Second tumor risk in children treated with proton therapy
These data were presented at the 2020 Annual Meeting of the American Society of Radiation Oncology (ASTRO), which took place virtually October 24–29, 2020.
Abstract
Background
Out-of-field neutron dissemination during double-scattered proton therapy has raised concerns of increased second malignancies, disproportionally affecting pediatric patients due to the proportion of body exposed to scatter dose and inherent radiosensitivity of developing tissue. We sought to provide empiric data on the incidence of early second tumors.
Methods
Between 2006 and 2019, 1713 consecutive children underwent double-scattered proton therapy. Median age at treatment was 9.1 years; 371 were ≤3 years old. Thirty-seven patients (2.2%) had tumor predisposition syndromes. Median prescription dose was 54 Gy (range 15–75.6). Median follow-up was 3.3 years (range 0.1–12.8), including 6587 total person-years. Five hundred forty-nine patients had ≥5 years of follow-up. A second tumor was defined as any solid neoplasm throughout the body.
Results
Eleven patients developed second tumors; the 5- and 10-year cumulative incidences were 0.8% (95% CI, 0.4–1.9%) and 3.1% (95% CI, 1.5–6.2%), respectively. Using age- and gender-specific data from the Surveillance, Epidemiology, and End Results (SEER) program, the standardized incidence ratio was 13.5; the absolute excess risk was 1.5/1000 person-years. All but one patient who developed second tumors were irradiated at ≤5 years old (p < .0005). There was also a statistically significant correlation between patients with tumor predisposition syndromes and second tumors (p < .0001). Excluding patients with tumor predisposition syndromes, 5- and 10-year rates were 0.6% (95% CI, 0.2–1.7%) and 1.7% (95% CI, 0.7–4.0%), respectively, with all five malignant second tumors occurring in the high-dose region.
Conclusion
Second tumors are rare within the decade following double-scattered proton therapy, particularly among children irradiated at >5 years old and those without tumor predisposition syndrome.
Abbreviations
-
- 3D
-
- three-dimensional
-
- AER
-
- absolute excess risk
-
- IMRT
-
- intensity-modulated photon therapy
-
- SEER
-
- Surveillance, Epidemiology, and End Results
-
- SIR
-
- standardized incidence ratio
1 INTRODUCTION
Through the characteristic Bragg peak, proton beam therapy spares patients the “exit” radiation dose associated with X-ray treatments, such as three-dimensional (3D) conformal photon therapy and intensity-modulated photon therapy (IMRT). Proton therapy confers numerous purported advantages, including the ability to escalate the radiation dose in tumors positioned near critical organs and reduce the overall volume of normal tissue exposed to low- and intermediate-radiation doses. The latter is particularly appealing in pediatric radiotherapy, where the potential for decades-long survivorship, inherent radiosensitivity of developing organs, and high rate of germline mutations in childhood cancer patients drives a constant pursuit to reduce or eliminate unnecessary radiation exposure. However, to date, the vast majority of pediatric patients worldwide have been treated using a passive modulation proton technique that employs a scattering foil to produce a clinically useful field size. This scattering foil is the source of neutrons that can expose the total body of a child to the damage of potent secondary ionization, estimated in doses distant from the field edge 10 times higher than those characteristic of IMRT.1 Such provocative models are an understandable source of both ongoing concern2, 3 and controversy,4, 5 calling for empiric validation in the real-world clinical setting. Therefore, we performed a comprehensive review of second tumors in pediatric patients treated with double-scattered proton therapy over the 13-year history of the University of Florida Health Proton Therapy Institute (UFHPTI).
2 METHODS
2.1 Patients and treatment
This was a single-institution retrospective study of pediatric patients (≤21 years of age) treated with double-scattered proton therapy for benign and malignant solid tumors at the UFHPTI from its opening in August 2006 through December 2019 (n = 1713). Patients who received prior radiation were excluded. The University of Florida Institutional Review Board (IRB201903320), including a waiver of consent, approved this retrospective noninterventional study. Data collected included age at treatment, sex, race, primary tumor histology and location, use of anesthesia, chemotherapy exposure and type, and radiation dose to the primary tumor. The presence of a tumor predisposition syndrome was assessed using standard methods of family history and clinical presentation, then confirmed with genetic testing. In applicable patients, age at the time of second tumor diagnosis, new histology and location, and radiation dose to the region obtained by fusing diagnostic imaging to the digital radiation treatment plan were also recorded.
2.2 Determination of second tumor development
The patient's UFHPTI medical record, including correspondence with medical professionals from other treating institutions, was carefully reviewed to assess the development of second tumors. Over 90% of patients were concurrently enrolled on a prospective clinical trial or outcome tracking registry, ensuring robust follow-up: 98.3% and 99.6% of living patients had a documented medical encounter within the past 2 and 3 years, respectively. Only seven patients (0.4%) were lost to follow-up. An event included any second solid tumor, benign or malignant, anywhere in the body. Hematologic malignancies following radiotherapy were recorded separately to allow both joint and independent analyses. Low-grade gliomas that transformed into biopsy-proven high-grade gliomas (n = 3) were not considered second tumors.
2.3 Statistical analyses
The Kaplan–Meier product limit method estimated freedom from second tumor and the log-rank test statistic estimated the level of statistical significance between strata of patients with tumor predisposition syndromes and those patients without. To ensure a comprehensive inventory of events, no minimum follow-up was required for the primary collection of second tumor information. This approach, consistent with prior analyses,6, 7 was designed with the intention to capture all second neoplasms following radiation, even those unrelated to radiation itself, such as sporadic lesions arising in patients with tumor predisposition syndrome or hematologic second malignancies, such as leukemia and lymphoma, which may occur shortly after chemotherapy. This approach was also selected due to the uncertainty surrounding the timing of neutron-induced carcinogenesis. Acknowledging the value of different perspectives on the data, however, additional analyses were performed on subsets with a minimum follow-up of 5 years and excluding those patients with a known tumor predisposition syndrome, and cohort rates were calculated including both solid and hematologic second tumors. For each, death was considered a competing event within both analyses, with surviving disease-free patients censored at the date of last follow-up. The standardized incidence ratio (SIR) was assessed as the ratio of the observed/expected cases for second tumor development using age- and gender-adjusted pediatric incidence rates from the Centers for Disease Control (CDC) National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) US Cancer Statistics Public Use Research Database covering the years 2001–2016.8 Absolute excess risk (AER) was computed by subtracting the expected number of second tumors from the observed number of cases, and dividing the difference by the number of person-years of follow-up.
3 RESULTS
Patient and treatment characteristics of the 1713 patients are detailed in Table 1. The 5- and 10-year overall survival rates were 83.5% and 82.2%, respectively. The median follow-up was 3.3 years (range 0.01–12.8) and included 6587 total person-years in follow-up. Overall, 549 patients had ≥5 years of follow-up. For this 549-patient subset, the follow-up duration was 7.1 years (range 5–12.8).
| Characteristic | No. of patients or other value |
|---|---|
| Median age at radiotherapy (range) | 9.1 (0.5–21.9) years |
| Patients age ≤3 years old | 371 |
| Male/female | 933/780 |
| Race | |
| White | 1355 |
| Black | 204 |
| Hispanic | 108 |
| Asian | 46 |
| Tumor predisposition syndrome | |
| Neurofibromatosis | 18 |
| Retinoblastoma | 12 |
| Li–Fraumeni | 6 |
| Ataxia-telangiesctasia | 1 |
| Tumor type | |
| Central nervous system | 1040 |
| Sarcoma | 478 |
| Head and neck carcinoma | 80 |
| Lymphoma | 74 |
| Other | 41 |
| Anesthesia during radiotherapy | 612 |
| Total radiation dose (range) | 54 (15–75.6) Gy |
| Craniospinal irradiation | 157 |
| Photon radiation component | 148 |
| Chemotherapy | |
| Any chemotherapy | 1003 |
| Alkylating chemotherapy | 837 |
3.1 Second solid tumor events
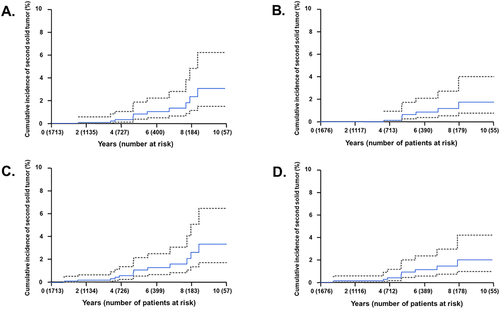
Overall, 11 patients developed second solid tumors resulting in a 5- and 10-year cumulative incidence of 0.8% (95% CI, 0.4–1.9%) and 3.1% (95% CI, 1.5–6.2%), respectively (Figure 1A). Table 2 contains second solid tumor details. The SIR was 13.5 and the AER was 1.5/1000 person-years. The median time to development of a second solid tumor was 66 months following irradiation (range 17–102 months). Eight of 11 patients had malignant tumors. The risk of developing a second solid tumor was 1.7% if irradiated at age ≤5 years versus 0.1% if older (p < .0005). The risk for patients with a known tumor predisposition syndrome developing a second tumor was 10.8% versus 0.4% in those without (p < .001). The second tumor risk was most pronounced in patients with Li–Fraumeni (33.3% vs. 0.5%; p < .001). Excluding patients with known tumor predisposition syndromes, the 5- and 10-year risks of second solid tumor development were 0.6% (95% CI, 0.2–1.7%) and 1.7% (95% CI, 0.7–4.0%), respectively (Figure 1B). Two of the four patients with known tumor predisposition syndromes developed multiple second tumors. Excluding the patients with known tumor predisposition syndrome, all of the malignant second tumors occurred in the high-dose region (Appendix SA1). In this subset of patients without a tumor predisposition syndrome, age ≤5 years remained a significant risk factor (p < .01). Examining the subset of 549 patients with at least 5-year follow-up reveals a 10-year second tumor cumulative incidence of 2.3% (95% CI, 0.9–5.7%). For this cohort, the SIR was 10.0 and AER was 1.2/1000 person-years. Among these patients, age ≤5 years (p < .05), tumor predisposition syndrome (p < .01), and the use of craniospinal radiation (p < .05) correlated with the development of a second solid tumor. When excluding the patients with known tumor predisposition syndrome from those with 5-year follow-up (n = 538), the 10-year second tumor cumulative incidence was 1.1% (95% CI, 0.3–3.6%). Overall and across the subset analyses, sex, histology, radiation dose, chemotherapy, and alkylating chemotherapy were not significantly associated with the development of a second solid tumor. The number of events precluded a valid multivariate analysis.
| Case # | Age at RT (years) | Primary tumor | Interval (months) | Secondary tumor |
Dose at site of secondary tumor (Gy) |
Genetic syndrome? | Chemotherapy? |
|---|---|---|---|---|---|---|---|
| 1 | 5 | Medulloblastoma | 66 | High-grade glioma | 54 | No | Yes |
| 2 | 4 | Ependymoma | 81 | High-grade glioma | 12.6–59.4 | No | No |
| 3 | 5 | Craniopharyngioma | 68 | High-grade glioma | 10.8–54 | No | No |
| 4 | 2 | Ependymoma | 96 | High-grade sarcoma | 10.8–54 | No | Yes |
| 5 | 3 | Ewing sarcoma | 56 | High-grade sarcoma | 54 | No | Yes |
| 6 | 2 | ATRT | 56 | Schwannoma | 10.8–50.4 | No | Yes |
| 7 | 7 | Ewing sarcoma | 44 | Inflammatory myofibroblastic tumor | 12–21 | No | Yes |
| 8 | 1 | S-PNET | 102 | High-grade bone sarcoma | 35–54 | ATM | Yes |
| 9 | 5 | Medulloblastoma | 93, 122 | Low-grade glioma, choroidal melanoma | 36, 36 | NF-1 | Yes |
| 10 | 3 | Rhabdomyosarcoma | 17 | Giant cell tumor | 0–41.4 | p53 | Yes |
| 11 | 2a | Rhabdomyosarcoma | 41, 66 | Medulloblastoma, high-grade soft tissue sarcoma | 0, 50.4 | p53 | Yes |
- Abbreviations: ATM, ataxia-telangiectasia mutation; ATRT, atypical teratoid rhabdoid tumor; RT, radiation therapy; S-PNET, supratentorial primitive neuroectodermal tumors.
- a Same patient as in Table 3.
3.2 All neoplasms (solid tumors and hematologic malignancies)
In addition to the 11 solid tumors, four patients developed hematologic malignancies following proton therapy, resulting in a 5- and 10-year cumulative incidence of all second neoplasms of 1.1% (95% CI, 0.6–2.2%) and 3.3% (95% CI, 1.7–6.4%), respectively (Figure 1C). Table 3 contains the hematologic malignancy details. The median time to development of a hematologic malignancy was 10 months following irradiation (range 9–66 months). The SIR was 12.0 and the AER was 1.9/1000 person-years for developing any second neoplasm (either a solid tumor or hematologic malignancy). The risk of developing any second neoplasm was 1.9% if irradiated at age ≤5 years versus 0.3% if older (p < .001). The risk for patients with a known tumor predisposition syndrome developing any second neoplasm was 10.8% versus 0.6% in those without (p < .0005). The second neoplasm rate was most pronounced in patients with Li–Fraumeni (33.3% vs. 0.7%; p < .001). Excluding patients with tumor predisposition syndromes, the 5- and 10-year risks of any second neoplasm were 0.9% (95% CI, 0.4–2.0%) and 2% (95% CI, 0.9–4.2%), respectively (Figure 1D). In this subset, age ≤5 years remained a significant risk factor (p < .05). Examining the subset of 549 patients with at least 5-year follow-up reveals a 10-year cumulative incidence of any second neoplasm of 2.3% (95% CI, 0.9–5.7%). For this cohort, the SIR was 7.6 and AER was 1.1/1000 person-years. Among these patients, age ≤5 years (p < .05), tumor predisposition syndrome (p < .01), and the use of craniospinal radiation (p < .05) correlated with the development of any second neoplasm. When excluding the patients with a known tumor predisposition syndrome from those with 5-year follow-up (n = 538), the 10-year second neoplasm cumulative incidence was 1.1% (95% CI, 0.3–3.6%). Overall and across the subset analyses, sex, tumor type, radiation dose, chemotherapy, and alkylating chemotherapy were not significantly associated with the development of a second neoplasm of any type. Again, the number of events precluded a valid multivariate analysis.
| Age at radiotherapy (years) | Primary tumor | Interval from completion of radiation (months) | Secondary malignancy | Genetic syndrome? | Chemotherapy? |
|---|---|---|---|---|---|
| 12 | CNS germinoma | 9 | Acute lymphoblastic leukemia | No | Yes |
| 17 | Ewing sarcoma | 10 | Myelodysplastic syndrome | No | Yes |
| 3.4 | Ependymoma | 48 | Acute myeloid leukemia | No | Yes |
| 2a | Rhabdomyosarcoma | 66 | Acute lymphoblastic leukemia | p53 | Yes |
- Abbreviation: CNS, central nervous system.
- a Same patient as in Table 2.
4 DISCUSSION
- 1. Do our empiric findings support the theory that double-scattered proton therapy results in an increased risk of second tumors in pediatric patients?
Similar to general age models, there is strong evidence supporting a linear dose–response for second solid tumors following radiation in children.12 This concept has been invoked by numerous proton therapy proponents who expect less carcinogenesis in children treated with proton therapy.13-15 However, a landmark article by Eric Hall published in 2006 indicated that the neutrons generated by the scattering foil used in passive-scattered radiation devices results in doses distant to the field edge that are 10 times greater than those characteristic of IMRT and even higher when compared to 3D conformal photon radiation.1 Furthermore, due to the smaller physical size of children undergoing radiation, the absolute risk of secondary neoplasms may be more pronounced in younger patients. The conclusions by Hall have subsequently been challenged from both physics and radiobiology perspectives,5, 16 but clinical evidence is lacking on both sides of the issue. An important barrier to resolution is the lack of suitable comparison data for second tumors following photon radiation. Many existing large cohort reports are based on survivorship clinic patients, excluding patients with less than 5 years of follow-up. Yet the relative risk of developing a subsequent malignancy after radiation is highest in the first 10 years after radiation for the primary cancer diagnosis,6, 17-19 and, like ours, multiple series report solid tumors occurring within 3 years following radiotherapy.6, 19, 20 Some reports exclude patients with cancer predisposition syndromes. For example, Berger et al. reported a cumulative incidence rate of 1.0% at 5 years, but excluded patients with neurofibromatosis and retinoblastoma.19 Other studies exclude “nonmalignant second neoplasms” or “nonglioma CNS neoplasms” from rate estimates.6, 7, 17, 18, 21, 22 Other reports generate SIRs using mixed therapy cohorts, including patients who did not receive radiotherapy.7, 18, 19, 23 Furthermore, the SIR in any given series may vary as a function of the irradiated primary tumor, where patients with CNS tumors, sarcoma, and Hodgkin lymphoma may have a two to three-times higher rate of developing a second malignancy compared to other types of tumors.6, 10, 19, 23 As a result of the wide variation in methodology, SIR estimates following photon radiation range broadly from 3.6 to 22.9.6, 7, 10, 17-19, 23
- 2. How does the rate of second neoplasms following double-scattered proton therapy compare to that following IMRT in children?
- 3. What does this large dataset contribute to our understanding of the safety of double-scattered proton therapy in children with known tumor predisposition syndromes and other risk factors for second neoplasms?
It is widely recognized that certain cancer predisposition syndromes, such as neurofibromatosis, Li–Fraumeni, retinoblastoma, and ataxia telangiectasia, increase sensitivity to ionizing photon radiation. The magnitude of elevated risk is uncertain as one cannot determine if, for any given event, radiation actually caused the second neoplasms so common in these patients. Furthermore, no meaningful clinical data exist on the relative impact of neutron scatter in children with cancer predisposition syndromes. Nonetheless, it is hoped that when these children do require therapeutic radiation, the risk of second tumors will be reduced if proton therapy is utilized. Given that 90% of children in our cohort with known tumor predisposition syndromes did not develop a second tumor, our early data are encouraging, especially considering the actual rate would be lower as some of the documented second tumors would likely occur in the absence of any radiation. Our report might serve as a useful foundation for future photon and proton radiation studies intended to address this knowledge gap. Beyond patients with a cancer predisposition syndrome, our observations also confirmed the elevated risk of second tumors in any patient irradiated during early childhood. When radiation cannot be delayed, however, double-scattered proton therapy seems to impart a fairly low risk in the first decade of follow-up: among our 1134 children older than 5 years at the time of treatment, the incidence of any second neoplasm was 0.3%.
Important limitations of our data must be acknowledged. Patients were screened for known predisposition syndromes using standard methods, but the true incidence of cancer predisposition genes in children may exceed 8%.28 Family history is an incomplete predictor in most patients and, therefore, the true number of children with cancer predisposition (and impact thereof) may be underestimated in our analysis. Moreover, despite >500 patients with over 5 years of follow-up, the latency of some second tumors requires decades of follow-up to truly characterize the risk faced by survivors. It is unclear how second cancer latency correlates with tissue dose: the lower doses afforded by proton therapy may simply delay, rather than eliminate, second neoplasm risks, although the limited existing clinical information shows no correlation between dose and latency.20 Given the high rate of participation in our prospective outcome study, we are hopeful that this patient cohort will continue to contribute to our future analyses of both incidence and timing of these second tumors. Our study is also susceptible to another limitation common to long-term research: evolving technology. Nearly all new proton therapy devices today have replaced passive modulation with a scanning beam. In theory, this will reduce the rate of second tumors even further.1 This limitation does not negate the value of our findings however, as thousands of survivors worldwide have been treated with double-scattered proton therapy and would benefit from more accurate risk estimates. Moreover, some scanning proton systems still require patient-specific apertures that generate neutron scatter29 and other heavy particles that are on the horizon, like carbon, generate secondary neutrons.30 Therefore, the data herein will remain useful and offer an opportunity to refine radiobiologic models and inform risk–benefit determination in the future.
CONFLICT OF INTEREST
In the last 36 months, Raymond B. Mailhot Vega and Julie A. Bradley were awarded unrestricted educational grants from IBA.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




