Novel variants in FERMT3 and RASGRP2—Genetic linkage in Glanzmann-like bleeding disorders
Funding information:
Günter Landbeck Excellence Award
Abstract
Defects of platelet intracellular signaling can result in severe platelet dysfunction. Several mutations in each of the linked genes FERMT3 and RASGRP2 on chromosome 11 causing a Glanzmann-like bleeding phenotype have been identified so far. We report on novel variants in two unrelated pediatric patients with severe bleeding diathesis—one with leukocyte adhesion deficiency type III due to a homozygous frameshift in FERMT3 and the other with homozygous variants in both, FERMT3 and RASGRP2. We focus on the challenging genetic and functional variant assessment and aim to accentuate the risk of obtaining misleading results due to the phenomenon of genetic linkage.
Abbreviations
-
- CalDAG-GEFI
-
- calcium and diacylglycerol-regulated guanine exchange factor I
-
- GT
-
- Glanzmann thrombasthenia
-
- LAD
-
- leukocyte adhesion deficiency
-
- Rap1
-
- Ras-related protein 1
-
- TRAP6
-
- thrombin receptor activating peptide 6
1 INTRODUCTION
Glanzmann thrombasthenia (GT) is a rare inherited platelet disorder caused by homozygous or compound-heterozygous pathogenic variants in the ITGA2B or ITGB3 genes leading to either absent expression or dysfunction of integrin αIIbβ3 on human platelets.1 However, mutations in the signaling molecules of platelets can lead to a similar bleeding phenotype. Since 2008, 15 homozygous, mainly stop-gain pathogenic variants in FERMT3 have been identified to account for a complete abrogation of Kindlin-3 expression, a subcellular focal adhesion protein that binds to the cytoplasmic tail of integrins, and hence, physiologically increases their stability and ligand affinity especially in neutrophils and platelets.2-11 The clinical phenotype of leukocyte adhesion deficiency (LAD) type III (LAD-III), formerly also classified as LAD type 1 variant (LAD-1/v) by Kuijpers et al, is characterized by early onset of recurrent nonpussing bacterial or fungal infections, a severe bleeding diathesis similar to that of GT, and, inconstantly, osteopetrosis.12
The calcium and diacylglycerol-regulated guanine exchange factor I (CalDAG-GEFI) has been proven to be a critical molecule regulating rapid Ca2+-dependent platelet activation by stimulating the GTP load of Ras-related protein 1 (Rap1), a central molecular switch that drives integrin-mediated platelet aggregation and granule release.13 In 2014, Canault et al provided the first evidence that a homozygous pathogenic variant in the CalDAG-GEFI encoding RASGRP2 gene caused a severe mucocutaneous bleeding disorder with impaired platelet, but normal neutrophil function in three affected siblings.14 Until now, 20 homozygous or compound-heterozygous pathogenic variants in RASGRP2 have been described.15, 16 In this report, we demonstrate that novel variants in FERMT3 and RASGRP2, both on chromosome 11q13.1, may mislead variant assessment due to genetic linkage in patients supposed to have LAD-III or platelet-type bleeding disorder 18.
2 RESULTS
2.1 Clinical symptoms and phenotype
Index patient 1.II.5 is the fifth child of asymptomatic Libyan parents who reported not to be consanguineous (Figure 1A). The eldest brother died at the age of 7 days due to neonatal sepsis, whereas the younger three siblings are healthy. The 4-year-old female patient with failure to thrive had a marked history of frequent mucocutaneous bleeding tendency, predominantly epistaxis, gum bleedings, and hematomas. After surviving neonatal sepsis, she suffered from recurrent nonpussing bacterial infections and chronic anemia, aggravated by repetitive acute blood loss, which regularly demanded antibiotic treatment and red blood cell transfusions. Severe bleeding episodes could hardly be overcome by tranexamic acid or desmopressin, but always terminated after platelet transfusion. She successfully underwent matched unrelated-donor hematopoietic stem cell transplantation at the age of 9 years following myeloablative conditioning with thiotepa (8 mg/kg), treosulfan (42 g/m2), fludarabine (150 mg/m2), and antithymocyte globulin (60 mg/kg). Prophylaxis of graft-versus-host disease was provided with methotrexate and cyclosporine A. Neutrophil engraftment was recorded at day 18 leading to discharge of the patient at day 34. Under complete donor chimerism, neither bacterial infections nor bleeding episodes occurred, and spleen size declined significantly.
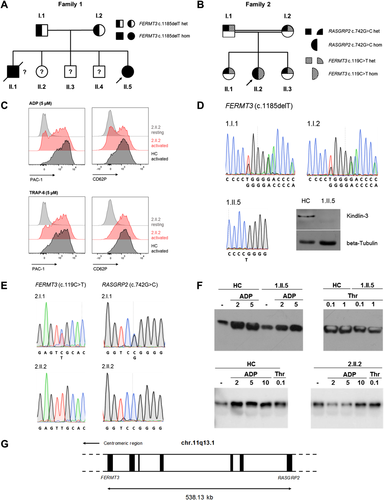
The 15-year-old female index patient 2.II.2 is the second child of asymptomatic, consanguineous parents (first-degree cousins) originating from Turkey (Figure 1B). Since early childhood, she suffered from a pronounced mucocutaneous bleeding tendency, in particular epistaxis, gum bleedings, hematomas, and prolonged menstrual hemorrhage. An adenoidectomy and a surgical abscess incision were complicated by diffuse postinterventional bleeding. The patient repetitively needs oral or even intravenous iron substitution due to secondary iron deficiency anemia. Perimenstrual treatment with tranexamic acid or desmopressin did not lead to significant improvement of the bleeding tendency. Recommended prophylaxis with an oral contraceptive to reduce menstrual bleeding episodes has been refused by her parents so far. The elder sister, 2.II.1, reported prolonged menstrual bleedings without additional bleeding symptoms.
2.2 Laboratory findings and functional assays
Accessible main laboratory findings of the investigated index patients and family members are summarized in Table 1. In all the investigated individuals, the platelet count and mean platelet volume were normal. The whole blood count of patient 1.II.5 showed marked leukocytosis with an elevated neutrophil count. PFA-100® (platelet function analyzer) closure times and light transmission aggregometry were significantly pathologic in both index patients and, to a milder extent, in other members of family 2, while von Willebrand and plasmatic coagulation factors were normal. Anemia was consistent with blood loss and secondary iron deficiency. Slightly reduced factor XI activity was recorded for two individuals.
| Family 1 | Family 2 | |||||
|---|---|---|---|---|---|---|
| Parametersa | Normal range | Patient (1.II.5) | Patient (2.II.2) | Sister (2.II.1) | Sister (2.II.3) | Mother (2.I.2) |
| Whole blood count | ||||||
| Leukocyte count (nL−1) | 4.5-13.0 | 23.39 | 8.64 | 7.76 | 8.72 | 8.45 |
| Neutrophils (%) | 36.0-72.0 | 66.0 | 61.9 | 66.1 | 52.9 | 70.0 |
| Hemoglobin (g/dL) | 12.0-16.0 | 8.2 | 9.6 | 11.5 | 12.6 | 12.2 |
| MCV (fL) | 78.0-95.0 | 78.0 | 72.6 | 69.3 | 75.7 | 84.2 |
| Platelet count (nL−1) | 150-450 | 189 | 272 | 223 | 214 | 190 |
| MPV (fL) | 7.0-11.0 | 11.0 | 9.1 | 8.4 | 7.9 | 9.0 |
| Coagulation factor activities | ||||||
| Factor XI (%) | 70-120 | 75 | 62 | n. d. | 61 | n. d. |
| vWF antigen (%) | 60-200 | 61 | 109 | 187 | 135 | 170 |
| vWF activity (%) | 50-180 | 82 | 131 | 158 | 143 | 154 |
| PFA-100® closure times | ||||||
| Col/Epi (s) | 84-160 | >300 | >300 | 257 | 119 | 189 |
| Col/ADP (s) | 68-121 | >300 | 231 | 116 | 71 | 94 |
| Born aggregometryb | ||||||
| ADP 4 µmol/L | Normal | n. a. | ↓↓ | ↓↓ | ↓ | ↓↓ |
| ADP 20 µmol/L | Normal | n. a. | ↓ | ↓ | n. d. | ↓ |
| Epinephrine 8 µmol/L | Normal | n. a. | ↓↓ | ↓↓ | ↓↓ | ↓↓ |
| Epinephrine 40 µmol/L | Normal | n. a. | ↓↓ | ↓↓ | n. d. | ↓↓ |
| Collagen 1 µg/mL | Normal | n. a. | ↓ | Normal | ↓ | Normal |
| Collagen 2 µg/mL | Normal | n. a. | Normal | n. d. | Normal | n. d. |
| Ristocetin 1.0 mg/mL | Normal | n. a. | ↓↓ | ↓↓ | ↓↓ | ↓↓ |
| Ristocetin 1.5 mg/mL | Normal | n. a. | Normal | Normal | Normal | Normal |
| Flow cytometry | ||||||
| CD41a expression | Normal | Normal | Normal | n. d. | n. d. | n. d. |
| CD61 expression | Normal | Normal | Normal | n. d. | n. d. | n. d. |
- Abbreviations: ADP, adenosine diphosphate; Col/ADP, collagen/ADP cartridge; Col/Epi, collagen/epinephrine cartridge; MCV, mean corpuscular volume; MPV, mean platelet volume; n. a., not available; n. d., not determined; PFA, platelet function analyzer; vWF, von Willebrand factor.
- a Results outside the reference range are expressed in bold numbers or symbols.
- b Results from at least two independent aggregation analyses are depicted. Aggregation curve assessment: normal (maximal aggregation >80%, aggregation >20% before 6 min, disaggregation <20%), ↓ = intermediate (maximal aggregation 60-80%), ↓↓ = pathologic (maximal aggregation <60%, aggregation <20% after 6 min and/or disaggregation ≥20%).
For both index patients, flow cytometric analysis of platelets proved normal expression of integrins αIIb (CD41a) and β3 (CD61), which form the fibrinogen receptor (Table 1), of the von Willebrand receptor GPIb/V/IX, and of the other investigated platelet receptors (data not shown).17 However, conformational change, and thus, activation of αIIbβ3 detected by PAC-1 antibody binding was diminished in both index patients upon low- (2 µM) and optimal-dose (5 µM) adenosine diphosphate (ADP) as well as thrombin receptor activating peptide 6 (TRAP6) treatment, though to a lesser extent (Figure 1C; data not shown). In contrast, P-selectin (CD62P) neo-expression upon TRAP6 was not impaired in patient 1.II.5 (data not shown), whereas CD62P neo-expression upon ADP treatment was clearly, and upon TRAP6 treatment, slightly reduced in patient 2.II.2 (Figure 1C).
2.3 Genetic analysis and variant assessment
Since the clinical phenotype and the functional assays suggested Kindlin-3 deficiency in patient 1.II.5, we sequenced the coding region of FERMT3 (NM_178443.2) and found a novel homozygous variant, c.1185delT (p.D397Tfs*29), leading to a premature stop codon and intracellular lack of the truncated protein (Figure 1D). Both parents 1.II.1 and 1.II.2 were heterozygous for the detected variant (Figures 1A and 1D). No variant was discovered in RASGRP2 (NM_153819.1) (data not shown).
In patient 2.II.2, we identified two homozygous variants in FERMT3 and RASGRP2 by targeted next generation sequencing, as previously described.18 Both variants were confirmed by Sanger sequencing (Figure 1E). The novel missense variant c.119C > T (p.S40L) in FERMT3, classified as a variant of unknown significance after applying in silico prediction tools (MutationTaster2: polymorphism; sorting intolerant from tolerant [SIFT]: tolerated; Polyphen-2: benign), cosegregated with the clinical phenotype in family 2 (Figures 1B and 1E). The variant c.742G > C (p.G248R) in RASGRP2 is a novel variant affecting the same amino acid position as that in a previously reported distinct missense variant (p.G248W).14 After applying in silico prediction tools (MutationTaster2: disease causing; SIFT: deleterious; Polyphen-2: probably damaging) and proving cosegregation in family 2, we classified this variant as likely pathogenic (Figures 1B and 1E).
We then verified the functional impact of the detected variants on intracellular platelet signaling in the two index patients by Rap1 pull-down assays, as previously reported.13 Normal Rap1 activation upon ADP and thrombin treatment confirmed functional signaling in patient 1.II.5. In contrast, ADP treatment showed absent Rap1 activation in patient 2.II.2, and hence, proved specifically impaired CalDAG-GEFI-mediated signaling (Figure 1F).13 With these results, we considered the RASGRP2 variant as causative in 2.II.2.
3 DISCUSSION
In this study, we describe a novel single nucleotide deletion variant in FERMT3 in a 4-year-old female with LAD-III, and detected a novel likely pathogenic variant in RASGRP2 in a female adolescent with a platelet-type bleeding disorder 18. We especially aim to underline the necessity for comprehensive diagnostic exploration of patients with similar severe bleeding diathesis, but distinct alteration of intracellular signaling, and to emphasize the pitfall of misleading variant interpretation due to chromosomal linkage of the affected genes (Figure 1G). In 2007, several partly conflicting reports on the role of CalDAG-GEFI and Rap1 in impaired integrin activation opened a research field for these signaling molecules.12, 13, 19 Likely due to the linkage disequilibrium, identification of the defective intracellular signaling molecule in LAD-III was controversially discussed. It remains unevaluated whether individual 2.II.1 in family 2 shows mild bleeding symptoms due to two heterozygous hits in the signaling pathway.
Although LAD-III can be differentiated from RASGRP2-associated platelet-type bleeding disorder 18 to a certain degree by marked leukocytosis and profoundly impaired neutrophil function, a clear distinction may not always be possible in daily clinical routine due to overlapping clinical phenotypes or restricted access to comprehensive functional platelet assays. Since current guidelines suggest high-throughput sequencing techniques early in the diagnostic algorithm for inherited platelet function disorders,20 we recommend careful assessment of the closely linked genes FERMT3 and RASGRP2 in context with a Glanzmann-like bleeding disorder.
ACKNOWLEDGMENTS
The authors would like to thank Silke Schwiebert (Laboratory for Pediatric Molecular Biology, Charité-University Hospital Berlin, Berlin, Germany) for excellent technical assistance in sequencing and data analysis. This work has been funded in part by the Günter Landbeck Excellence Award (to Harald Schulze).
CONFLICT OF INTEREST
The authors report no conflict of interest.




