Multimodality treatment for recurrent neuroblastoma in the central nervous system
Abstract
Survival for patients with recurrent central nervous system (CNS) neuroblastoma remains poor. A single-institutional study demonstrated the potential of multimodality therapy, including compartmental intrathecal radioimmunotherapy (cRIT) with 131I-3F8 or 131I-8H9 to increase the survival of neuroblastoma patients with CNS relapse. However, not all patients are able to receive this therapy. We report three patients with CNS neuroblastoma who remain disease-free 3–9 years after receiving multimodality treatment without cRIT. Additional studies to identify patients most likely to benefit from cRIT are warranted.
Abbreviations
-
- CNS
-
- central nervous system
-
- cRIT
-
- compartmental intrathecal radioimmunotherapy
-
- INSS
-
- International Neuroblastoma Staging System
-
- MIBG
-
- meta-iodobenzylguanidine
-
- MRI
-
- magnetic resonance imaging
-
- PR
-
- partial response
1 INTRODUCTION
The outcome for high-risk neuroblastoma patients who relapse is poor,1, 2 and survival is dismal for patients with central nervous system (CNS) relapse.3-5 In a single-institutional report, 17 neuroblastoma patients with CNS relapse had favorable outcome following surgical resection of CNS disease, craniospinal irradiation, multiagent chemotherapy, compartmental intrathecal radioimmunotherapy (cRIT) with 131I-3F8 or 131I-8H9, and systemic immunotherapy with 3F8 antibody.6 These promising results have led many providers to recommend cRIT for children with CNS relapse. However, many children are not able to receive this treatment due to rapidly progressive disease or severe illness that prevents their ability to travel. Here, we report three patients who were diagnosed in the past decade at Comer Children's Hospital or Nationwide Children's Hospital with isolated relapsed CNS neuroblastoma, defined as primary brain parenchymal involvement or dural-based tumors with radiographic or histologic penetration of the dura. All three patients were treated with multimodality therapy. Although the patients were clinically stable to travel for cRIT, their families declined this treatment due to limited resources and social challenges. The patients remain alive without disease 3–9 years following CNS relapse.
2 CASE REPORTS
2.1 Case 1
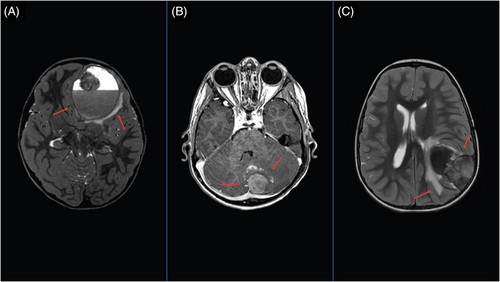
The patient was diagnosed with high-risk International Neuroblastoma Staging System (INSS)7 stage 3 neuroblastoma at 22 months of age.8 Tumor characteristics and treatment are summarized in Table 1. At the end of upfront therapy, the patient achieved a very good partial response (VGPR).7 Twelve months later, the patient developed altered mental status, and a large hemorrhagic lesion in the left frontal region projecting into the frontal lobe was seen on brain magnetic resonance imaging (MRI) (Figure 1A). The patient underwent gross total surgical resection of the CNS tumor. Targeted sequencing panel tests demonstrated the relapsed neuroblastoma was MYCN amplified with wild-type ALK.9 No other sites of disease were detected. Treatment for the CNS relapse is shown in Table 1.10 The patient remains alive and disease-free more than 4.4 years following the CNS relapse.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| INSS stage | Stage 3 | Stage 4 | Stage 4 |
| INPC histology | Unfavorable histology | Unfavorable histology | Unfavorable histology |
| Diagnostic tumor molecular characteristics | MYCN nonamplified, unknown SCA and ALK status | MYCN amplified, Unknown SCA and ALK status | MYCN nonamplified, 11q LOH, wild-type ALK |
| Upfront therapy and response | |||
| Induction | Per ANBL0532 (NCT00567567) | Per ANBL0532 (NCT00567567) | Per ANBL12P1 (NCT01798004) |
| Overall response after induction | VGPR per 1993 INRC7 | PR per 1993 INRC7 | PR per 2017 INRC11 |
| Consolidation | Per ANBL0532 (NCT00567567)
|
Per ANBL0532 (NCT00567567)
|
|
| Overall response after consolidation | VGPR per 1993 INRC7 | PR per 1993 INRC7 | PR per 2017 INRC11 |
| Post-consolidation | Per ANBL0032 (NCT01418495), Regimen B, dinutuximab, cytokines, isotretinoin | Enrolled on ANBL0032 (NCT01418495), Regimen B, dinutuximab, cytokines, isotretinoin | Per ANBL0032 (NCT01418495), Regimen B, dinutuximab, cytokines, isotretinoin |
| Overall response after consolidation (end of therapy) | VGPR per 1993 INRC7 | PR per 1993 INRC7 | PR per 2017 INRC11 |
| CNS relapse | |||
| Symptoms at the time of relapse; initial imaging of CNS relapse | Altered mental status and emesis; CNS relapse identified on brain CT | Headaches; CNS relapse identified on brain MRI | Headaches; CNS relapse identified on brain MRI |
| Sites of relapse after upfront therapy | Isolated CNS relapse (hemorrhagic lesion in the left frontal region projecting into the frontal lobe with a solid component along the left ethmoid skull base and medial orbital roof) | Isolated CNS relapse (dural-based extra-axial mass in the left aspect of the posterior fossa with hemorrhage in the adjacent cerebellar parenchyma and modest mass-effect) | Isolated CNS relapse (dural-based parietal mass with extension into the posterior frontal and temporal lobes) |
| Molecular characteristics of the relapsed CNS neuroblastoma | MYCN amplified, wild-type ALK status, SCA unknown | Unknown MYCN, ALK, and SCA | MYCN nonamplified; 11q LOH; wild-typeALK |
| CNS multimodality therapy |
|
|
|
- Abbreviations: BuMel, busulfan and melphalan; Carbo/Thiotepa, carboplatin, thiotepa; CEM, carboplatin, etoposide, melphalan; CNS, central nervous system; CR, complete response; GTR, gross total resection; Gy, Gray; INPC, The International Neuroblastoma Pathology Classification; INRC, International Neuroblastoma, Response Criteria; INSS, International Neuroblastoma Staging System; LOH, loss of heterozygosity; PR, partial response; SCA, segmental chromosomal aberrations; VGPR, very good partial response.
2.2 Case 2
The patient was diagnosed at 3 years of age with high-risk, INSS stage 4 neuroblastoma.8 Tumor characteristics and upfront treatment are summarized in Table 1. At the end of upfront therapy, the patient achieved a partial response (PR),7 with decreased size of the retroperitoneal soft tissue mass and faint MIBG (meta-iodobenzylguanidine) uptake in the occipital region. Two months later, the patient developed headaches, and a brain MRI revealed a dural-based metastatic tumor in the left aspect of the posterior fossa with hemorrhage in the adjacent cerebellar parenchyma and modest mass-effect (Figure 1B). The patient underwent gross total surgical resection of the posterior fossa tumor. Disease evaluation revealed no other sites of disease. Treatment for the CNS relapsed neuroblastoma is shown in Table 1. The patient remains alive and disease-free 9.6 years following the CNS relapse.
2.3 Case 3
At 33 months of age, the patient was diagnosed with high-risk INSS stage 4 neuroblastoma.8 She presented with a primary paraspinal thoracic tumor (T3–T9) with metastatic soft tissue, bone, and bone marrow. A metastatic lesion in the left sphenoid wing with extension into the temporal lobe was also identified. Tumor characteristics and the upfront treatment is shown in Table 1. The patient achieved a PR11 with persistent MIBG uptake in the left lateral orbit and the primary T7–T8 tumor site. The patient developed headaches 4 months later, and a brain MRI revealed a dural-based parietal mass with extension into the posterior frontal and temporal lobes (Figure 1C). The patient underwent gross total surgical resection of the CNS tumor. Disease evaluation following surgery showed MIBG-avid soft tissue at T7–T8 tumor.6 The CNS relapse treatment is summarized in Table 1. The patient remains alive in a PR11 with a soft tissue mass between T7 and T8 more than 3 years after the CNS relapse.
3 DISCUSSION
Multimodality therapy without cRIT resulted in favorable outcome for the three neuroblastoma patients with isolated CNS relapse reported here. Others have also reported small numbers of children with CNS relapse with favorable survival following treatment with regimens that did not include cRIT. In a Chinese study of 106 stage 4 neuroblastoma patients, two of eight patients with recurrent CNS disease were alive 29–47 months following relapse after treatment with surgery, radiation, and chemotherapy.12 Similarly, among 286 patients reported by the Polish Paediatric Solid Tumours Study Group (PPSTSG), two of 13 with isolated CNS relapse remained alive and disease-free 9 and 2.5 years following multimodality therapy.13 More recently, Berlanga and colleagues reported very poor survival among 53 high-risk stage 4 patients enrolled on the high-risk NBL1/SIOPEN study who developed a first relapse in the CNS.5 However, three patients with isolated CNS recurrence remained alive 4.7, 4.9, and 10.1 years following complete surgical excision of the tumor and craniospinal radiotherapy, with or without a temozolomide-containing regimen.
Differences in treatment may have influenced the striking variation in survival observed in the single-institutional report by Kramer et al.6 compared to the NBL1/SIOPEN study.5 However, it is important to recognize that in contrast to the unselected, large cohort enrolled on the SIOPEN trial,5 only small numbers of patients were included in the report by Kramer and colleagues.6 Further, the patients treated with cRIT were highly selected, as those who had rapid tumor progression or were too ill to travel were excluded. Recently, social determinants of health have been identified as independent risk factors for poor outcome among patients with high-risk neuroblastoma.14, 15 As illustrated by the cases reported here, lack of resources may also influence whether a patient is able to travel to receive cRIT.
Three additional patients with recurrent CNS disease were diagnosed at our institutions in the past decade. None of these patients received cRIT, and all have died. One patient was treated only with supportive care after a neurologically devastating CNS tumor hemorrhage. The second patient had a combined systemic and CNS relapse. He did achieve a complete response (CR) in the CNS with multimodal therapy without cRIT, but died of progressive disease outside the CNS. The third patient developed rapidly progressive leptomeningeal disease weeks after treatment with surgery, palliative focal radiation, and oral temozolomide for relapsed disease in the internal auditory canal and the cerebellopontine angle.
Kramer and colleagues have reported preliminary results for 80 patients with recurrent CNS neuroblastoma enrolled on a single-institutional phase I study (NCT0008924) treated with 131I-8H9 (131I-omburtamab) cRIT.16 Forty-five patients (56%) were reported to be alive for a median 58 months after the CNS relapse. An industry-sponsored multicenter single-arm phase 2/3 trial (NCT03275402) to evaluate the safety and feasibly of treating patients’ recurrent CNS neuroblastoma with 131I-omburtamab is ongoing. Our cases and others5, 12, 13 indicate that favorable survival can be achieved in a subset of patients with isolated CNS relapsed neuroblastoma following gross total resection of the tumor and multimodality therapy without cRIT. Future studies testing the benefit of adding cRIT to multimodality therapy in patients with isolated CNS relapse and the efficacy of this treatment in unresectable recurrent CNS disease are warranted.
CONFLICT OF INTEREST
Ami V. Desai: stock ownership in Pfizer and Viatris; consultancy/advisory board fees from Merck, Ology Medical Education, YMabs Therapeutics, Glaxo Smith Kline; travel and accommodation expenses from GlaxoSmithKline and YMabs Therapeutics. Keri Streby: consulting fees from YmAbs Therapeutics, Inc and Illumina Radiopharmaceuticals, LLC. Susan L. Cohn: stock ownership in Pfizer, Merck, and Lilly; served on an advisory board for YMabs Therapeutics. The remaining authors made no disclosures.




