Multimodality detection of tumour rupture in children with Wilms tumour
Kristina Dzhuma and Minou Oostveen contributed equally to this work and share first authorship.
Level of evidence: Level II.
Abstract presented and published: CCLG annual conference, 21–23 April 2024, abstract book. ESPR annual meeting, 3–7 June 2024, abstract book.
Abstract
Background and aims
Tumour rupture (TR) signifies stage III disease and requires treatment intensification, which includes radiotherapy. We studied the associations between radiological, surgical and pathology TR in children with Wilms tumour (WT) in a United Kingdom multicentre clinical study.
Patients and methods
The IMPORT (Improving Population Outcomes for Renal Tumours of Childhood) study registered 712 patients between 2012 and 2021. Children with TR on central radiology review (CRR) at diagnosis and/or indication of preoperative TR on surgical forms were included. Correlation between radiology/surgery/pathology findings was made.
Results
Total 141 patients had TR identified (69 on CRR, 43 on surgical form and 29 on both), and 124/141 had images available for CRR, and 98/124 had features suggestive of TR on diagnostic CRR (63 magnetic resonance imaging/35 computed tomography). TR was limited to the renal fossa in 47/98 (48%) and intraperitoneal in 51/98 (52%). Three of 98(3%) had upfront surgery, and 87/95 (92%) had TR confirmed on post-chemotherapy preoperative scans. Among 80/98 (82%) cases with TR on CRR and available surgical forms, TR was not confirmed on surgery or pathology in 38/80, giving a false-positive rate of 48%. Preoperative TR was indicated on 72 surgical forms, with images available for CRR in 55. Twenty-six of 55 (47%) were false-negative for TR on CRR and of those 10/26 (38%) had TR confirmed on pathology.
Conclusions
Radiology alone should not be used to define TR, as it does not accurately correlate with surgical or pathology findings, and therefore cannot be relied upon for definitive staging and treatment. A multidisciplinary team should take the decision regarding the final abdominal stage and treatment using a multimodality approach considering clinical, radiological, surgical and pathological findings.
Abbreviations
-
- CCLG
-
- Children's Cancer and Leukaemia Group
-
- COG
-
- Children's Oncology Group
-
- CPR
-
- central pathology review
-
- CRF
-
- case report form
-
- CRR
-
- central radiology review
-
- IMPORT
-
- Improving Population Outcomes for Renal Tumours of Childhood
-
- MDT
-
- multidisciplinary team
-
- SIOP
-
- International Society of Paediatric Oncology
-
- TR
-
- tumour rupture
-
- WART
-
- whole abdominal radiotherapy
-
- WT
-
- Wilms tumour
1 INTRODUCTION
According to the International Society of Paediatric Oncology (SIOP) staging criteria for renal tumours of childhood,1 tumour rupture (TR) before surgery or intraoperatively results in upstaging to local stage III because of an increased risk of abdominal recurrence.2 Local stage III requires intensification of postoperative treatment, including radiotherapy to flank or whole abdomen (WART).3 It has been shown that WART carries a high risk of long-term infertility and moderately increased lifelong risk for secondary tumours, particularly bowel cancer.4 Hence, the definition and diagnostic criteria for TR should be very accurate.
Intraoperative TR has clearly defined diagnostic criteria based on macroscopic appearances during surgery.5 Preoperative TR is more difficult to define,6 and there is no ‘gold standard’ for its diagnosis. Clinically, it may manifest with acute abdominal pain, haemorrhagic anaemia or shock,6 which may require an upfront surgery.7 However, none of these clinical symptoms can be used independently to predict TR because of insufficient positive predictive value, estimated between 16% and 54%.6 Moreover, it is also well known that TR may not be confirmed at pathological examination after neoadjuvant chemotherapy due to formation of a pseudocapsule, which may cover the site of rupture.8-11
Radiological diagnostic criteria for preoperative TR are poorly defined, and in the absence of clinical signs, using radiological criteria alone to diagnose preoperative TR can be misleading.12 Moreover, studies have shown that both diagnostic13 and preoperative14 computed tomography (CT) have low sensitivity and specificity in detecting preoperative TR regarding concomitant histological analysis. It is important to recognise the different approaches to renal tumour management between protocols of the Children's Oncology Group (COG) and the International Society of Paediatric Oncology Renal Tumour Study Group (SIOP-RTSG). Findings reported by COG mainly apply to the diagnostic scan, whereas the SIOP use of neoadjuvant chemotherapy provides two time points for assessment of preoperative scans.
The aim of this study is to describe the associations between radiological, surgical and pathology-detected TR in children with Wilms tumour (WT) in a nationwide multicentre clinical study.
2 PATIENTS AND METHODS
2.1 Import study
Patients for inclusion in this analysis were drawn from the IMPORT (Improving Population Outcomes for Renal Tumours of Childhood) study in the United Kingdom and Republic of Ireland. The IMPORT study is a prospective clinical study of imaging and molecular biomarkers in children treated according to the national clinical guidelines of the Children's Cancer and Leukaemia Group (CCLG).3 These are based on the results of the SIOP WT 2001 trial and study,15, 16 and were updated to include the tumour volume after preoperative chemotherapy in the risk stratification of July 2019.16 It aims to maintain the success of first-line therapy whilst improving risk stratification to reduce the burden of therapy for the overall population of children with WT.
2.2 Inclusion and exclusion criteria
There were 712 patients registered in the IMPORT study from 20 centres between September 2012 and March 2021. Inclusion criteria were patients with newly diagnosed WT and indication of TR on the central radiology review (CRR) case report form (CRF) of the diagnostic cross-sectional abdominal imaging and/or on the surgical CRF. Exclusion criteria were patients with non-WT, registered at relapse, extrarenal site tumour and diagnosed abroad, or if the timing of TR was indicated on the surgical CRF as intraoperative (Figure 1). Clinical data for the included newly diagnosed patients with WT were extracted from the IMPORT study CRFs and central pathology review (CPR) letters. Correlation between radiology, surgery and pathology findings was made.
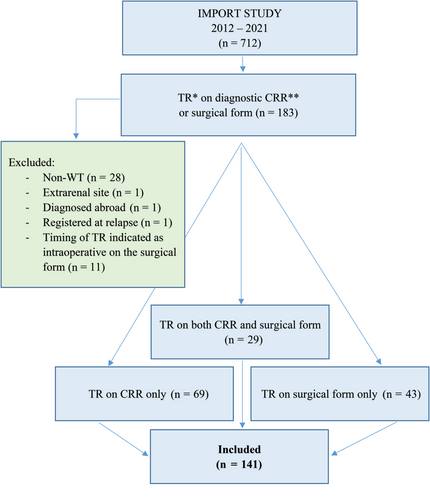
2.3 Central radiology review
CRR was offered for all patients registered in the IMPORT study. CRR panel consisted of three radiologists with expertise in paediatric oncology imaging (Oystein Olsen with 20 years of experience, Tom Watson with 11 years of experience, Susan C. Shelmerdine with 14 years of experience). The diagnostic and preoperative cross-sectional imaging was reviewed by a member of the panel and findings recorded on a standardised CRR form. Where possible, the CRR was done in ‘real time’, that is at the appropriate times in the patient's treatment pathway to avoid potential hindsight bias. Radiologists taking part in CRR were blinded to patient demographics and surgical/pathology findings. On the CRR form, the reviewing radiologist could classify the images as either adequate or suboptimal according to how closely they followed the image acquisition guidelines described in the national clinical guidelines.3 In some cases, this assessment was not recorded.
-
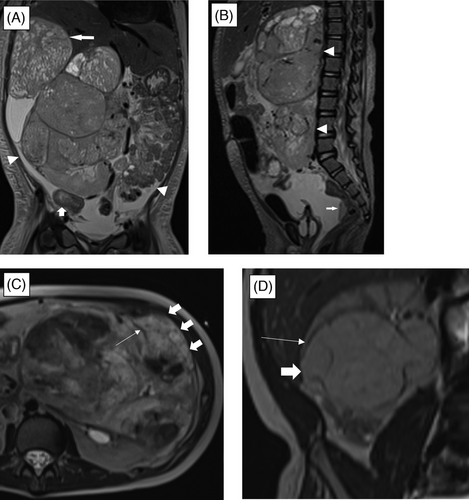
Significant amount of perinephric, retroperitoneal or peritoneal fluid (Figure 2A,B)
-
Perinephric fluid: defined as fluid in the perinephric space surrounding tumour and/or normal kidney.
-
Retroperitoneal fluid: fluid seen in the retroperitoneal space either as extension of perinephric fluid or adjacent to a large renal mass.
-
Peritoneal fluid: defined as peritoneal fluid beyond the rectovesical or rectouterine reflections.
-
-
Poorly circumscribed mass (Figure 2C,D)
-
A renal tumour without a definable pseudocapsule on magnetic resonance imaging (MRI) or irregular border with clear extension into the perinephric tissues on CT.
-
-
Capsular breach (Figure 2C,D)
-
Clear extension of tumour through its pseudocapsule into the perinephric tissues often in no more than one to five locations (otherwise tumour described as a poorly circumscribed mass).
-
-
Haemoperitoneum (Figure 2A,B)
-
MRI: presence of T1 hyperintense and mixed T2 intensity ascites. CT: dense ascites with Hounsfield units greater than 30.
-
-
Peritoneal implants (Figure 2A,B)
-
Solid tumour deposits within the peritoneal cavity and/or pelvis.
-
2.4 Surgical reporting
Findings at the time of operation were recorded on the surgical form. There is an option to indicate the timing of TR to allow distinction between preoperative and intraoperative. Intraoperative TR was defined by visual tumour spillage during the surgery or cutting through the tumour. Preoperative TR was considered if there was a presence of capsular breach, presence of blood in the peritoneal cavity or peritoneal implants at laparotomy, before intervention on the tumour.
2.5 Pathology reporting
CPR was performed according to the SIOP 2001 classification and staging criteria,1 and was achieved in 141/141 (100%) of cases. Local pathology form was available in 100/141 (71%). TR on pathology was defined as the presence of capsular rupture macroscopically, and microscopically positive resection margins in the areas of suspected rupture and/or viable or non-viable peritoneal tumour deposits.
2.6 Statistical methods
The cross-sectional imaging findings were noted, and correlation was made with surgical and pathology findings using descriptive statistics in Microsoft Excel. Continuous variables were expressed in medians.
3 RESULTS
3.1 Patients’ characteristics
The demographic data, tumour laterality, disease extent and preoperative treatment for the included 141 patients are summarised in Table 1.
| n | % | |
|---|---|---|
| Total | 141 | 100 |
| Male | 61 | 43 |
| Female | 80 | 57 |
| Median age at diagnosis, months | 50 (range 14–199) | |
| Right | 78 | 55 |
| Left | 63 | 45 |
| Localised | 85 | 60 |
| Metastatic | 56 | 40 |
| PCNB | 101 | 72 |
| Preoperative chemotherapy | 135 | 96 |
| Upfront surgery | 6 | 4 |
| High-risk histology | 17 | 12 |
| Intermediate-risk histology | 112 | 79 |
| Low-risk histology | 12 | 9 |
| Median follow-up, months | 48 (range 7–135) |
- Abbreviation: PCNB, percutaneous needle biopsy.
3.2 Tumour rupture on central radiology review
From the included cohort, 124/141 (86%) patients had images available for CRR. Imaging quality was recorded as 66 adequate, 22 suboptimal and 10 quality unrecorded. Ninety-eight patients had radiological signs suggestive of TR on the diagnostic cross-sectional abdominal imaging (63 MRI/35 CT). In 47/98 (48%), TR was limited to the renal fossa and in 51/98 (52%) involved peritoneum. Three patients had upfront surgery, 87/95 (92%) had findings of TR confirmed on the post-chemotherapy preoperative scan, whilst eight of 95 (8%) did not. Among 80/98 (82%) cases with TR on diagnostic CRR and available surgical forms, TR was confirmed on surgery in 29/80 (36%); on both surgery and pathology in 15/80 (15%), only on pathology in 13/80 (16%), not confirmed on either surgery or pathology (false positive) in 38/80 (48%) (Figure 3).
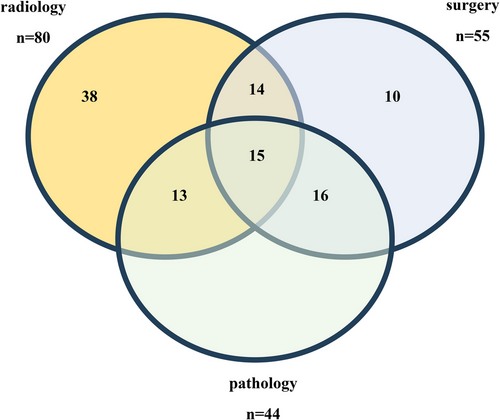
TR confirmed on pathology as a sole reason or in combination with other reasons (positive lymph nodes, incomplete tumour resection, tumour thrombus-related reasons) was responsible for stage III in 38/98 (39%) with subsequent flank or WART. Four of 98 (4%) were upstaged to stage III and received WART due to radiological TR confirmed at nephrectomy, but not on pathology. Twenty of 98 (20%) received flank or WART for stage III due to other reasons for stage III. Three of 98 (3%) with stage II received radiotherapy due to diffuse anaplasia on pathology (Table 2).
| TR confirmed on surgery | TR not confirmed on surgery | Missing surgical forms | Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Number of patients | 29 | 30 | 51 | 52 | 18 | 18 | 98 | 100 |
| Finala abdominal stage | ||||||||
| Stage I | 0 | 0 | 17 | 33 | 2 | 11 | 19 | 19 |
| Stage II | 1 | 3 | 10 | 20 | 6 | 33 | 17 | 17 |
| Stage III | 28 | 97 | 24 | 47 | 10 | 56 | 62 | 63 |
| Reasons for stage III | ||||||||
| Histological features of TR with/without other reasonsb | 20 | 71 | 12 | 50 | 6 | 60 | 38 | 61 |
| No histological features of TR but upstaged only due to preoperative TR | 4 | 14 | 0 | 0 | 0 | 0 | 4 | 6 |
| Other histological reasons | 4 | 14 | 12 | 50 | 4 | 40 | 20 | 32 |
| Radiotherapy | ||||||||
| Flank | 8 | 26 | 14 | 27 | 4 | 22 | 26 | 27 |
| WART | 18 | 62 | 5 | 10 | 4 | 22 | 27 | 28 |
| Missing postoperative treatment forms | 3 | 10 | 6 | 12 | 2 | 11 | 11 | 11 |
| Clinical outcomes | ||||||||
| Distant relapse | 2 | 7 | 3 | 6 | 1 | 6 | 6 | 6 |
| Abdominal relapse | 2 | 7 | 3 | 6 | 1 | 6 | 6 | 6 |
| Combined relapse | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 1 |
| Death | 3 | 10 | 1 | 2 | 0 | 0 | 4 | 4 |
- Abbreviations: TR, tumour rupture; WART, whole abdominal radiotherapy.
- a Assigned by the oncology multidisciplinary team.
- b Positive lymph nodes, incomplete tumour resection, tumour thrombus-related reasons.
3.3 Reporting of tumour rupture on the surgical CRF
The surgical CRF was available in 123/141 (87%) cases, and the timing of TR was indicated as preoperative on 72. Fifty-five of 72 (76%) had images available for CRR. TR was seen on CRR in 29/55 (53%), further described in the radiology section of the results. Twenty-six of 55 (47%) were negative for TR on diagnostic CRR, and 30/55 (55%) for preoperative. Twenty-two of 26 (84%) were assigned stage III: 16/22 (73%) had histological features in favour of TR with or without other reasons, three of 22 (17%) did not have confirmation of TR on histology nor other reasons for stage III but were upstaged due to preoperative TR and received radiotherapy, two of 22 (9%) were assigned stage III due to other reasons, one of 22 with stage II received radiotherapy due to diffuse anaplasia on pathology (Table 3).
| Positive CRR* | Negative CRR | No images | Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Number of patients | 29 | 40 | 26 | 36 | 17 | 24 | 72 | 100 |
| Finala abdominal stage | ||||||||
| Stage I | 0 | 0 | 2 | 8 | 2 | 12 | 4 | 6 |
| Stage II | 1 | 3 | 3 | 12 | 0 | 0 | 4 | 6 |
| Stage III | 28 | 97 | 21 | 80 | 15 | 88 | 64 | 88 |
| Reasons for stage III | ||||||||
| Histological features of TR with/without other reasonsb | 20 | 72 | 16 | 76 | 12 | 80 | 48 | 75 |
| No histological features of TR but upstaged only due to preoperative TR | 4 | 14 | 3 | 14 | 2 | 13 | 9 | 14 |
| Other reasons | 4 | 14 | 2 | 10 | 1 | 7 | 7 | 11 |
| Radiotherapy | ||||||||
| Flank | 8 | 26 | 12 | 55 | 3 | 20 | 23 | 35 |
| WART | 18 | 62 | 9 | 41 | 9 | 60 | 36 | 55 |
| Missing postoperative treatment forms | 3 | 10 | 1 | 4 | 2 | 13 | 6 | 9 |
| Not received radiotherapy | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 1 |
| Clinical outcomes | ||||||||
| Distant relapse | 2 | 7 | 1 | 4 | 0 | 0 | 3 | 4 |
| Abdominal relapse | 2 | 7 | 3 | 12 | 2 | 13 | 7 | 10 |
| Combined relapse | 0 | 0 | 1 | 4 | 1 | 7 | 2 | 3 |
| Death | 3 | 10 | 2 | 8 | 1 | 7 | 6 | 8 |
- Abbreviations: CRR, central radiology review; TR, tumour rupture; WART, whole abdominal radiotherapy.
- a Assigned by the oncology multidisciplinary team.
- b Positive lymph nodes, incomplete tumour resection, tumour thrombus-related reasons.
There were six of 141 (4%) patients who had upfront surgery, with five of six (83%) due to clinical emergency because of abdominal pain with intra-abdominal bleeding suggestive of TR. Four of six (67%) patients with upfront surgery had images available for CRR with three of four (75%) having positive findings of TR. Four of five (80%) for whom the surgical form was available had preoperative TR documented by the surgeon. Five of six (83%) had features of TR on pathology and were assigned stage III with subsequent radiotherapy (flank = 2, WART = 3); of those one had a combined relapse and survived. One of six (17%) with no clinical signs of TR was assigned stage III due to positive lymph node and had flank radiotherapy.
4 DISCUSSION
This study aimed to describe associations between TR detected on radiology, with subsequent findings at surgery and pathology and vice versa in a large cohort of children newly diagnosed with a renal tumour registered in a multicentre prospective clinical study that included CRR. We showed that diagnostic abdominal cross-sectional imaging has a high false-positive and false-negative rate for detection of TR 48% and 47%, respectively, with both surgery and pathology being the reference. These findings suggest that imaging should not be relied upon to detect TR nor used to make treatment decisions in this respect. It is important to understand the tumour anatomy, where it extends to, the presence of locoregional or distant disease and the treatment response, but imaging is not a reliable method for detection of TR using current radiological criteria. These are subjective and include perinephric or intraperitoneal fluid,6, 12, 13 intraperitoneal nodules,17 retroperitoneal tumour extension or ‘capsule breach’.13 Each of these features were considered by the reviewing radiologists as indicative of or suspicious for TR, but this needs further consensus and building work.
We suggest that the term ‘tumour rupture’ is a fundamentally misleading term to be used by radiologists. Instead, clearly defined terms such as ‘extracapsular tumour extension’ (meaning that tumour extends beyond the kidney and its own tumour pseudocapsule) and ‘intraperitoneal tumour spread’ (meaning that there are tumour deposits separate to the main tumour within the peritoneal cavity) should be adopted. ‘Radiological features highly suggestive of tumour rupture’ should only be used where a combination of findings is present: large volume ascites, extracapsular tumour extension and intraperitoneal tumour spread.
Our data show that there were nine of 141 (6%) children who had pathology stage I or II, but were upstaged to stage III and treated with radiotherapy due to preoperative TR. None of those were patients with peritoneal tumour implants. All these patients are in the first complete remission. Similar results have been shown in the study conducted by Le Rouzic et al.,14 where they have overtreated/stage V patients. Therefore, dilemma arises if this cohort of patients should be upstaged and treated with intensified chemotherapy and WART as stage III. Few studies have shown that the prognosis seems not to be better in patients treated with intensified postoperative chemotherapy and radiotherapy versus patients treated less aggressively for preoperative TR alone.6, 18 These studies are small series; therefore, larger clinical trials are required to determine whether preoperative TR suspected on imaging, clinical grounds or by the surgeon but without pathological confirmation has prognostic significance in the long-term survival for these patients.
Finally, our study showed that all six patients with upfront surgery due to acute bleeding had TR confirmed either on surgery or pathology or both, but only half of them had TR seen on radiology review. All these patients were assigned stage III and treated accordingly. The rate of the emergency surgery reported in literature is low, between 0.8%6 and 3%,7 and the predictive value of clinical signs of pain and haemorrhage seems to be low varying from 13%6 to 54%.19 We did not systematically collect data on clinical signs of TR at the time of diagnosis, and therefore cannot include this in our analysis.
A limitation of our study was that we did not perform an assessment of interobserver variation in diagnosing TR on CRR, and therefore are not able to establish specificity and sensitivity for CT and MRI in detecting TR. This is an important area for further research. Images were obtained from different centres, with different imaging protocols, on different scanners and in the case of MRI, on both 1.5 and 3 T, around 20% of the studies with deemed to be of suboptimal quality at CRR. Another limitation is that we had around 13% of patients with missing images for CRR and 13% missing surgical forms. Additionally, the documentation of preoperative TR on the surgical form might be biased if it was based on the radiological conclusions/MDT (multidisciplinary team) discussion regarding TR rather than intraoperative findings.
5 CONCLUSIONS
Radiology alone should not be used to define TR as it has not been shown to accurately correlate with surgery and pathology findings. Management and staging decisions should be based on multimodality approach with MDT considering clinical, radiological, surgical and pathology findings.
Future areas for research could be to identify accurate imaging markers that can detect TR, but this does rely on an accurate gold standard to validate against. Novel imaging biomarkers including multiparametric analysis and machine learning may be avenues for exploration, as the current imaging markers have proven insufficient.
ACKNOWLEDGEMENTS
The authors thank clinicians who helped to set up the IMPORT study: Dr Marks Week, Dr Jessica Bates and Dr Vesna Pavasovic. Authors also thank patients and clinicians from all IMPORT UK and Ireland treatment centres who contributed data to this analysis. This work was supported by the Children's Cancer and Leukaemia Group/Little Princess Trust (Grant ref: CCLGA 2019 10). The IMPORT study is funded by CCLG/Little Princess Trust (Grant ref: CCLGA 2019 27) and received past funding from Children's Cancer and Leukaemia Group/Bethany's Wish (Grant ref: CCLGA 2017 02), EU-FP7 Grant refs: 261474 (ENCCA) and 270089 (P-medicine), Great Ormond Street Children's Charity (Grant ref: W1090) and Cancer Research UK (Grant ref: C1188/A17297), and benefits from the infrastructural support of the UK National Cancer Research Network and the CCLG.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




