A Phase 1/2 Study of Lenvatinib in Combination With Everolimus in Recurrent and Refractory Pediatric and Young Adult Solid Tumors
Karen O'Hara and Cixin S. He formerly of Eisai.
Funding: This study was sponsored by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA. Medical writing was provided by Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA.
Prior related presentations: Data from this manuscript have been presented, in part, at the International Society of Paediatric Oncology Congress (October 11–14, 2023; Ottawa, Canada).
ABSTRACT
Introduction
Developing targeted therapies with manageable toxicities remains a high priority for pediatric cancer. We sought to determine the recommended Phase 2 dose (RP2D) and evaluate the antitumor activity of lenvatinib+everolimus in children/young adults with select recurrent/refractory solid tumors.
Methods
Patients 2–21 years old were eligible. Phase 1 used a rolling-six design. Phase 2 was limited to patients with Ewing sarcoma (EWS), rhabdomyosarcoma (RMS), or high-grade glioma (HGG), and ≤2 prior VEGF/VEGFR-targeted therapies. Primary endpoints included the determination of maximum tolerated dose (MTD), RP2D, safety/toxicity (Phase 1), and objective response rate (ORR) per RECIST version 1.1 (RANO for HGG) at Week 16 (Phase 2).
Results
In Phase 1, 23 patients received lenvatinib 11 mg/m2 (dose level [DL] 1, n = 18) or 8 mg/m2 (DL −1, n = 5) combined with everolimus 3 mg/m2 orally once daily. DL1 was declared the MTD/RP2D given dose-limiting toxicities (proteinuria [n = 1]; hypertriglyceridemia and hypercholesterolemia [n = 1]) observed in two of 12 patients treated at DL1. In Phase 2, 41 patients (EWS, n = 10; RMS, n = 20; HGG, n = 11) were treated with the RP2D. Two patients with RMS experienced partial response by Week 16. No other objective responses were observed. Two patients with EWS experienced prolonged disease control (≥23 weeks). No new safety signals were identified. The safety profile was similar to those of treated adults with renal cell carcinoma.
Conclusion
Lenvatinib+everolimus has a manageable safety profile in this pediatric population. Despite unmet efficacy endpoints, the antitumor activity observed in RMS and EWS may warrant further study in select pediatric solid tumors.
ClinicalTrials.gov number
NCT03245151
Abbreviations
-
- AE
-
- adverse event
-
- AUC
-
- area under the curve
-
- BOR
-
- best overall response
-
- CBR
-
- clinical benefit rate
-
- Cmax
-
- peak plasma concentration
-
- CNS
-
- central nervous system
-
- COG
-
- Children's Oncology Group
-
- CR
-
- complete response
-
- DL
-
- dose level
-
- DLT
-
- dose-limiting toxicity
-
- DOR
-
- duration of response
-
- EWS
-
- Ewing sarcoma
-
- FDA
-
- US Food and Drug Administration
-
- FGF
-
- fibroblast growth factor
-
- HGG
-
- high-grade glioma
-
- KPS
-
- Karnofsky Performance Status
-
- LPS
-
- Lansky play score
-
- MTD
-
- maximum tolerated dose
-
- mTOR
-
- mammalian target of rapamycin
-
- ORR
-
- objective response rate
-
- PDGF
-
- platelet-derived growth factor
-
- PFS
-
- progression-free survival
-
- PK
-
- pharmacokinetic
-
- PR
-
- partial response
-
- RANO
-
- Response Assessment in Neuro-Oncology
-
- RCC
-
- renal cell carcinoma
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- RMS
-
- rhabdomyosarcoma
-
- RP2D
-
- recommended Phase 2 dose
-
- RTKI
-
- receptor tyrosine kinase inhibitor
-
- SD
-
- stable disease
-
- TEAE
-
- treatment-emergent adverse event
-
- VEGF
-
- vascular endothelial growth factor
-
- VEGFR
-
- vascular endothelial growth factor receptor
1 Introduction
Although conventional multimodal therapies for pediatric solid and central nervous system (CNS) tumors have improved survival over the last several decades, approximately 20% of patients with pediatric cancers do not survive 5 years after diagnosis, and long-term survivors carry a lifelong burden of morbidity [1]. Thus, a significant unmet medical need remains for more effective treatment options with manageable safety profiles.
Overexpression of angiogenic factors across a variety of pediatric extracranial solid tumors including rhabdomyosarcoma (RMS), Ewing sarcoma (EWS), and other bone sarcomas [2, 3], along with platelet-derived growth factor (PDGF) receptor-α amplification and/or mutations in pediatric high-grade glioma (HGG) [4], have supported testing multitargeted receptor tyrosine kinase inhibitors (RTKIs) as a potential therapeutic strategy. The potential benefit of RTKIs is further supported by their preclinical activity across a variety of pediatric solid tumors [5, 6]. Lenvatinib is an oral RTKI of vascular endothelial growth factor (VEGF) receptors (VEGFRs) 1–3, fibroblast growth factor (FGF) receptors 1–4, PDGF receptor-α, KIT, and RET. In addition to targeting VEGF-mediated angiogenesis and lymphangiogenesis, lenvatinib also demonstrates more potent inhibitory activity toward FGF receptors compared with other RTKIs [7-11]. Lenvatinib has also demonstrated antitumor activity and CNS penetration by tandem mass spectrometry imaging in preclinical models of CNS metastasis [12].
Everolimus is an oral rapamycin analog that acts as a highly selective allosteric inhibitor of the mammalian target of rapamycin (mTOR) complex 1 [13]. When given in combination with the multi-RTKI sorafenib, everolimus demonstrated enhancement of the anti-angiogenic, anti-proliferative, and pro-apoptotic effects of RTK inhibition in various in vitro and in vivo solid tumor models [14-17], suggesting that dual inhibition may overcome mechanisms of drug resistance. Proangiogenic signaling pathways targeted by lenvatinib (VEGF, FGF) cooperate with mTOR signaling to mediate tumor cell growth and maintenance in pediatric solid tumors [3, 15, 18]. Hence, dual targeting of VEGF-mediated and mTOR signaling pathways is hypothesized to overcome therapy resistance inevitable with monotherapy, and result in enhanced and prolonged efficacy. Notably, the combination of lenvatinib and everolimus (18 mg/day and 5 mg/day, respectively) significantly prolonged progression-free survival when compared with everolimus monotherapy in adults with previously treated advanced renal cell carcinoma (RCC) [19], leading to the US Food and Drug Administration (FDA) approval of the combination for this population [20].
A Phase 1 dose-finding study of lenvatinib monotherapy in pediatric and young adult patients with relapsed/refractory solid tumors established lenvatinib 14 mg/m2 as the recommended Phase 2 dose (RP2D) (NCT02432274) [21]. The treatment demonstrated a safety profile that was overall consistent with that observed in adults with differentiated thyroid cancer [22] and showed promising activity in relapsed/refractory osteosarcoma (progression-free survival rate at 4 months of 37.8% [21]). Everolimus alone (5 mg/m2/day) [23], and in combination with bevacizumab [24], was also deemed tolerable in pediatric patients with recurrent/refractory solid tumors.
We present the results of a Phase 1/2 study evaluating lenvatinib in combination with everolimus in pediatric patients with recurrent/refractory solid and CNS tumors, including RMS, EWS, and HGG, using a novel drug combination to target pro-tumorigenic and resistance mechanisms driven by VEGF/FGF/PDGF and mTOR signaling.
2 Methods
2.1 Patients
Children and young adults (aged ≥2 to ≤18 years in Phase 1, and ≥2 to ≤21 years in Phase 2) with a histologically or cytologically confirmed diagnosis of recurrent or refractory malignant solid tumor (excluding hepatoblastoma and lymphomas), including primary CNS tumors, were eligible for Phase 1. Patients were required to have adequate organ function and a Karnofsky Performance Status (KPS; patients >16 years of age) or Lansky play score (LPS, patients ≤16 years of age) of ≥50. In Phase 1, patients with evaluable disease (non-target lesions only) or measurable disease (target and/or non-target lesions) per Response Evaluation Criteria in Solid Tumors (RECIST) version (v) 1.1 or Response Assessment in Neuro-Oncology (RANO), as appropriate, were eligible. Phase 2 was limited to patients with a diagnosis of EWS, RMS, or HGG (excluding patients with diffuse intrinsic pontine glioma) with measurable disease and ≤2 prior VEGF/VEGFR-targeted therapies (but no prior VEGF/VEGFR-targeted therapy in combination with an mTOR inhibitor).
This study was conducted in collaboration with the Children's Oncology Group (COG) in accordance with the Good Clinical Practice guidelines of the International Council for Harmonization and ethical principles originating from the Declaration of Helsinki. All patients and/or their parent(s) or legally authorized representative(s) signed an informed consent/assent form before any study-specific procedures were performed.
2.2 Study Design and Treatments
Study 216/COG-ADVL1711 (NCT03245151) was a multicenter, open-label, single-arm, Phase 1/2 trial of lenvatinib in combination with everolimus, each administered orally once daily in 28-day treatment cycles. Lenvatinib was provided as hard capsules, and everolimus was administered as a dispersible tablet. Younger patients who were unable to swallow tablets received an oral extemporaneous solution of lenvatinib. The Phase 1 portion used a rolling-six design [25] with dose escalation in sequential cohorts. Dose-limiting toxicity (DLT) assessment for dose escalation was limited to the first cycle; therefore, the “treatment phase” was defined as 4 weeks of therapy in Phase 1. Patients with nonprogressive disease could subsequently receive up to 24 cycles of treatment. The initial dose level (dose level 1; DL1) for lenvatinib was 11 mg/m2 (the maximum dose was 18 mg/day), representing approximately 80% of the single-agent maximum tolerated dose (MTD) of lenvatinib in pediatric and young adult patients with solid tumors (14 mg/m2) [21]. The initial dose of everolimus was 3 mg/m2, which is 66% of the FDA-approved dose for adults and pediatric patients with tuberous sclerosis complex-associated subependymal giant cell astrocytoma [26]. The maximum everolimus dose was 5 mg/day (equivalent to the adult dose when used in combination with lenvatinib for advanced RCC [19]). A single de-escalation to dose level −1 (DL –1), consisting of lenvatinib 8 mg/m2 plus everolimus 3 mg/m2, was incorporated as a contingency for toxicity at DL1. The Phase 2 portion was initiated at RP2D in patients with a diagnosis of EWS (Cohort 1), RMS (Cohort 2), or HGG (Cohort 3). In Phase 2, the “treatment phase” was defined as 16 weeks of therapy. Patients could subsequently proceed with treatment in an “extension phase” for up to 24 cycles. All patients could receive study treatment until disease progression, lack of clinical benefit (per investigator's judgment), unacceptable toxicity, withdrawal from the study for any reason, or termination of the study by the sponsor. The study design is shown in Figure S1.
2.3 Study Assessments and Endpoints
DLT and safety assessments included monitoring and recording all adverse events (AEs) per the Common Terminology Criteria for Adverse Events version 4.03. DLTs attributable to the investigational agents were to include Grade 4 thrombocytopenia or neutropenia; any Grade ≥2 arterial thromboembolic events; any nonhematologic Grade 4 toxicity with the exception of fever <5 days; Grade 3 or 4 febrile neutropenia; any Grade 3 nonhematologic toxicity with exceptions of nausea, vomiting, diarrhea, or headache <3-day duration; weight loss; fever or infection <5 days; liver enzyme elevation (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, bilirubin, or alkaline phosphatase), amylase, lipase, or triglycerides that improve to Grade ≤1 or baseline within 7 days; proteinuria unless confirmed within 72 hours; any Grade 2 toxicity persisting for ≥7 days and considered sufficiently medically significant or intolerable by patients despite optimal supportive care; and any dose interruption or reduction due to toxicity resulting in administration of <75% of the planned dosage of lenvatinib and/or everolimus. Dose-limiting hypertension was defined as Grade 4 hypertension with confirmed systolic or diastolic blood pressure >25 mmHg above the 95th percentile for sex, age, height/length; or elevated diastolic blood pressure (i.e., >95th percentile for age) not controlled by a single antihypertensive medication within 14 days of study drug use. Investigator-determined response assessments were based on RECIST v1.1 for all tumor types except HGG, where assessments were based on RANO (see Supporting Information Methods for details). Serial blood samples for plasma concentrations of lenvatinib and whole blood concentrations of everolimus were collected for pharmacokinetic (PK) analyses.
For Phase 1, the primary endpoints were MTD, RP2D, and safety/toxicity. PK was a secondary endpoint, and provisions were made to ensure that data were available from at least six patients younger than the age of 6 years. For Phase 2, the primary endpoint was the objective response rate (ORR) at Week 16. ORR was defined as the sum of complete response (CR) and partial response (PR). For both phases, secondary endpoints included ORR at the time of data cutoff, disease control rate (proportion of patients who had the best overall response [BOR] of CR, PR, or stable disease [SD; duration of ≥7 weeks since the first dose of the study treatment]), clinical benefit rate (CBR; defined as the proportion of patients who had a BOR of CR, PR, or durable SD [duration ≥23 weeks since the first dose of the study treatment]), and duration of response (DOR).
Safety was evaluated in all patients who received at least one dose of the study drug (safety analysis set). In the safety analysis set, PK analyses were conducted in all patients with at least one measurable post-dose plasma concentration of lenvatinib and an adequately documented dosing history. Plasma concentrations of lenvatinib were determined using validated liquid chromatography with tandem mass spectrometry. The lower limit of quantitation for lenvatinib was 0.250 ng/mL in human plasma. Phase 2 efficacy endpoints were evaluated in all patients with measurable disease at baseline and underwent at least one post-baseline efficacy assessment (evaluable analysis set; additional details are included in the Supporting Information Methods).
2.4 Statistical Methods
Dose escalation utilized a rolling-six design [25] in which the MTD was considered exceeded, and dose escalation stopped if more than 33% of patients (≥2/6) experienced DLTs. If two DLTs at a given dose level were of different AE classes, at least one did not appear to be dose related, and both were readily reversible, then expansion of the cohort could be considered. RP2D was to be declared based on the totality of safety data observed during Phase 1. Secondary safety endpoints were summarized descriptively. ORR was assessed only in patients with measurable disease at screening/baseline. Patients with evaluable disease were analyzed separately for BOR. None of the patients treated in Phase 1 were included in Phase 2 cohorts. In Phase 2, a 10+10 Simon's optimal two-stage design was used for each cohort (10 evaluable patients per stage; see Supporting Information Methods for additional details) with the goal of detecting an ORR of 20% (88% power to detect a 20% increase in response rate with one-sided alpha = 0.07 assuming a null response rate of 5% and alternative response rate of ≥25%). If ≥1 responses were observed after the first 10 patients were treated, enrollment in the cohort would continue for up to 20 patients total. If no objective responses (CR/PR) were observed in the first 10 patients, enrollment was ceased, and the therapy was deemed inactive for the cohort. For an expanded cohort, if ≤2 objective responses were observed, the lenvatinib/everolimus combination was deemed insufficiently active for that cohort. ORR (at Week 16 and the cutoff date) and CBR were summarized descriptively, with 95% confidence intervals (CIs) determined using the Clopper–Pearson exact method. DOR was analyzed using the Kaplan–Meier method. Database lock occurred after all patients had withdrawn from the study and were no longer in survival follow-up.
3 Results
3.1 Patient Disposition and Baseline Characteristics
In Phase 1, 23 patients met eligibility criteria and were treated at dose level 1 (DL1) or −1 (DL −1). In Phase 2, 41 patients met eligibility criteria and received ≥1 doses of study treatment (EWS, n = 10; RMS, n = 20; HGG, n = 11, with one patient not evaluable for tumor response). At the time of database lock (November 14, 2022), all patients had discontinued study treatment. Additional details on patient disposition are shown in Figure S2.
Baseline demographic and disease characteristics are shown in Table 1 and Table S1. In Phase 1, most patients were White (60.9%), had a KPS/LPS of ≥80 (82.6%), were <17 years of age (91.3%, including 30.4% <6 years of age), and were female (52.2%). In Phase 2, most patients were White (75.6%), had a KPS/LPS of ≥80 (83%), were <17 years of age (61%), and were male (53.7%). Patients in both Phase 1 and Phase 2 were heavily pretreated with a median (range) number of prior regimens of two (1–7) and two (1–10), respectively. Of patients in Phase 1 and Phase 2, 78.3% and 85.4% had received ≥2 prior regimens, respectively, and 21.7% and 14.6%, respectively, had received prior VEGF-targeted therapy (Table 1).
| Phase 1 | Phase 2 LEN 11 mg/m2 + EVE 3 mg/m2 |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Dose level −1: LEN 8 mg/m2 + EVE 3 mg/m2 (n = 5) |
Dose level 1: LEN 11 mg/m2 + EVE 3 mg/m2 (n = 18) |
Total (N = 23) |
EWS (n = 10) |
RMS (n = 20) |
HGG (n = 11) |
Total (N = 41) |
| Median age, years (range) | 15.0 (12–21) | 7.0 (3–19) | 9.0 (3–21) | 16.5 (3–19) | 15.0 (2–21) | 14.0 (9–18) | 15.0 (2–21) |
| Sex, n (%) | |||||||
Male Female |
3 (60.0) 2 (40.0) |
8 (44.4) 10 (55.6) |
11 (47.8) 12 (52.2) |
7 (70.0) 3 (30.0) |
12 (60.0) 8 (40.0) |
3 (27.3) 8 (72.7) |
22 (53.7) 19 (46.3) |
| Race, n (%) | |||||||
White Black or African American Asian American Indian or Alaskan Native Othera |
3 (60.0) 1 (20.0) 0 0 1 (20.0) |
11 (61.1) 1 (5.6) 1 (5.6) 1 (5.6) 4 (22.2) |
14 (60.9) 2 (8.7) 1 (4.3) 1 (4.3) 5 (21.7) |
9 (90.0) 0 1 (10.0) 0 0 |
16 (80.0) 1 (5.0) 0 0 3 (15.0) |
6 (54.5) 3 (27.3) 0 0 2 (18.2) |
31 (75.6) 4 (9.8) 1 (2.4) 0 5 (12.2) |
| Median BSA, m2 (range) | 1.560 (1.48–1.92) | 0.870 (0.60–2.13) | 1.000 (0.60–2.13) | 1.680 (0.65–2.34) | 1.525 (0.60–2.15) | 1.550 (1.02–2.27) | 1.630 (0.60–2.34) |
| KPS/Lansky play score, n (%) | |||||||
50 60 70 80 90 100 |
0 0 0 2 (40.0) 2 (40.0) 1 (20.0) |
0 1 (5.6) 3 (16.7) 2 (11.1) 7 (38.9) 5 (27.8) |
0 1 (4.3) 3 (13.0) 4 (17.4) 9 (39.1) 6 (26.1) |
0 0 1 (10.0) 2 (20.0) 4 (40.0) 3 (30.0) |
1 (5.0) 0 2 (10.0) 6 (30.0) 4 (20.0) 7 (35.0) |
0 2 (18.2) 1 (9.1) 3 (27.3) 4 (36.4) 1 (9.1) |
1 (2.4) 2 (4.9) 4 (9.8) 11 (26.8) 12 (29.3) 11 (26.8) |
| Median age at first tumor diagnosis, years (range) | 13.0 (5–19) | 4.5 (0–16) | 5.0 (0–19) | 13.0 (1–14) | 12.5 (1–18) | 12.0 (0–17) | 12.0 (0–18) |
| Median time since metastatic diagnosis to first dose of study drug, months (range)b | 22.78 (19.6–48.6) | 21.52 (0.5–46.2) | 21.52 (0.5–48.6) | 20.55 (0.7–81.0) | 11.83 (0.5–45.9) | 1.58 (0.4–50.9) | 10.83 (0.4–81.0) |
| Median age at metastatic diagnosis, years (range)b | 13.5 (9–19) | 7.0 (1–16) | 8.0 (1–19) | 13.0 (3–18) | 13.0 (1–20) | 12.0 (9–18) | 13.0 (1–20) |
| Metastatic sites at baseline, n (%) | |||||||
1 2 ≥3 |
3 (60.0) 2 (40.0) 0 |
15 (83.3) 2 (11.1) 1 (5.6) |
18 (78.3) 4 (17.4) 1 (4.3) |
5 (50.0) 5 (50.0) 0 |
10 (50.0) 7 (35.0) 3 (15.0) |
11 (100) 0 0 |
26 (63.4) 12 (29.3) 3 (7.3) |
| Site of lesion, n (%)c, d | |||||||
Lung Bone Brain Liver Lymph node Kidney Lung and bone Other |
1 (20.0) 2 (40.0) 3 (60.0) 0 0 0 1 (20.0) 1 (20.0) |
8 (44.4) 1 (5.6) 9 (50.0) 0 3 (16.7) 0 1 (5.6) 2 (11.1) |
9 (39.1) 3 (13.0) 12 (52.2) 0 3 (13.0) 0 2 (8.7) 3 (13.0) |
7 (70.0) 5 (50.0) 0 0 0 0 3 (30.0) 3 (30.0) |
8 (40.0) 3 (15.0) 0 1 (5.0) 5 (25.0) 2 (10.0) 1 (5.0) 15 (75.0) |
0 0 11 (100) 0 0 0 0 0 |
15 (36.6) 8 (19.5) 11 (26.8) 1 (2.4) 5 (12.2) 2 (4.9) 4 (9.8) 18 (43.9) |
Non-HGG patients, n |
4 |
13 |
17 |
10e |
20e |
n/a |
n/a |
Target lesions, n (%) |
|||||||
Lymph node |
0 |
1 (7.7) |
1 (5.9) |
0 |
3 (15.0) |
||
Non-lymph node |
3 (75.0) |
11 (84.6) |
14 (82.4) |
10 (100) |
20 (100) |
||
Non-target lesions, n (%) |
|||||||
| Yes | 3 (75.0) |
10 (76.9) |
13 (76.5) |
6 (60.0) |
9 (45.0) |
||
| No | 1 (25.0) |
3 (23.1) |
4 (23.5) |
4 (40.0) |
11 (55.5) |
||
HGG patients (RANO), n |
1 |
5 |
6 |
n/a |
n/a |
11 |
n/a |
Target lesions, n (%) |
|||||||
Yes |
0 |
4 (80.0) |
4 (66.7) |
11 (100) |
|||
No |
1 (100) |
1 (20.2) |
2 (33.3) |
0 |
|||
Non-target lesions, n (%) |
|||||||
Yes |
1 (100) |
1 (20.0) |
2 (33.3) |
3 (27.3) |
|||
No |
0 |
4 (80.0) |
4 (66.7) |
8 (72.7) |
|||
| Patients who received previous anticancer medications,f n (%) | 5 (100) | 15 (83.3) | 20 (87.0) | 10 (100) | 20 (100) | 10 (90.9) | 40 (97.6) |
| Patients who received previous VEGF-targeted therapy | 1 (20.0) | 4 (22.2) | 5 (21.7) | 1 (10.0) | 3 (15.0) | 2 (18.2) | 6 (14.6) |
| Patients who received previous anthracycline therapy | 1 (20.0) | 7 (38.9) | 8 (34.8) | 10 (100) | 9 (45.0) | 0 (0.0) | 19 (46.3) |
Number of previous therapy regimens,g n (%) |
|||||||
1 2 ≥3 |
0 5 (100) 0 |
2 (11.1) 5 (27.8) 8 (44.4) |
2 (8.7) 10 (43.5) 8 (34.8) |
0 6 (60.0) 4 (10.0) |
3 (15.0) 7 (35.0) 10 (50.0) |
2 (18.2) 7 (63.6) 1 (9.1) |
5 (12.2) 20 (48.8) 15 (36.6) |
Best response for last therapy,h-j n (%) Complete response Partial response Stable disease Progressive disease Not applicable/Not evaluable Unknown |
n = 3 0 0 0 3 (60.0) 0 0 |
n = 14 2 (11.1) 0 3 (16.7) 8 (44.4) 1 (5.6) 0 |
n = 17 2 (8.7) 0 3 (13.0) 11 (47.8) 1 (4.3) 0 |
n = 9 2 (20.0) 1 (10.0) 0 5 (50.0) 0 1 (10.0) |
n = 17 4 (20.0) 3 (15.0) 2 (10.0) 4 (20.0) 1 (5.0) 3 (15.0) |
n = 9 0 1 (9.1) 1 (9.1) 5 (45.5) 0 2 (18.2) |
n = 35 6 (14.6) 5 (12.2) 3 (7.3) 14 (34.1) 1 (2.4) 6 (14.6) |
- Abbreviations: BSA, body surface area; EVE, everolimus; EWS, Ewing sarcoma; HGG, high-grade glioma; KPS, Karnofsky performance status; LEN, lenvatinib; mTOR, mammalian target of rapamycin; n/a, not applicable; RANO, Response Assessment in Neuro-Oncology; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; RMS, rhabdomyosarcoma.
- a “Other” refers to Black or African American and White, unknown or unspecified.
- b Reported for 17 total patients from Phase 1 (DL −1: 4; DL1: 13) and 38 total patients from Phase 2 (EWS, n = 10; RMS, n = 19; HGG, n = 9).
- c Patients may be counted in multiple categories.
- d Rows containing only zeroes are omitted from the in-text table.
- e By RECIST v1.1.
- f Excluding anthracycline, anti-VEGF/VEGFR, and mTOR medications.
- g Each patient with previous anticancer medications was counted only once within each category.
- h Based on patients who received previous anticancer medications.
- i Best response was summarized only for patients who received any previous anticancer medications.
- j Rows containing only zeroes are omitted from the in-text table.
3.2 DLTs (Phase 1)
Three patients were initially enrolled at DL1 (i.e., lenvatinib 11 mg/m2 plus everolimus 3 mg/m2); two of these patients had Grade 3 AEs that were initially thought to meet DLT criteria (proteinuria, n = 1; headache, n = 1). Five patients were subsequently enrolled at DL −1 (lenvatinib 8 mg/m2 plus everolimus 3.0 mg/m2) with no observed DLTs. On re-review, one of the potential DLTs (Grade 3 headache) reported at DL1 did not meet the definition of a DLT (for details, see Supporting Information Results), and hence, additional enrollment reopened at DL1. Of three additional patients enrolled at DL1, one patient had DLTs of Grade 3 hypertriglyceridemia and Grade 4 hypercholesterolemia. The DLTs resulting in dose modifications during Cycle 1 are summarized in Table 2. Because the two patients treated at DL1 had DLTs from different classes of AEs (proteinuria and lipid abnormalities), the cohort was expanded to 12 patients with no additional DLTs observed. Hence, DL1 (lenvatinib 11 mg/m2 plus everolimus 3 mg/m2) was determined to be the MTD and RP2D. After the RP2D was defined, six additional patients were enrolled at DL1 to obtain evaluable PK data from at least six patients aged 2 to <6 years. No DLTs were observed in these six patients.
| Patient | Assigned dose level | DLT (AE preferred term) | Study day of DLTa | Grade | Study drug action taken | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age, years | Sex | Race | LEN | EVE | Outcome | ||||
| 8 | Male | White |
LEN 11 mg/m2 EVE 3 mg/m2 |
Proteinuria | 22 | 3 | Interrupted | Interrupted | The patient discontinued both study drugs on Day 23 due to PD. DLT resolved on Day 27 |
| 7 | Female | White |
LENb 11 mg/m2 EVE 3 mg/m2 |
Hypertriglyceridemia Hypercholesterolemia |
15 15 |
3 4 |
Interrupted Interrupted |
Reduced Reduced |
Hypercholesterolemia resolved on Day 29. LEN was resumed at the same dose and EVE was resumed at the same dose at a reduced frequency due to hypertriglyceridemia and hypercholesterolemia (every other day)c |
- Abbreviations: AE, adverse event; DLT, dose-limiting toxicity; EVE, everolimus; LEN, lenvatinib.
- a Study day listed is the first day (start) of the DLT.
- b Patient received LEN 9 mg on Cycle 1, Day 1, equivalent to 10.71 mg/m2.
- c On Day 43, the patient experienced hypercholesterolemia (Grade 3) and hypertriglyceridemia (Grade 2). On Day 43, EVE was withdrawn due to hypercholesterolemia and hypertriglyceridemia. The patient did not recover from these events. On Day 99, the patient discontinued LEN due to progressive disease.
3.3 Safety
Treatment-emergent AEs (TEAEs) and treatment-related TEAEs observed in Phases 1 and 2 are presented in Table 3, Tables S2 and S3, and Supporting Information Results. Two patients (8.7%), both receiving DL1 in Phase 1, discontinued treatment with lenvatinib (4.3%), everolimus (8.7%), or both (4.3%) due to a treatment-related TEAE (Patient 1: hypercholesterolemia and hypertriglyceridemia; Patient 2: tendon rupture). Treatment-related TEAEs of any grade occurred in all patients treated in Phase 1; the most common treatment-related TEAEs (>40% of patients) were hypertension (60.9%), hypothyroidism (52.2%), hypertriglyceridemia (47.8%), abdominal pain (43.5%), and diarrhea (43.5%). Grade ≥3 severity treatment-related TEAEs were observed in 13 (56.5%) patients. Treatment-related pneumothorax was observed in two patients treated at DL1. The most common Grade 3 treatment-related TEAEs (occurring in three patients each) were hypertriglyceridemia and proteinuria.
| Phase 1 | Phase 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
LEN 8 mg/m2 + EVE 3 mg/m2 (n = 5) |
LEN 11 mg/m2 + EVE 3 mg/m2 (n = 18) |
Total (n = 23) |
EWS (n = 10) |
Rhabdomyosarcoma (n = 20) |
HGG (n = 11) |
Total (N = 41) |
||||||||
|
Preferred term, n (%) |
Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Any treatment-related TEAE | 5 (100) | 0 | 18 (100) | 13 (72.2) | 23 (100) | 13 (56.5) | 8 (80.0) | 6 (60.0) | 19 (95.0) | 12 (60.0) | 11 (100) | 6 (54.5) | 38 (92.7) | 24 (58.5) |
| Hypertension | 3 (60.0) | 0 | 11 (61.1) | 1 (5.6) | 14 (60.9) | 1 (4.3) | 3 (30.0) | 0 | 8 (40.0) | 1 (5.0) | 3 (27.3) | 0 | 14 (34.1) | 1 (2.4) |
| Hypothyroidism | 2 (40.0) | 0 | 10 (55.6) | 0 | 12 (52.2) | 0 | 2 (20.0) | 0 | 7 (35.0) | 0 | 5 (45.5) | 0 | 14 (34.1) | 0 |
| Hypertriglyceridemia | 1 (20.0) | 0 | 10 (55.6) | 3 (16.7) | 11 (47.8) | 3 (13.0) | 5 (50.0) | 2 (20.0) | 12 (60.0) | 2 (10.0) | 6 (54.5) | 3 (27.3) | 23 (56.1) | 7 (17.1) |
| Abdominal pain | 3 (60.0) | 0 | 7 (38.9) | 0 | 10 (43.5) | 0 | 0 | 0 | 5 (25.0) | 1 (5.0) | 4 (36.4) | 0 | 9 (22.2) | 1 (2.4) |
| Diarrhea | 2 (40.0) | 0 | 8 (44.4) | 1 (5.6) | 10 (43.5) | 1 (4.3) | 4 (40.0) | 2 (20.0) | 6 (30.0) | 0 | 6 (54.5) | 1 (9.1) | 16 (39.0) | 3 (7.3) |
| Proteinuria | 1 (20.0) | 0 | 7 (38.9) | 3 (16.7) | 8 (34.8) | 3 (13.0) | 6 (60.0) | 1 (10.0) | 9 (45.0) | 2 (10.0) | 3 (27.3) | 0 | 18 (43.9) | 3 (7.3) |
| Stomatitis | 3 (60.0) | 0 | 5 (27.8) | 0 | 8 (34.8) | 0 | 1 (10.0) | 0 | 9 (45.0) | 3 (15.0) | 2 (18.2) | 0 | 12 (29.3) | 3 (7.3) |
| Blood cholesterol increased | 1 (20.0) | 0 | 6 (33.3) | 1 (5.6) | 7 (30.4) | 1 (4.3) | 1 (10.0) | 0 | 11 (55.5) | 0 | 3 (27.3) | 1 (9.1) | 15 (36.6) | 1 (2.4) |
| Decreased appetite | 4 (80.0) | 0 | 3 (16.7) | 0 | 7 (30.4) | 0 | 1 (10.0) | 0 | 4 (20.0) | 2 (10.0) | 2 (18.2) | 0 | 7 (17.1) | 2 (4.9) |
| Fatigue | 2 (40.0) | 0 | 5 (27.8) | 0 | 7 (30.4) | 0 | 3 (30.0) | 0 | 9 (45.0) | 0 | 3 (27.3) | 0 | 15 (36.6) | 0 |
| Lymphocyte count decreased | 2 (40.0) | 0 | 5 (27.8) | 1 (5.6) | 7 (30.4) | 1 (4.3) | 5 (50.0) | 3 (30.0) | 10 (50.0) | 3 (15.0) | 1 (9.1) | 1 (9.1) | 16 (39.0) | 7 (17.1) |
| Nausea | 4 (80.0) | 0 | 3 (16.7) | 0 | 7 (30.4) | 0 | 2 (20.0) | 0 | 5 (25.0) | 1 (5.0) | 4 (36.4) | 0 | 11 (26.8) | 1 (2.4) |
| Vomiting | 2 (40.0) | 0 | 5 (27.8) | 0 | 7 (30.4) | 0 | 2 (20.0) | 0 | 4 (20.0) | 0 | 4 (36.4) | 0 | 10 (24.4) | 0 |
| Headache | 2 (40.0) | 0 | 4 (22.2) | 2 (11.1) | 6 (26.1) | 2 (8.7) | 1 (10.0) | 0 | 3 (15.0) | 0 | 3 (27.3) | 0 | 7 (17.1) | 0 |
| Neutrophil count decreased | 2 (40.0) | 0 | 4 (22.2) | 0 | 6 (26.1) | 0 | 2 (20.0) | 0 | 6 (30.0) | 0 | 2 (18.2) | 1 (9.1) | 10 (24.4) | 1 (2.4) |
| Platelet count decreased | 0 | 0 | 6 (33.3) | 2 (11.1) | 6 (26.1) | 2 (8.7) | 4 (40.0) | 1 (10.0) | 8 (40.0) | 1 (5.0) | 3 (27.3) | 0 | 15 (36.6) | 2 (4.9) |
| WBC count decreased | 1 (20.0) | 0 | 5 (27.8) | 0 | 6 (26.1) | 0 | 6 (60.0) | 1 (10.0) | 8 (40.0) | 0 | 2 (18.2) | 0 | 16 (39.0) | 1 (2.4) |
| ALT increased | 0 | 0 | 5 (27.8) | 1 (5.6) | 5 (21.7) | 1 (4.3) | 2 (20.0) | 0 | 5 (25.0) | 1 (5.0) | 3 (27.3) | 0 | 10 (24.4) | 1 (2.4) |
| Anemia | 1 (20.0) | 0 | 4 (22.2) | 1 (5.6) | 5 (21.7) | 1 (4.3) | 2 (20.0) | 1 (10.0) | 5 (25.0) | 1 (5.0) | 1 (9.1) | 1 (9.1) | 8 (19.5) | 3 (7.3) |
| AST increased | 0 | 0 | 5 (27.8) | 0 | 5 (21.7) | 0 | 4 (40.0) | 0 | 5 (25.0) | 0 | 4 (36.4) | 0 | 13 (31.7) | 0 |
| Dry skin | 1 (20.0) | 0 | 4 (22.2) | 0 | 5 (21.7) | 0 | 0 | 0 | 2 (10.0) | 0 | 1 (9.1) | 0 | 3 (7.3) | 0 |
| Weight decreased | 1 (20.0) | 0 | 4 (22.2) | 0 | 5 (21.7) | 0 | 1 (10.0) | 0 | 5 (25.0) | 0 | 0 | 0 | 6 (14.6) | 0 |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; EVE, everolimus; EWS, Ewing sarcoma; HGG, high-grade glioma; LEN, lenvatinib; RMS, rhabdomyosarcoma; TEAE, treatment-emergent adverse event; WBC, white blood cell.
In Phase 2, three (7.3%) patients (one in RMS and two in HGG cohorts) discontinued treatment with lenvatinib (4.9%), everolimus (7.3%), or both (2.4%) due to a treatment-related TEAE (Patient 1: pancreatitis, increased lipase, and increased amylase; Patient 2: hypercholesterolemia and hypertriglyceridemia; Patient 3: hypertriglyceridemia, skin ulcer). Treatment-related TEAEs were reported in 38 (92.7%) patients. The most common treatment-related TEAEs (occurring in >40% of patients) were hypertriglyceridemia (n = 23, 56.1%) and proteinuria (n = 18, 43.9%). Treatment-related pneumothorax was observed in one patient (RMS cohort). The most common Grade ≥3 treatment-related TEAEs (occurring in seven [17.1%] patients each) were hypertriglyceridemia and lymphopenia.
Of the 34 deaths observed in Phase 2, there were eight within 28 days of the patient's last dose of the study drug. Seven patients died from disease progression, and one patient died due to disease-related encephalopathy that was considered unrelated to study treatment.
3.4 Efficacy
Secondary efficacy endpoints for Phase 1 are summarized in Table S4. Eighteen patients with measurable disease and five with evaluable disease were evaluated for BOR. No objective responses occurred at either dose level. At DL1, seven (38.9%) patients with measurable disease had a BOR of SD, and two (11.1%) patients with evaluable disease had a BOR of non-CR/non-PD (equivalent to SD in patients with measurable disease). The remainder had PD, except for one patient whose BOR was not evaluable because no post-baseline tumor assessment was available. At DL −1, a BOR of SD was reported in one patient with measurable disease; the remainder had PD. In Phase 1, a total of five patients (one treated at DL −1 and four at DL1) had durable SD (i.e., SD ≥23 weeks) with the following diagnoses: astrocytoma (DL −1), ependymoma, HGG, osteosarcoma, and Wilms tumor.
BOR and duration of treatment for Phase 2 patients are shown in Figure 1. In Phase 2, no objective responses (PR/CR) were observed in the EWS and HGG cohorts at Week 16, and further enrollment was stopped due to futility after Stage 1. In the RMS cohort, one patient in Stage 1 had a confirmed PR at Week 16, prompting cohort expansion to 20 patients. One additional patient demonstrated a PR at Week 16, resulting in an ORR of 10% for the RMS cohort (Table 4). However, the ORR for the RMS cohort did not meet the statistical threshold for activity. The DORs for the two patients with PRs were 2.10 and 2.76 months, respectively. The molecular characteristics of these two responders are included in the Supporting Information Results. An additional 13 (32.5%) patients had BORs of SD, which were similarly distributed across disease types (EWS n = 4, 40%; RMS n = 6, 30%; HGG n = 3, 30%). Patients with nonprogressive disease (n = 5) proceeded with treatment in the extension phase. The CBRs for patients with EWS, RMS, and HGG were 20%, 10%, and 0%, respectively (Table 4). Notably, two patients with EWS experienced SD lasting ≥23 weeks.
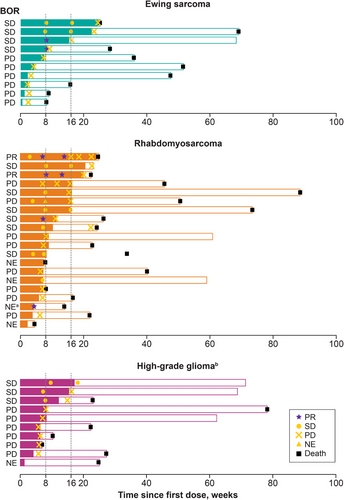
| Tumor responses |
EWS (n = 10) |
RMS (n = 20) |
HGG (n = 10)a |
Total (n = 40) |
|---|---|---|---|---|
| Responses at Week 16 | ||||
|
BOR, n (%) CR PR SD PD Unknown/Not evaluableb No post-baseline tumor assessment Early SD (SD <7 weeks) |
0 0 4 (40.0) 6 (60.0) 0 0 0 |
0 2 (10.0) 6 (30.0) 8 (40.0) 4 (20.0) 2 (10.0) 2 (10.0) |
0 0 3 (30.0) 6 (60.0) 1 (10.0) 1 (10.0) 0 |
0 2 (5.0) 13 (32.5) 20 (50.0) 5 (12.5) 3 (7.5) 2 (5.0) |
|
ORR (CR + PR), n (%) (95% CI)c |
0 (0–30.8) |
2 (10.0) (1.2–31.7) |
0 (0–30.8) |
2 (5.0) (0.6–16.9) |
| Responses at data cutoff date | ||||
|
BOR, n (%) CR PR SD PD Unknown/Not evaluableb No post-baseline tumor assessment Early SD (SD <7 weeks) |
0 0 4 (40.0) 6 (60.0) 0 0 0 |
0 2 (10.0) 6 (30.0) 8 (40.0) 4 (20.0) 2 (10.0) 2 (10.0) |
0 0 3 (30.0) 6 (60.0) 1 (10.0) 1 (10.0) 0 |
0 2 (5.0) 13 (32.5) 20 (50.0) 5 (12.5) 3 (7.5) 2 (5.0) |
|
ORR (CR + PR), n (%) (95% CI)c |
0 (0–30.8) |
2 (10.0) (1.2–31.7) |
0 (0–30.8) |
2 (5.0) (0.6–16.9) |
|
Duration of objective response, months Median (95% CI) Range |
n = 0 n/a n/a |
n = 2 2.4 (2.1–NE) (2.10, 2.76) |
n = 0 n/a n/a |
n = 2 2.4 (2.1–NE) (2.10, 2.76) |
|
DCR,d n (%) (95% CI) |
4 (40.0) (12.2–73.8) |
8 (40.0) (19.1–63.9) |
3 (30.0) (6.7–65.2) |
15 (37.5) (22.7–54.2) |
|
CBR,e n (%) (95% CI) |
2 (20.0) (2.5–55.6) |
2 (10.0) (1.2–31.7) |
0 (0–30.8) |
4 (10.0) (2.8–23.7) |
- Note: Tumor assessments were based on RECIST v1.1 for EWS and rhabdomyosarcoma, and RANO for HGG.
- Abbreviations: BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DCR, disease control rate; EWS, Ewing sarcoma; HGG, high-grade glioma; n/a, not applicable; NE, not estimable; ORR, objective response rate; PD, progressive disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RECIST, Response Evaluation Criteria for Solid Tumors; RMS, rhabdomyosarcoma; SD, stable disease.
- a One patient with HGG withdrew before the first imaging assessment and thus was unevaluable for a response.
- b Rows containing only zeroes are omitted from the in-text table.
- c 95% CI was determined using the Clopper–Pearson exact method.
- d DCR was defined as CR +PR +SD ≥7 weeks.
- e CBR was defined as CR + PR + durable SD (SD ≥23 weeks).
3.5 Pharmacokinetics
A summary of PK parameters of lenvatinib for Phase 1 is provided in Table 5. At DL −1, Cmax and area under the curve (AUC) means were similar between Cycle 1 Day 1 (Cmax: 240 ng/mL [standard deviation: 131]; AUC: 1230 ng*h/mL [standard deviation: 740]) and Cycle 1 Day 15 (Cmax: 314 ng/mL [standard deviation: 150]; AUC: 1330 ng*h/mL [standard deviation: 521]). At DL1, both Cmax and AUC were also similar between Cycle 1 Day 1 (Cmax: 404 ng/mL [standard deviation: 121]; AUC: 1880 ng*h/mL [standard deviation: 549]) and Cycle 1 Day 15 (Cmax: 448 ng/mL [standard deviation: 273]; AUC: 2140 ng*h/mL [standard deviation: 1160]). Cmax and AUC were approximately dose-proportional between DL −1 and DL1 on both cycle days. Lenvatinib plasma concentration data for Phase 1 are shown in Figure S3. The DLTs experienced by patients at DL1 (i.e., lenvatinib 11 mg/m2) were not associated with high plasma concentrations of lenvatinib (Figure S4). Population PK analyses for everolimus were not performed because of the lack of efficacy for the combination.
| Parameter | Cmax (ng/mL) | Tmax (h) | AUC(0–8 h) (ng*h/mL) |
|---|---|---|---|
| Lenvatinib 8 mg/m2 + everolimus 3 mg/m2 Cycle 1 Day 1 | |||
| Patient n | 5 | 5 | 5 |
| Mean (SD) | 240 (131) | 3.20 (0.83) | 1230 (740) |
| Median | 200 | 3.00 | 1110 |
| Minimum, maximum | 124, 463 | 2.00, 4.00 | 640, 2480 |
| Geometric mean (% CV) | 217 (51.4) | — | 1090 (57.4) |
| Lenvatinib 8 mg/m2 + everolimus 3 mg/m2 Cycle 1 Day 15 | |||
| Patient n | 5 | 5 | 5 |
| Mean (SD) | 314 (150) | 4.00 (2.46) | 1330 (521) |
| Median | 276 | 3.95 | 1120 |
| Minimum, maximum | 166, 548 | 2.00, 8.05 | 780, 2060 |
| Geometric mean (% CV) | 288 (48.5) | — | 1250 (40.4) |
| Lenvatinib 11 mg/m2 + everolimus 3 mg/m2 Cycle 1 Day 1 | |||
| Patient n | 18 | 18 | 18 |
| Mean (SD) | 404 (121) | 3.04 (1.50) | 1880 (549) |
| Median | 410 | 2.89 | 1910 |
| Minimum, maximum | 90.3, 633 | 1.00, 7.78 | 288, 2880 |
| Geometric mean (% CV) | 379 (44.2) | — | 1740 (51.7) |
| Lenvatinib 11 mg/m2 + everolimus 3 mg/m2 Cycle 1 Day 15 | |||
| Patient n | 17 | 17 | 17 |
| Mean (SD) | 448 (273) | 3.09 (2.63) | 2140 (1160) |
| Median | 417 | 2.95 | 1960 |
| Minimum, maximum | 70.6, 1000 | 0, 8.02 | 474, 3900 |
| Geometric mean (% CV) | 356 (88.5) | — | 1787 (75.5) |
- Abbreviations: AUC(0-8 h), the area under the baseline-corrected plasma concentration versus time curve from time 0 to 8 hours; Cmax, peak plasma concentration; CV, coefficient of variance; SD, standard deviation; Tmax, time to reach Cmax.
4 Discussion
The safety profile for lenvatinib in combination with everolimus observed in the current study was as expected and consistent with the safety profile observed with this combination in adults with RCC [19]. Treatment-related TEAEs per investigator assessment occurred in all patients in Phase 1 and 92.7% in Phase 2. This observed toxicity rate was comparable to the toxicity rate observed in a study of pediatric and young adult patients receiving single-agent lenvatinib, wherein treatment-related TEAEs affected 87.0% of patients in Phase 1 and 90.3% of patients in Phase 2 [21]. In patients who received lenvatinib 11 mg/m2 plus everolimus 3 mg/m2 (18 patients in Phase 1 and all patients in Phase 2), the most frequent treatment-related TEAEs (observed in ≥40% of patients) included hypertriglyceridemia (55.9%), proteinuria (42.4%), hypertension (42.4%), diarrhea (40.7%), and hypothyroidism (40.7%). Notably, lipid abnormalities are known on-target effects associated with mTOR inhibitors [27]. Overall, most treatment-related TEAEs were considered low severity (most Grade ≥3 treatment-related TEAEs had an incidence of <20%), no new safety signals were observed, and TEAEs were effectively medically managed.
In the assessment of antitumor activity, no objective responses were observed in Phase 1. In Phase 2, two patients with RMS had confirmed PRs by Week 16, but responses were transient (DORs of 2.10 and 2.76 months, respectively) and likely did not meet the proposed benchmarks for activity (∼17% 6-month event-free survival) in patients with relapsed/refractory RMS [28]. Next-generation targeted-gene-panel testing showed no identifiable molecular alterations known to be targetable by either lenvatinib or everolimus, and neither responder had previously received VEGF-targeted or mTOR-targeted therapy. Notably, the highest CBR (20%) was observed in the EWS cohort, including two patients with prolonged SD (≥23 weeks).
The characteristics of the study population may have contributed to the paucity of observed responses: most patients in both phases had ≥2 prior anticancer therapies, a high proportion of patients had metastatic disease at baseline, and the study population was relatively small. Additionally, despite prior evidence suggesting biologic relevance for VEGF and mTOR signaling across pediatric solid tumors [2, 3], the lack of significant antitumor responses observed in this trial may suggest a relatively lower degree of pathway dependency by the histologies evaluated, rather than true single-agent resistance that might be overcome by concurrent inhibition of a compensatory pathway. Notably, in the tumor types studied, there was an absence of known genetic modifications that result in hyperactivation of VEGF signaling (as observed in Von Hippel–Lindau-mutant clear cell RCC [29-31]) or mTOR signaling (as observed in patients with tuberous sclerosis and subependymal giant cell astrocytomas [32, 33]). Lower reliance on pathways targeted by lenvatinib and everolimus and the absence of genetically encoded addiction to VEGF or mTOR signaling may have influenced the modest antitumor activity observed.
This trial identified a tolerable dose of lenvatinib in combination with everolimus in the pediatric population and established its safety and PK profile in pediatric and young adult patients with relapsed/refractory solid tumors. PK exposures of lenvatinib observed in Phase 1 were comparable to those previously observed in children and young adults receiving lenvatinib monotherapy [21] and in adults receiving the lenvatinib plus everolimus combination [19]. No clear relationship between DLTs and high plasma concentrations of lenvatinib was observed. Although lenvatinib in combination with everolimus did not meet statistical thresholds for antitumor activity in RMS, EWS, or HGG, it remains notable that several patients—particularly in the EWS cohort—demonstrated prolonged disease stabilization (≥23 weeks), which may be of more clinical significance than the relatively short objective responses described in 10% of patients in the RMS cohort. This observation highlights the need for further studies to better understand the biologic drivers of tumorigenesis and relative pathway dependencies to enrich treatment for those patients most likely to benefit.
Author Contributions
Study concept and design: CSH, CO, FDC, JGB, KOH, BJW. Data acquisition: SGD, MEM, KTV, DAM, TW, AK, FDC, JGB, JK, GKF, JMC, BJW. Data analysis: CSH, CO, FDC, JGB, KOH, JA. Data interpretation: SGD, MEM, KTV, DAM, CSH, CO, FDC, JGB, TW, KOH, JA. Review of the manuscript drafts, and approval of the final version for submission: SGD, MEM, KTV, DAM, CSH, TW, AK, CO, FDC, JGB, JK, GKF, JMC, KOH, BJW, JA.
Acknowledgments
The authors would like to acknowledge Yan Jia (Biostatistics, Eisai Inc., Nutley, NJ, USA). This study was sponsored by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Medical writing was provided by Oxford PharmaGenesis Inc., Newtown, PA, USA, and was funded by Eisai Inc., Nutley, NJ, USA, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA.
Conflicts of Interest
Filemon S. Dela Cruz: institutional research support: Eisai and Y-mAbs Therapeutics; member of Protocol Steering Committee for Study 216: Eisai.
Steven G. DuBois: consulting fees: Amgen, Bayer, Inhibrx, and Jazz; travel expenses: Loxo Oncology, Roche, and Salarius.
Gregory K. Friedman: prior contracts to the University of Alabama at Birmingham from Eisai; Pfizer, Eli Lilly & Company, Bristol Myers Squibb Company.
James M. Croop: grants/contracts: patient case reimbursement from COG.
Daniel A. Morgenstern: consulting fees: Y-mAbs Therapeutics, Regeneron, RayzeBio, AbbVie, US World Meds; travel expenses: AbbVie, Lilly; participation on a data safety monitoring board or advisory board: Clarity Pharmaceuticals; speaker fees: Takeda Israel, Y-mAbs Therapeutics; unpaid consultancy: Oncoheroes Biosciences.
Frank M. Balis: grant: Alex Lemonade Stand Center of Excellence Award in Drug Development.
Margaret E. Macy: grants/contracts to institution: Bayer, Loxo/Lilly, Turning Point Therapeutics, Merck, Roche, AbbVie; travel expenses: Bayer; participation on a data safety monitoring board or advisory board: Y-mAbs Therapeutics; consulting fees: Recordati, Kestel Therapeutics; stock or stock options: Johnson and Johnson.
Elizabeth Fox, AeRang Kim, Joseph Pressey, Tanya Watt, Julie I. Krystal, Kieuhoa T. Vo, and Brenda Weigel: have no conflicts of interest.
Rajen Mody and Theodore W. Laetsch: grants/contracts: Lilly, Roche/Genentech, Taiho Oncology, Advanced Accelerator Applications, Bristol Myers Squibb, BioAtla, Roche, Pfizer, Bayer, Turning Point Therapeutics; consulting fees: Advanced Microbubbles, AI Therapeutics, Bayer, GSK, ITM Oncologics, Jazz Pharmaceuticals, Massive Bio; payment/honoraria: Aptitude Health, Medscape, Targeted Oncology; stock or stock options: Advanced Microbubbles.
Karen O'Hara, Cixin S. He, Jagadeesh Aluri, and Chinyere E. Okpara: employment: Eisai.
Julia L. Glade Bender: grant/contracts (to institution): Lilly, Loxo-oncology, Eisai, Cellectar and Bayer, Amgen; consulting fees: Jazz Pharmaceuticals; participation on a data safety monitoring board or advisory board: Springworks Therapeutics, Merck Sharp & Dohme, Pfizer; royalties from patent on a T-lymphoblastic lymphoma cell line: CUTLL1.
Open Research
Data Availability Statement
The data are commercially confidential and will not be available for sharing; however, Eisai will consider written requests to share the data on a case-by-case basis.




