Cerebral venous sinus thrombosis after a third dose of mRNA COVID-19 vaccine in an adolescent
Since the first reports in 2019, coronavirus disease 2019 (COVID-19) has spread worldwide, resulting in rapid production of several effective vaccines. The Pfizer-BioNTech and Moderna vaccines are both messenger ribonucleic acid (mRNA)-based, while the AstraZeneca and Johnson and Johnson/Janssen vaccines are adenoviral vector-based. All were designed to produce coronavirus spike proteins and trigger an immune response.1 Both mRNA-based vaccines have been approved in Japan for children. Initial trials of these vaccines reported rare anaphylaxis and low-rate serious adverse events. However, since the vaccines became widely used, some serious adverse effects were reported, including cerebral venous sinus thrombosis (CVST).2-7 CVST is a serious neurovascular condition defined as the occlusion of venous sinuses, causing increased venous pressure. This disrupts venous return, resulting in infarction and hemorrhage.8 CVST usually affects young-adult women.9, 10 Risk factors for pediatric CVST include birth complications, infections, cancer, traumatic head injury, acquired or inherited thrombophilia, and use of hormonal contraceptives.8, 11, 12 A few cases of thrombosis with thrombocytopenia syndrome (TTS) were reported following vector-based vaccinations.2, 6, 13 However, cases of CVST in children after mRNA-based vaccinations were rarely reported.
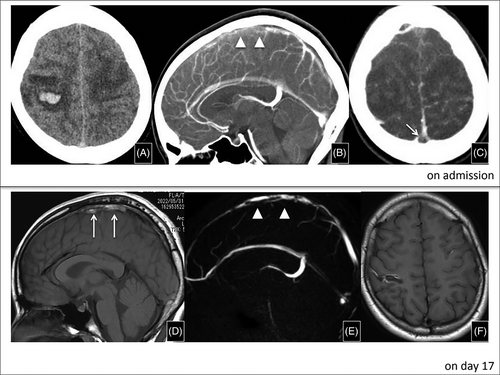
Here, we report a case of CVST following administration of the BNT162b2 (Pfizer-BioNTech, USA/Germany) vaccine to an adolescent with no relevant medical history. Informed consent was obtained from patient and patient's parents. A 14-year-old female presented with a 1-day history of persistent headache, vomiting, paresthesia of the bilateral extremities, and seizure. She reported no recent infection, including COVID-19. Medical, surgical, and family histories were unremarkable. She received the third Pfizer-BioNTech mRNA vaccine 7 days prior to presentation and reported no use of oral contraceptive pills or any other clinically relevant medications. Neurological examination revealed disturbed consciousness and bilateral weakness of the upper extremities (manual muscle testing: four in the upper limbs; five in the lower limbs). Table 1 shows summarized laboratory findings. Brain computed tomography (CT) and magnetic resonance imaging (MRI) showed hemorrhage in the right temporal region with a filling defect within the superior sagittal sinus on day 1 (Figure 1). As no clear precipitating factors were found in her clinical history, we ordered a thrombophilia workup. Protein S (PS) activity on admission was low and returned to normal on day 4. However, low values presented again during her follow-up as an outpatient. Anti-platelet factor-4 (PF-4) antibodies were negative. A polymerase chain reaction assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was negative. We administered argatroban, instead of heparin, because TTS was suspected. The patient did not meet the criteria for a TTS case definition due to the absent low platelet count and anti-PF-4 antibodies; however, we continued argatroban for 11 days, and then switched to warfarin. Her symptoms improved greatly and subsided completely by day 3. MRI performed on day 7 and magnetic resonance venography on day 17 showed improvement of the abnormal hyperintensity area at sagittal sinus (Figure 1). The patient was discharged with mildly impaired finger coordination/movement on day 17. Further investigation for PS deficiency revealed no mutation in the PROS1 gene encoding PS.
| Day 1 | Day2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| Complete blood count | ||||
| White blood cell count (×109/L)c | 13.7 | 142 | 12.4 | 12.3 |
| Hb (g/dL) | 13.1 | 12.6 | 12.4 | 13.4 |
| Platelets (×109/L) | 182 | 169 | 185 | 214 |
| Coagulation profile | ||||
| PT INR | 1.03 | 1.11 | 1.37 | 1.64 |
| APTT (seconds) | 33.3 | 34.1 | 37.3 | 49.7 |
| D dimer (µg/mL) | ― | 4.1 | 6.0 | 1.7 |
| Liver function test | ||||
| AST (U/L) | 18 | 20 | 17 | 17 |
| ALT (U/L) | 19 | 18 | 18 | 20 |
| Total bilirubin (mg/dL) | 0.52 | 0.54 | ||
| Renal function test | ||||
| Urea nitrogen (mg/dL) | 14.1 | 11.3 | 11.9 | 8.1 |
| Creatinine (mg/dL) | 0.42 | 0.3 8 | 0.3 5 | 0.3 6 |
| Thrombophilla workup | ||||
| Anti-beta 2 glycoprotein antibody IgG (CU) | <0.7 | |||
| Anti-beta 2 glycoprotein antibody IgM (CU) | <0.4 | |||
| Anticardiolipin antibody IgG (CU) | <4.0 | |||
| Anticardiolipin antibody IgM (CU) | <2.5 | |||
| Lupus anticoagulant | 0.9 | |||
| Protein C (%) | 103 | |||
| Protein S (%) | 18 | 57 | ||
| Antithrombin III (%) | 97.0 | |||
| Other special tests | ||||
| Anti PF-4/H | Negative | |||
| SARS-CoV-2 PCR | Negative | |||
- Abbreviations: ALT, alanine aminotransferase; anti-PF-4/H, anti-platelet factor-4/heparin; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; Hb, hemoglobin; IgG, immunoglobulin G; IgM, immunoglobulin M; INR, international normalized ratio; PCR, polymerase chain reaction; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In most instances, CVST is triggered by predisposing factors, and at least one precipitant risk factor is identified in more than 85% of the cases.5, 11, 12 In our patient, a recent mRNA vaccination and low-level PS activity were noted by precise history and detailed laboratory workup. Most COVID-19 vaccine-related cases of CVST are associated with vector-based vaccines and usually accompanied by TTS.2, 6, 13, 14 However, our patient developed CVST without TTS following an mRNA vaccination. Few adult cases of mRNA vaccine-related CVST in the absence of TSS were reported.14-16 The reporting event rate of the Pfizer-BioNTech vaccine-related CVST from December 12, 2020 to March 16, 2021 was 0.4%, less than that of the viral vector vaccine-related CVST within the same period, which was 1.1%.17 Likewise, a CVST analysis post-vaccination in Europe noted that it occurred far more frequently after a vector-based vaccination than after an mRNA vaccination.6 Nevertheless, SARS-CoV-2 infection itself shows high incidence of CVST compared with the general and mRNA-vaccinated population.18 The mechanism underlying mRNA vaccine-related CVST is unclear, although the following have been proposed: first, the interaction between the spike glycoprotein and platelets leads to platelet aggregation19; second, binding of the spike glycoprotein to the angiotensin converting enzyme receptor activates endothelial cells and upregulates expression of cell adhesion molecules, promoting thrombogenesis and causing CVST20; and third, in vitro studies showed that spike proteins can activate the alternative complement pathway, which is involved in immune-mediated thrombogenesis.21 In our patient, additionally to these hypotheses, acquired PS deficiency, secondary to the immune-mediated reaction to the mRNA vaccine, may have contributed to the CVST onset. PROS1, found on chromosome 3 (3q11.1), presents a reported detection rate of causative gene mutation in suspected PS deficiency of approximately 50%.22 Therefore, the possibility of an inherited PS deficiency was considered. The exact mechanism of acquired PS deficiency is not known, although autoantibodies and epigenetic could be involved.
This case may add to the evidence supporting a possible relationship between mRNA vaccines and thrombotic events, and raises the awareness among pediatric healthcare providers regarding this rare, critical adverse event; early recognition facilitates early treatment to prevent complications. Further investigations are needed to establish whether thrombotic events are merely incidental or are mRNA-based vaccine-associated complications.
AUTHOR CONTRIBUTIONS
Shinsuke Mizuno conceptualized the study, collected, analyzed, and interpreted the data, drafted the initial manuscript, and critically reviewed and revised the manuscript. Junji Koyama, Shogo Horikawa, Kenji Kishimoto, Daiichiro Hasegawa, Yoshiyuki Kosaka, and Masashi Kasai collected the data, drafted the initial manuscript, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
We thank Dr. Hiroshi Kurosawa for his contribution in collecting patient's clinical data. We also thank Dr. Naoya Morisada for his help in collecting and interpreting the genetic data.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to [email protected].




