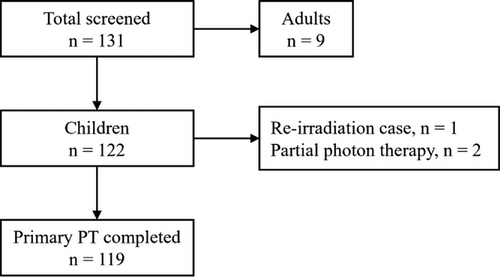
A total of 131 patients with an intracranial ependymoma were identified in our institutional database within the 20-year study period. Nine adult patients were excluded. Among the 122 children, one was retreated with protons, and two underwent part of the treatment with photons. As such, 119 patients treated with PT only were finally included in the analyses (Figure 1).
The median age of the patients at the time of PT was 36.3 months (range: 10–214.1). The cohort included a vast majority of patients with infra-tentorial tumor (70.6%), the main histology was anaplastic ependymoma (82.4%), and most of the patients were referred for primary treatment (89.9%). Other patient and tumor characteristics are described in Table 1.
| Number of patients | n = 119 (100%) |
|---|---|
| Age at diagnosis: median (range) [months] | 29.0 (3.6–212.3) |
| Age at proton therapy: median (range) [months] | 36.3 (10–214.1) |
| Gender | |
| Male | 77 (64.7%) |
| Female | 42 (35.3%) |
| Tumor grade | |
| Classic (WHO Grade 2) | 20 (16.8%) |
| Anaplastic (WHO Grade 3) | 98 (82.4%) |
| Ependymoblastoma (WHO Grade 4) | 1 (0.8%) |
| Tumor location | |
| Supra-tentorial | 35 (29.4%) |
| Infra-tentorial | 84 (70.6%) |
| Reason for referral | |
| Primary treatment | 107 (89.9%) |
| Relapse treatment (with no previous irradiation) | 12 (10.1%) |
| Extent of resection | |
| Biopsy only | 1 (0.8%) |
| Subtotal resection | 45 (37.8%) |
| Complete resection | 73 (61.4%) |
| Chemotherapy | |
| Yes | 73 (61.4%) |
| No | 46 (38.6%) |
| Proton therapy prescription | |
| General anesthesia | |
| * Yes | 102 (85.7%) |
| * No | 17 (14.3%) |
| Field of treatment: | |
| * Cranial | 96 (80.7%) |
| * Cranio-cervical | 22 (18.5%) |
| * Cranio-spinal | 1 (0.8%) |
| Total prescribed dose: median (range) [Gy, RBE] | 59.4 (44.0–63.4) |
| Number of fractions: median (range) | 33 (22–35) |
| Dose per fraction: median (range) [Gy, RBE] | 1.8 (1.8–2.0) |
| Proton therapy target volumes | |
| CTV Dmean: median (range) [Gy, RBE] | 58.6 (46.7–61.0) |
| CTV D95%: median (range) [Gy, RBE] | 54.8 (36.0–59.1) |
| PTV (range) [cm3] | 131.0 (42.3–407.5) |
| Organs at risk dosimetry | |
| Chiasm Dmax: median (range) [Gy, RBE] | 10.5 (0–56.5) |
| Optic nerves Dmax: median (range) [Gy, RBE] | 0.11 (0–55.9) |
| Brainstem Dmax: median (range) [Gy, RBE] | 58.2 (0–64.4) |
| Brainstem Dmean: median (range) [Gy, RBE] | 42.6 (0–58.5) |
| Brainstem D0.1cc: median (range) [Gy, RBE] | 57.4 (0–63.8) |
| Brainstem D50%: median (range) [Gy, RBE] | 46.9 (0–58.0) |
| Cochlea Dmax: median (range) [Gy, RBE] | 44.0 (0–64.7) |
| Pituitary Dmean: median (range) [Gy, RBE] | 0.75 (0–57.2) |
- Abbreviaions: CTV, clinical target volume; GTV, gross tumor volume; PTV, planning target volume; RBE, relative biological effectiveness; WHO, World Health Organization.
3.2 Multimodal Treatment Characteristics
The multimodal treatment strategy generally involved complete resection (61.4%), whereas sequential chemotherapy was performed in 61.4% of cases (Table 1). With regard to PT planning, most of children underwent the treatment under general anesthesia (85.7%). Most patients received cranial (80.7%) or cranio-cervical (18.5%) irradiation. Only one patient (0.8%) received cranio-spinal irradiation (CSI) for an anaplastic ependymoma (WHO Grade 3) with malignant cells on cerebrospinal fluid cytology. The median margin from GTV to CTV was 10 mm (range: 4–20). The median PT dose was 59.4 GyRBE (range: 44.0–63.4) delivered in a median of 1.8 GyRBE/fraction (range: 1.8–2.0). The details of dosimetric data are presented in Table 1.
3.3 Disease Control
The median time of follow-up was 63.3 months (range: 1.4–218.5). The median age of patients at the last follow-up was 10.2 years (3.3–22). After a 5-year follow-up, we estimated an LC of 70.4% (confidence interval [CI]: 62.2–79.7) (Figure 2), a PFS of 63.5% (CI: 55–73.3), and an OS of 82.2% (CI: 75–90.1).
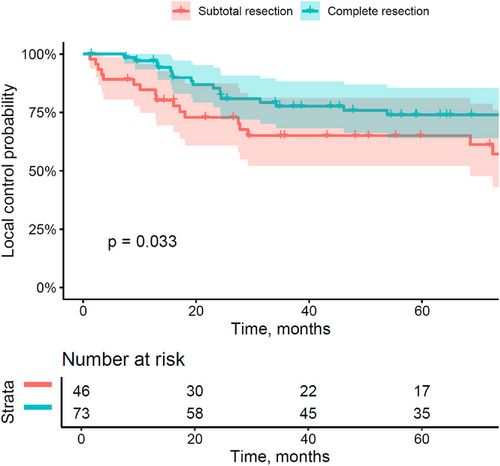
The patients referred for relapse treatment (but without previous RT) presented a worse OS than those referred for upfront treatment (5-year OS of 69.8% [CI: 46–100] vs. 83.7% [CI: 76.5–91.7]; p = 0.024). The extent of resection was also associated with a poorer prognosis, as a complete resection in comparison to a subtotal resection was associated with better local control (5-year LC of 74% [CI: 64–85.6] vs. 65.1% [CI: 52.2–81.2]; p = 0.033), but also higher PFS (5-year PFS of 67.5% [CI: 57.1–79.8] vs. 57.1% [CI: 43.7–74.6]; p = 0.049).
A total of 37 local failures (31.1%) occurred within a median of 18 months (1.2–205.1). Among them, five (13.5%) occurred more than 5 years after PT, at 5.7, 6, 8, 9, and 17 years, respectively. All patterns of failures, including distant failures, are described in Table 2.
| Number of patients | n = 119 (100%) |
|---|---|
| Median time of follow-up: median (range) [months] | 63.3 (1.4–218.5) |
| Local failure | 37 (31.1%) |
| In-field | 22/37 (59.5%) |
| Marginal | 5/37 (13.5%) |
| No data available | 10/37 (27.0%) |
| Time to local failure: median (range) [months] | 18.0 (1.2–205.1) |
| Distant failure | 13 (10.9%) |
| Brain | 8/13 (61.5%) |
| Spine | 4/13 (30.8%) |
| Brain and spine | 1/13 (7.7%) |
| Time to distant failure: median (range) [months] | 16.0 (3.7–94.1) |
3.4 Toxicity
Early toxicity (patchy alopecia, radiodermatitis) was frequent (77.3%), but high-grade toxicity (Grade 3 or higher) was reported in only 5% of patients.
A vast majority of patients (n = 86; 72.3%) did not experience any late toxicity. Late toxicity occurred in 33 patients (27.7%) at a median of 23 months (range: 3.4–177.4) after PT. The most relevant late toxicities included 17 cases (14.3%) of hormone deficiency, six cases (5.0%) of hearing loss, three cases (2.5%) of brain necrosis (one case Grade 1, one case Grade 4, one case Grade 5), and one case (0.8%) of Moyamoya vasculopathy Grade 2. Of note, the Dmax and Dmean of the three brainstem necrosis cases were: 60.1 Gy and 57.2 Gy for Grade 1, 61.2 Gy and 51.6 Gy for Grade 4, and 59.9 Gy and 47.8 Gy for Grade 5, respectively. No visual impairment was observed. Among them, seven cases (5.9%) of late toxicity Grade 3 or more were observed. The estimated TFS for high-grade toxicity at 5 years was 94.9% (CI: 90.6–99.5). The only patient treated with CSI showed delayed neurocognitive development 1.5 years after PT. All toxicities are detailed in Table 3.
| Number of patients | n = 119 (100%) |
|---|---|
| Early toxicity | |
| No | 27 (22.7%) |
| Yes | 92 (77.3%) |
| * Early toxicity Grade ≥2 | 20 (16.8%) |
| * Early toxicity Grade ≥3 | 6 (5.0%) |
| - Hearing loss Grade 3 | 1 (0.8%) |
| - Brainstem necrosis Grade 3 | 1 (0.8%) |
| - Chemotherapy-induced myelotoxicity Grade 3 | 1 (0.8%) |
| * Early toxicity Grade ≥4 | 3 (2.5%) |
| - Chemotherapy-induced myelotoxicity Grade 4 | 3 (2.5%) |
| Late toxicity | |
| No | 86 (72.3%) |
| Yes | 33 (27.7%) |
| * Time to first late toxicity: median (range) [months] | 23.0 (3.4–177.4) |
| * Late hearing loss | 6 (5.0%) |
| - Hearing loss, Grade 1 | 1 (0.8%) |
| - Hearing loss, Grade 2 | 2 (1.7%) |
| - Hearing loss, Grade 3 | 3 (2.5%) |
| * Late visual impairment | 0 |
| * Late brainstem radiation necrosis | 3 (2.5%) |
| - Brainstem radiation necrosis, Grade 1 | 1 (0.8%) |
| - Brainstem radiation necrosis, Grade 4 | 1 (0.8%) |
| - Brainstem radiation necrosis, Grade 5 | 1 (0.8%) |
| * Late hormone deficiency | 17 (14.3%) |
| - Hormone deficiency, Grade 1 | 2 (1.7%) |
| - Hormone deficiency, Grade 2 | 13 (10.9%) |
| - Hormone deficiency, Grade 3 | 2 (1.7%) |
| * Other late toxicity, Grade ≥2 | 6 (5.0%) |
| - Concentration difficulties, Grade 2 | 3 (2.5%) |
| - Ataxia, Grade 2 | 1 (0.8%) |
| - SMART syndrome, Grade 2 | 1 (0.8%) |
| - Moyamoya (vasculopathy), Grade 2 | 1 (0.8%) |
| TFS: Kaplan–Meier estimate at 5 years (CI) | 94.9% (90.6–99.5) |
| Number of secondary tumors | 4 (3.4%) |
| Meningioma, WHO Grade 1 [time from start of proton therapy] | 1 (0.8%) [180.5 months] |
| Astrocytoma, WHO Grade 3 [time from start of proton therapy] | 1 (0.8%) [44.8 months] |
| Glioblastoma [time from start of proton therapy] | 1 (0.8%) [100.0 months] |
| Glioblastoma [time from start of proton therapy] | 1 (0.8%) [123.8 months] |
- Note: Toxicity grades are determined according to the Common Terminology Criteria for Adverse Events (CTCAE) classification, version 5.0.
- Abbreviations: SMART, stroke-like migraine attacks after radiation therapy; WHO, World Health Organization.
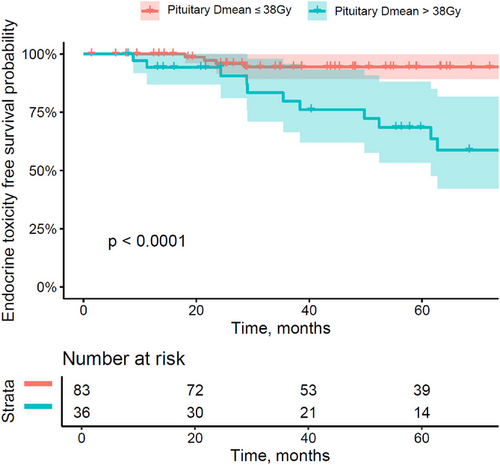
We did not observe any hearing loss in patients receiving a cochlea Dmax less or equal to 48 GyRBE as compared to the patients with a cochlea Dmax over 48 GyRBE, where hearing loss occurred in a significantly higher portion of the patients (10.5% vs. 0%; p = 0.0097). Similarly, the retrospective dosimetric analysis showed that Dmean to pituitary gland over 38 GyRBE was significantly associated with hormone deficiency—developed in 33.3% of patients of this group in comparison to 6.0% of patients with Dmean to pituitary gland ≤38 GyRBE (p = 0.00007) (Figure 3).
Secondary brain tumors occurred in four cases (3.4%), within a median time to event of 9.3 years after PT (range: 3.7–15). The first patient was a male aged 13 years at the time of diagnosis. The initial surgery was limited to a supra-tentorial tumor biopsy, revealing an ependymoma Grade 2. He underwent adjuvant chemotherapy with irinotecan/cisplatin (Eurodract protocol), as well as adjuvant cranial PT up to 59.4 GyRBE, delivered in 33 fractions of 1.8 GyRBE/fraction. Seven months after PT, he presented with a hormone deficiency Grade 2 (thyroid-stimulating hormone [TSH] and cortisol), associated with concentration difficulties. A Grade 3 astrocytoma was diagnosed in the periventricular region (encompassing 10–58 Gy isodoses), after 3.7 years of follow-up. The patient finally died from primary disease 4 years after PT, at the age of 18 years.
The second case of the secondary tumor was a 10-month-old male with infra-tentorial ependymoma Grade 3, treated with upfront subtotal resection, chemotherapy (irinotecan/cisplatin), and adjuvant cranial PT (59.4 GyRBE). No early toxicity was reported, but he developed a Grade 3 growth hormone deficiency 7 years after PT. One year later, he was diagnosed with a secondary glioblastoma in the posterior fossa (from 20 to 55 Gy isodoses), whose postoperative complications led to the patient's death at the age of 10 years.
The third patient was a 19-month-old male, diagnosed with an infra-tentorial ependymoma Grade 3. He initially underwent complete surgery, followed by chemotherapy including carboplatin/etoposide/ifosfamide (Children's Oncology Group [COG] ACNS 0122 protocol). The adjuvant cranial PT delivered 59.4 GyRBE and was responsible for an asymptomatic early brainstem necrosis. A glioblastoma of the posterior fossa (within the 59.4 Gy isodose region) was discovered 10.3 years after PT, and caused the patient's death at the age of 14 years.
The fourth case of secondary brain tumor involved a male aged 23 months at the time of diagnosis. The treatment included a complete resection of an infra-tentorial ependymoma Grade 3, chemotherapy according to HIT-2000 protocol, and a 54 GyRBE adjuvant cranial PT, delivered at 1.8 GyRBE/fraction. The child presented with a Grade 1 growth hormone deficiency, 4.3 years after the end of PT. After 15 years of follow-up, a Grade 1 meningioma was diagnosed in the posterior fossa (from 17 to 50 Gy isodoses). Almost 18 years after PT, the patient was still alive with no sign of primary disease.
3.5 Quality of Life
In the PedsQL subcohort (patients under 4 years of age), 55 children were proxy-evaluated before PT (E1), and 24 after 2 years of PT (E4). At both time points, parents rated their children QoL significantly worse than the norm group in physical, emotional, and psychosocial domains (Table 4).
| PedsQL (t-tests) |
Physical (p-value) |
Emotional (p-value) |
Social (p-value) |
School (p-value) |
Psychosocial (p-value) |
Total (p-value) |
|---|---|---|---|---|---|---|
| Number of control children in the norm group = 2883 | 89.8 | 84.3 | 88.4 | 87.8 | 86.5 | 87.8 |
| Number of patients before the start of proton therapy (E1, baseline) = 55 | 72.6 (<0.001*) | 70.2 (<0.001*) | 78.8 (0.006*) | 68.2 (0.165) | 74.2 (<0.001*) | 73.4 (0.006*) |
| Number of patients 2 years after proton therapy (E4, 2 years after PT) = 24 | 76.7 (0.013*) | 73.5 (0.003*) | 81.5 (0.105) | 77.0 (0.010*) | 77.3 (0.004*) | 77.0 (0.005*) |
- Note: Ependymoma cohort (at baseline and 2 years after PT) and control children, including t-test for comparison to the norm group, respectively.
- Abbreviations: PedsQL, Pediatric Quality of Life Inventory; PT, proton therapy.
- *A p-value of less than 0.05 between patient and norm group was considered significant.
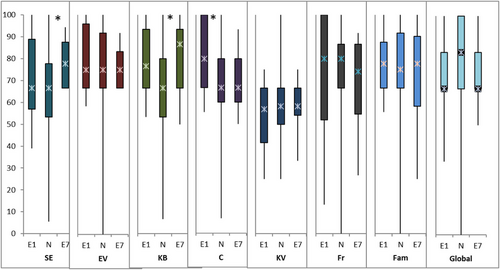
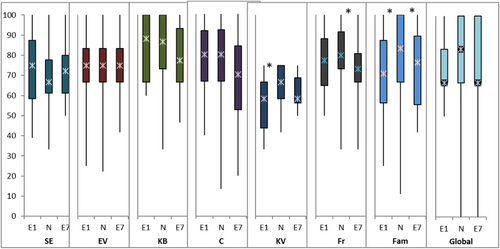
In the PedQoL subcohort (children over the age of 4 years), the QoL domains were either self- and/or proxy-assessed. At the start of PT (E1), 13 children with a median age of 10 years (5–17 years) rated their QoL in the “cognition” domain significantly better than the norm group (Figure 4), whereas the parents of 20 children aged 7.5 years (range: 4–17) proxy-rated “physical functioning” and “social functioning in family” domains significantly worse than the norm group (Figure 5). Five years later (E7), 17 children rated their QoL in the domains “autonomy” and “body image” significantly better than the norm group (Figure 4), while QoL of 26 children was proxy-rated significantly worse than the norm group in the domains of peer and family social functioning (Figure 5). All results of self- and proxy-assessments, and their comparisons to the norm groups, are summarized in Table S1.
4 Discussion
4.1 Disease Control
Our 5-year follow-up showed a 5-year PFS of 64%, similar to the PFS rates described for a decade in other series of pediatric intracranial ependymoma PT, ranging from 55.6% [23] to 82% [7] at 3 years. The 5-year OS of 82% in our cohort was lower than others’ OS with shorter follow-up [7, 16, 23], but matching the one described after 7 years in the world's largest cohorts of PT ependymoma [12, 24]. Given the significant OS deterioration in the case of salvage PT (69.8%) compared with adjuvant PT (83.7%), emphasis should be placed on the latter strategy in the first-line setting.
Our 70% 5-year LC was somewhat identical to the 74% at 3 years reported by the German group in 2022 [23], but lower than the 85.4% at 3 years described by the University of Florida Health Proton Therapy Institute in 2018 [11], and still under the 76%–77% reported after a longer follow-up of 7 years [12, 24] (Table 5).
| Institution | Reference | Treatment period | PT technique | No. patients | Median dose, Gy (RBE) | Median follow-up (years) | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Disease control | Toxicity | |||||||
| University of Florida Health Proton Therapy Institute |
Indelicato et al. Int J Radiat Oncol Biol Phys, 2021 [12] |
2000–2019 | PS | 386 | 55.8 | 5 |
7-year LC = 77% 7-year PFS = 63.8% 7-year OS = 82.2% |
Hearing loss = 5.4% Hormone deficiency = 11.7% Fatal brainstem RN = 2 (0.5%) Second tumor = 5 (3 malignant) |
|
Indelicato et al. Acta Oncol, 2018 [11] |
2007–2017 | PS | 179 | 59.4 | 3.2 | 3-year LC = 85.4% |
Acute Grade ≥2 = 10% Late Grade ≥2 = 7.3%: • Hearing loss = 6.1% • Fatal brainstem RN = 1 (0.5%) |
|
| MD Anderson |
Sato et al. Cancer, 2017 [7] |
2000–2013 | PS | 41 | 55.8 | 2.6 |
3-year PFS = 82% 3-year OS = 97% |
RN = 7.3% |
| France |
Ducassou et al. IJROBP, 2018 [15] |
2000–2013 | N/A | 17 | 57.6 | 4.5 | N/A | N/A |
|
Dalmasso et al. Radiother Oncol, 2024 [14] |
2007–2020 | PBS |
35 PS 7 PBS |
59.4 | 5.6 | N/A | N/A | |
| Germany |
Peters et al. Neuro Oncol, 2022 [21] |
2013–2017 | PS or PBS |
35 PBS 45 PS 5 PBS+PS |
59.4 | 3.3 |
3-year LC = 74.1% 3-year PFS = 55.6% 3-year OS = 93.7% |
Acute Grade ≥3 = 8.6% Late Grade ≥3 = 10.5% |
| MGH |
MacDonald et al. Neuro Oncol, 2013 [16] |
2000–2011 | PS | 70 | 55.8 | 3.8 |
3-year PFS = 76% 3-year OS = 95% |
Median height-loss = 2.6% Hearing loss = 2.9% Second malignancy = 0 |
| PSI |
Ares et al. J Neurooncol, 2016 [10] |
2004–2013 | PBS | 50 | 59.4 | 3.6 |
5-year LC = 78% 5-year OS = 84% |
Late Grade 1–2 = 38% Late Grade ≥3 = 6%: • Unilateral deafness = 2 • Fatal brainstem RN = 1 |
|
Tran et al. Pediatr Blood Cancer, 2020 [30] |
1999–2017 | PBS | 88 | 54 | 4.3 | 5-year OS = 80% | Second malignancy = 2 | |
- Abbreviations: LC, local control; N/A, not applicable; OS, overall survival; PBS, pencil beam scanning; PFS, progression-free survival; PS, passive scattering; PSI, Paul Scherrer Institute; PT, proton therapy; RBE, relative biological effectiveness; RN, radiation necrosis.
Our lower LC rate could be explained by the lower amount of complete resection in our cohort (61.4% vs. 85% in [12])—given the prognostic value of the resection extent [25]—and also by the higher amount of patients requiring chemotherapy prior to PT, suggestive of a more aggressive tumor (61.4% in our cohort vs. 27.2% in [12]) occurring in our cohort. Our increased rate of local failures (31%) does not result from the very narrow penumbra of proton radiation and/or the margins used for PT, but remains difficult to interpret due to the lack of data concerning the patterns of local failure in 10 cases (Table 2).
The choice of PT technique could also explain the discrepancies in disease control. Indeed, the PT field arrangement changed after the Grade 5 brainstem necrosis event. The patient who presented this fatal toxicity was treated with narrow fields ranging out in the brainstem, which was partially included in the PTV. After a radiophysics internal study, the use of narrow fields has been limited to approximately 20 GyRBE, lateral and posterior–anterior fields and three non-coplanar fields were used for the treatment. Lastly, the difference between PBS and passive scattering in terms of disease control remains difficult to assess, given the preponderance of passive scattering series in the literature [7, 11, 12, 16, 24].
4.2 Toxicity and Risk of Secondary Tumor
The rate of early toxicity in our cohort was well in line with the literature [11, 23] (Table 5). Within the minority of our patients who presented late toxicity (n = 33; 27.7%), the rate of hormone deficiency (14.3%) corresponded roughly to that of the largest series of pediatric ependymoma PT (11.7%) [12] (Table 5). The Dmean threshold of 38 GyRBE to the pituitary gland that we identified as a prognostic factor for hormone deficiency is similar to the hypothalamic and pituitary median dose of 40 Gy that Vatner et al. used as a predictor for high risk of hormone deficiency [26].
In terms of hearing loss, the 5.0% rate in our cohort falls within the range of the literature, between the 2.9% of MacDonald et al. [16] and the most recent results of 5.4% [12]. Once again, the Dmax threshold of 48 GyRBE at the cochlea is in line with the literature for predicting the risk of hearing loss [27].
The 2.5% rate of late brainstem necrosis in our cohort was lower than that of MD Anderson's cohort (7.3%) [7], bearing in mind that the latter used a passive scattering technique. The rate of fatal brainstem necrosis (0.8%) was congruent with the most recent data from bigger cohort series (0.5%) [12].
Regarding the risk of secondary tumors, literature data on ependymoma PT are scarce. In their series of 386 patients, Indelicato et al. reported five cases of secondary tumors (1.3%), three of them malignant (0.8%) [12] (Table 5). As a comparison, we reported four secondary tumors (3.4%), including three malignant ones (2.5%).
4.3 Quality of Life
Data on QoL in children treated with PT for ependymoma are scarce [28, 29]. This pediatric series is the first to the best of our knowledge to assess QoL in intracranial ependymoma patients treated with PBS-PT. At 5 years of follow-up (E7), patients’ overall QoL was good, according to both proxy- and self-assessment.
Interestingly, patients themselves assessed their own QoL better than their parents did. They even evaluated their own “autonomy” and “body image” better than the norm group did. This inter-rater discrepancy is a well-known effect reported in the literature, especially for children with severe diseases such as cancer [30]. Parents are very concerned about their children and may be overcautious, which may lead to an overrating of limitations and therefore worse assessment of their quality of life. Whereas the children themselves may be very attentive to changes in their bodies and their social environment due to their disease and appreciate every small improvement more, which may lead to a better assessment of their own quality of life.
4.4 Limitations
There were several limitations of our study. First, the study design was retrospective in nature and thus lacked complete data for certain variables such as cognitive functions. The small sample size of 119 patients limited the statistical power to detect independent associations between treatment failure and some clinical factors examined. Second, although the inherent limitations of retrospective chart review regarding toxicity assessment are acknowledged, most (84.1%) of the late toxicities attributed to PT in our study were Grade 1 (36.4%) or Grade 2 (47.7%). Additionally, no molecular data were available for the vast majority of patients and the outcome/recurrence pattern was assessed agnostic of any molecular classification, although it is known that EP is not a uniform disease but represents different disease types with clinical and biological heterogeneity. Finally, for the QoL, results must be interpreted with caution, given the external nature of proxy evaluations and the potential lack of discernment of children in self-evaluations. While a self-assessment of “body image” seems appropriate, the “autonomy” domain, on the other hand, might be better assessed by proxy. Another limitation of this study is that the PedQoL questionnaire has been in use for over 20 years, but has only been applied to a few series of cephalic RTs [31-34], so the interpretation of the results is open to question.
5 Conclusion
This study, which represents the world's largest series of pediatric intracranial ependymomas treated with PBS-PT with QoL data, confirmed the importance of complete resection for LC and PFS, and highlighted the crucial role of early PT in OS. No hearing loss was observed with cochlea Dmax not exceeding 48 GyRBE, whereas the risk of hormone deficiency was significantly increased with pituitary Dmean above 38 GyRBE. Beyond clinical outcomes, intracranial PBS-PT revealed good overall quality of life in children with ependymoma, both by proxy- and self-assessment. The rate of secondary tumors should be the subject of a specific in-depth study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.




