Common bone marrow signature in COVID-19-associated multisystem inflammatory syndrome in children: A first-wave small case series experience
Mastronuzzi Angela and De Vito Rita contributed equally to this work.
Abstract
The hyper-inflammatory response, also known as multisystem inflammatory syndrome in children (MIS-C), represents a major concern in children with SARS-CoV-2 infection. We report bone marrow features of three patients with MIS-C who were diagnosed during the first wave of the SARS-CoV-2 pandemic. A bone marrow evaluation was performed at onset of the inflammatory condition in order to exclude secondary hemophagocytic lymphohistiocytosis (sHLH). The bone marrows of the patients presented common features: the erythroid and megakaryocytic lineages were prominently affected and hemophagocytosis was moderately increased, differently than observed in sHLH. Megakaryocytopoiesis was increased, representing a peculiar feature of MIS-C differing from sHLH. SARS-CoV-2 RT-PCR and viral panel were studied in bone marrow aspiration samples. MIS-C is a rare complication of SARS-CoV-2 infections in children. An immuno-dysregulation considering both innate and adaptive immunity together with vascular inflammation and endothelial dysfunction play a major role. Our observations, although limited due to the small sample size, suggest that there are unique features in the bone marrow of patients with MIS-C that are likely secondary to immuno-dysregulation, and there are notable differences in bone marrow features compared to those reported in sHLH.
Abbreviations
-
- BM
-
- bone marrow
-
- CXCL10
-
- C-X-C motif chemokine ligand 10
-
- CXCL9
-
- C-X-C motif chemokine ligand 9
-
- GvHD
-
- graft-versus-host disease
-
- IL
-
- interleukin
-
- MIS-C
-
- multisystem inflammatory syndrome in children; MPO, myeloperoxidase
-
- OPBG
-
- Bambino Gesù Children's Hospital
-
- sHLH
-
- secondary hemophagocytic lymphohistiocytosis
-
- TFNα
-
- tumor necrosis factor α
1 INTRODUCTION
Children are susceptible to SARS-CoV-2 infection, but are less likely to be symptomatic or present severe respiratory symptoms with less than 1% of critically ill patients1-5; however, a hyperinflammatory response, also known as multisystem inflammatory syndrome in children (MIS-C), represents a major concern in pediatric population.6-13 MIS-C occurs in children 4–6 weeks after mild or asymptomatic SARS-CoV-2 infection due to a delayed immune response. The case definition for MIS-C is broad, including the presence of fever with laboratory evidence of inflammation and of severe multisystem involvement: cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurologic.5-13
During the first SARS-CoV-2 wave, specifically at the first observations of hyperinflammatory syndrome, we performed a bone marrow (BM) evaluation in order to detect secondary hemophagocytic lymphohistiocytosis (sHLH). We report the BM features with plasma cytokine profiles of three children who met the criteria for the diagnosis of MIS-C. We compared BM with patients who had sHLH.
2 METHODS
We focused on three patients admitted at Bambino Gesù Children's Hospital (OPBG) with a hyperinflammatory syndrome consistent with MIS-C13 in which a BM evaluation was performed at diagnosis. The hyperinflammatory status of all three patients was diagnosed by the end of April 2020; at that time, MIS-C had not yet been described as a pathological entity uniquely associated with the SARS-CoV-2 infection, and a BM evaluation was performed to exclude/confirm an sHLH. The plasma from the three patients was collected before any anti-inflammatory therapy. Clinical characteristics are summarized in Table 1 and Table S1. All patients were alive 24 months after MIS-C diagnosis. A viral, bacterial, and fungal infection workup was performed on blood, BM, nasopharyngeal swab, and urine and stool samples. All three patients presented a positive RT-PCR nasopharyngeal swab for SARS-CoV-2 within the last 4 weeks prior to MIS-C diagnosis.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Lymphocyte count per mm3, N (%) (5.5–15) (40%–57%) | 600 (10%) | 810 (7.9%) | 1430 (11%) |
| Hemoglobin, g/dl (10.5–15) | 10.7 | 11.3 | 10.4 |
| Platelets per mm3 (150–450) | 116,000 | 215,000 | 186,000 |
| C-reactive protein, mg/dl (<0.5) | 19 | 22 | 28 |
| Procalcitonin, ng/ml (<0.5) | 7.9 | 2.28 | 1.2 |
| Erythrocyte sedimentation rate, mm/h (<20) | 57 | 53 | 74 |
| ThsTnT (<14 pg/ml) | 86.5 | 93 | 173 |
| NT-proBNP (<230 pg/ml) | 812 | 2950 | 190 |
| D-dimer (>0.5 mg/L) | 5.42 | 3.18 | >20 |
| Ferritin (<300 μg/ml) | 772 | 23.345 | 419 |
| Cholesterol, mg/dl (<170) | 76 | 85 | 151 |
| Triglyceride, mg/dl(<0.5) | 86 | 196 | 124 |
| Alanine aminotransferase (<41 U/L) | 19 | 90 | 21 |
| Aspartate aminotransferase (<40 U/L) | 25 | 97 | 27 |
| Lactate dehydrogenase (120–300 U/L) | 228 | 569 | 255 |
| Uric acid (3.4–7 mg/dl) | 3 | 3.2 | 4.9 |
| Serum albumin (3.8–5.4 g/dl) | 3.3 | 2.6 | 4.0 |
| Fibrinogen (162–401 mg/dl) | 564 | 400 | 643 |
| Sodium (136–145 mEq/L) | 135 | 137 | 137 |
| IL-1b, pg/ml (HD <0.04) | 1.3 | 6.2 | 2.5 |
| IL-6, pg/ml (HD 1.2; 0.91–1.89) | 76.4 | 90.7 | 50.3 |
| IL-8, pg/ml (HD 1.55–1.6) | 40.9 | 408.1 | 209.7 |
| TNF-a, pg/ml (HD 7.47; 5.4–9.4) | 27.3 | 20.9 | 20.9 |
| CXCL9*, pg/ml (NV 429.5–801.5) | 2760 | 1267 | 515 |
| CXCL10*, pg/ml (NV 111.0–379.3) | 2034 | 560 | 244 |
| IL-18*, pg/ml (NV <300 pg/ml) | <2048 | 246,492 | <2048 |
| Perforin | Normal | Normal | Normal |
| Cytotoxicity assay | Reduced | Reduced | Reduced |
| Degranulation assays | Reduced | Reduced | Reduced |
- Note: N, absolute count. For ThsTnT, NT-proBNP, D-dimer, and ferritin are added at the upper local limit; for IL1b, IL 6, IL8, and TNF-a are added median value and range in healthy donors (HD).
Cytokine profile data were obtained at diagnosis: interleukin (IL)-1β), IL-6, IL-8, tumor necrosis factor α (TFNα), C-X-C motif chemokine ligand 9 (CXCL9), C-X-C motif chemokine ligand 10 (CXCL10), and IL-18 were considered for this report. Cytokine levels were measured using an automated ELISA assay (ELLA microfluidic analyzer, Protein Simple) according to the manufacturer's instructions.
2.1 Bone marrow morphological study
BM biopsies were fixed in acetic zinc formalin, decalcified in ethylenediaminetetraacetic acid (EDTA), and paraffin embedded. Hematoxylin and eosin, periodic acid-Schiff, May–Grunwald–Giemsa, reticulin, and Pearls iron stains were performed. The immunohistochemical panel included glycophorin, CD71, myeloperoxidase (MPO), CD61, CD10, TdT, CD20, CD79, CD3, CD4, CD8, CD68, CD163, CD138, and CD117. As controls, BM biopsies of five patients with sHLH diagnosed in the last 3 years at OPBG were compared to BM biopsies of MIS-C patients. The patients with sHLH had an HLH of unknown etiology, and no infection or underline disease had been identified. The Ethics Committee (2159_OPBG_2020) approved this retrospective study. All investigations were conducted in accordance with principles expressed in the Declaration of Helsinki.
3 RESULTS
A BM evaluation was performed in three pediatric patients with MIS-C. Co-infection were excluded. A viral panel including RT-PCR for Cytomegalovirus, Epstein–Barr virus, parvovirus B19, herpes simplex virus 1 and 2, human herpes virus 6, and adenovirus were negative on peripheral and BM blood. Blood, urine, and sputum cultures were negative, as was the viral panel on nasopharyngeal swab. The level of plasma cytokines increased in all patients (Table 1).
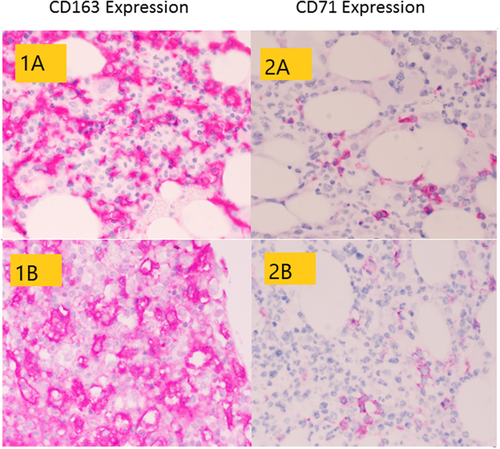
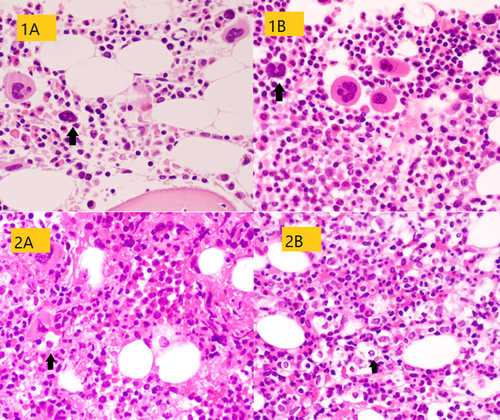
Pathology findings of BM biopsies are summarized in Table 2. Cellularity was normal in two patients and decreased in one. Erythroid precursors were reduced in all BM biopsies, with isolated early erythroblasts that rarely formed small aggregates. Myeloid population (stained with MPO) was relatively increased with left shift and focal disarray. Megakaryocytes (identified with a CD61 staining) were increased in number, with cell size variable from small to large along with the presence of rare micro-megakaryocytes. The nuclei appeared hypo- or hyper-lobated and hyper-chromatic. A mild lymphocytic infiltrate was seen with a prevalent T phenotype. CD68 and CD163 highlighted mild histiocytic hyperplasia, with a small number of histiocytes containing vacuoles, granules, and ingested blood elements (Figure 1). The plasma cell and mast cell counts were normal. No vascular alterations were found (Figure 2). Reticulin stain showed a mildly increased reticulin network.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Cellularity | Decreased | Normal | Normal |
| Reticulin fibrosis | Focally increased | Focally increased | Focally increased |
| Collagen fibrosis | Not increased | Not increased | Not increased |
| Abnormal iron storage | No | No | No |
| Erythropoiesis | Decreased | Decreased | Decreased |
| Small island | Small island | Small island | |
| Frequent single immature cells | Frequent single immature cells | Frequent single immature cells | |
| Myelopoiesis | Left shift | Left shift | Left shift |
| Mild disarray | Mild disarray | Mild disarray | |
| Megakaryocytopoiesis | Increased | Increased | Increased |
| Pleomorphic | Pleomorphic | Pleomorphic | |
| Megakaryocytes: small hypolobated and large hypolobated | Megakaryocytes: small hypolobated and large hypolobated | Megakaryocytes: small hypolobated and large hypolobated | |
| Micromegakaryocytes | Micromegakaryocytes | Micromegakaryocytes | |
| Hyperchromatic nuclei | Hyperchromatic nuclei | Hyperchromatic nuclei | |
| Lymphocytes | Mild increased | Mild increased | Mild increased |
| Plasma cells | Not increased | Not increased | Not increased |
| Hematogones | Not increased | Not increased | Not increased |
| Histiocytes | Moderately increased | Moderately increased | Moderately increased |
| Mast cells | Not increased | Not increased | Not increased |
| Vascularity | Not increased | Not increased | Not increased |
4 DISCUSSION
In the first wave of the COVID-19 pandemic, the pediatric population had been considered less affected by COVID-19.3-5 By the end of April 2020, clinicians and scientists were warned over a rising number of children presenting with a multisystem inflammatory condition needing intensive care, in connection to the pandemic; the presenting signs and symptoms overlapped with another inflammatory disease such as Kawasaki disease and macrophage activation syndrome (MAS)/sHLH.5-14 The acronym MIS-C is used to identify a clinical condition characterized by an inflammatory status with frequent gastrointestinal manifestations and circulatory failure, including myocardial injury. Despite a severe disease with often emerging life-threatening symptoms, the mortality rate is low at 1.9%.4-14 The pathogenesis is not yet fully understood, but it seems to be due to a delayed immunological phenomenon associated with proinflammatory triggers following either symptomatic or asymptomatic SARS-CoV-2 infection related to age-specific immune response mechanisms.15-18
There is scarce information reporting on BM evaluation in COVID-19 patients that showed hemophagocytosis; the COVID-19-related hyperinflammatory status is considered as a form of sHLH.19-29 Recently, Lacinel et al. reported the presence of hemophagocytosis on BM aspirates from three children with MIS-C.30
The three reported children presented an inflammatory condition consistent with MIS-C after the SARS-CoV-2 infection. Plasma cytokine levels were increased in all patients, as observed in recent MIS-C immunological studies.15-18 Surprisingly, the BM presented common features in all three patients; the erythroid and megakaryocytic lineages were prominently affected and hemophagocytosis was evident. Hemophagocytosis was previously described in adults on BM, lymph nodes, and spleen (mostly in autopsy findings) and can be found in critically ill patients with no other secondary HLH features, most likely as a result of increased BM turnover.19-26, 31, 32 It is noteworthy that in the presented MIS-C cases, only a mild hemophagocytosis was observed with almost normal cellularity, while the erythroid and megakaryocytic lineages were found to be prominently affected with almost normal peripheral blood findings. The erythroid hypoplasia with lack of erythroid maturation resembles the primary cytopathic effect of the parvovirus B19 infection on BM,33 while the mild peripheral anemia could be explained by a compensatory extension of the half-life of red blood cells, as discussed by Patel et al.34
The megakaryocytopoiesis alterations seem to be a peculiar feature of MIS-C differing from sHLH; the BM of sHLS do not show any significant alterations of megakaryocytopoiesis (see Table S2). The hyperplasia of megakaryocytes in addition to the presence of polynuclear cells and immature forms with asynchrony nucleus/cytoplasm could be due to an overstimulation by thrombopoietin as a compensatory mechanism of peripheral autoimmune thrombocytopenia, as reported in adults.35 Nevertheless, a prominent thrombocytopenia was not observed in our patients. Moreover, the nuclear hyperchromasia could be a sign of virus colonization and virus-induced apoptosis, as seen in HIV infection.36 However, as underlined before, we did not find SARS-CoV-2 RNA or any other virus on BM, and co-infection were excluded. The BM alterations seem to be a consequence of an inflammatory microenvironment as suggested by the plasma cytokine profile.
As reported, the inflammatory response in MIS-C presents differences from the cytokine storm of severe acute COVID-19, sharing several features with Kawasaki disease, while autoantibodies could be involved in the pathogenesis.16 Recently, particularly marked elevations of IL-1, IL-10, and TFNα were observed in patients affected by MIS-C compared to patients with severe COVID-19 infection, as observed in our series.37, 38
More likely, the aberrant activation of the immune network caused by the cell-extrinsic microenvironmental factors driven by IL-6 and IL-8 plays a fundamental role, leading to a final common pathway of organ damage that involves BM as observed in other conditions such as graft-versus-host disease (GvHD). Acute GvHD is a major life-threatening complication after allogeneic hematopoietic cell transplantation, with classical target organs including the intestines, liver, and skin and nonclassical such as BM; since the early 1990s, the cytokine dysregulation in GVDH has been well known, which reflects an immune network and underlying complex mechanism.39-43
MIS-C is a rare complication of SARS-CoV-2 infection in children; great efforts were made to distinguish this entity from other inflammatory states of childhood and clarify its pathogenesis. An immuno-dysregulation, considering both innate and adaptive immunity together with vascular inflammation and endothelial dysfunction, plays a major role with a distinct, but not specific, cytokine signature. Our observations, although limited due to the small sample size, suggest that there are unique features in the BM of patients with MIS-C that are likely secondary to immuno-dysregulation, and there are notable differences in BM features compared to those reported in sHLH.
ACKNOWLEDGMENTS
We would like to thank the entire medical and nursing staff members who cared for patients and parents at their personal risk in this time of the epidemic, and we also thank Megan Eckley for manuscript editing.
Open access funding provided by Ospedale Pediatrico Bambino Gesu.
[Correction added on 25 November 2022, after first online publication: BIBLIOSAN funding statement has been added.]
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Maria Antonietta De Ioris and Rita De Vito conceived the study. Maria Antonietta De Ioris, Alessia Scarselli, Angela Mastronuzzi, and Claudia Bracaglia drafted the manuscript. Rita De Vito, Maria Antonietta De Ioris, Chiara Agrati, and Claudia Bracaglia performed the data analysis. The remaining authors participated in its experimentation and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




