Stage III cystic partially differentiated nephroblastoma recurring after nephrectomy and chemotherapy
Abstract
Cystic partially differentiated nephroblastoma (CPDN) has low malignant potential. We report a 1-year-old with stage III CPDN of the right kidney that recurred following radical nephrectomy and chemotherapy. There was evidence of tumor spillage pre-operatively and intra-operatively. During chemotherapy the disease recurred in the omentum and the peritoneum. Pathology of the recurrent resected cysts revealed a more differentiated biphasic tumor without blastemal elements. It appears that spillage of CPDN in our patient led to dissemination of disease. Chemotherapy failed to prevent recurrence but only mature elements were present following this treatment. The intensity of therapy required to treat CPDN remains undefined. Pediatr Blood Cancer 2008;50:129–131. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Cystic partially differentiated nephroblastoma (CPDN) is a rare variant of Wilms tumor considered to have unique pathology and clinical behavior 1,2. Pathologically, CPDN is distinct from Wilms tumor in that the tumor is entirely cystic with no solid component. The thin septa typically house blastema, with or without embryonal stromal or epithelial elements. Clinically, CPDN presents similarly to Wilms tumor, but it has been described as a relatively benign tumor with low malignant potential 2-4.
Evidence suggests that stage I CPDN may be treated with nephrectomy alone, while chemotherapy is reserved for higher stage disease 2-8. Stages II–V disease is uncommon, therefore, there is less experience with these patients, and little is known about the best way to treat them. In this report, we describe the case of a 1-year-old diagnosed with stage III CPDN having recurrent disease after total nephrectomy and chemotherapy.
CASE REPORT
A 1-year-old child was seen at a local hospital with a 1-month history of abdominal distension, rapidly increasing over 3 days. Further history revealed mild motor delay and difficulty standing. The patient was irritable, constipated and had a decreased appetite.
On presentation, the child appeared well with a heart rate and respiratory rate of 120 and 36, respectively, shallow respirations with no distress, and a blood pressure of 124/60. Abdominal examination revealed a distended abdomen with right-sided prominence. A large, firm mass was palpable extending from the right side across the abdomen. Neurological assessment was within normal limits. The child sat well but was unable to crawl or stand. The patient had no dysmorphic features, hemi hypertrophy, or aniridia.
Initial investigations included hemoglobin of 105 g/L, with an MCV of 74.4 fL and a normal hemoglobin electrophoresis. An abdominal ultrasound and CT scan showed a mass arising from the right kidney. Management of hypertension was instituted prior to transfer to our center. Pre-operative imaging characterized the tumor as a large, right renal multi-loculated cystic mass measuring 19 cm × 13 cm × 12 cm, crossing the midline and having mass effect on the IVC and aorta. The left kidney was normal. Imaging revealed no signs of metastatic disease.
The patient underwent a right radical nephrectomy with excision of multiple para-aortic and para-caval lymph nodes. The tumor consisted of large, thin-walled, urine-filled cysts (Fig. 1). Several of the cysts had ruptured pre-operatively and further intra-operative rupture occurred while removing the tumor. The mass was adherent to the abdominal wall and liver, however, quick section pathology ruled out invasion of these structures. Pathology of the cystic mass was consistent with CPDN. The tumor was entirely cystic, tissue reminiscent of triphasic Wilms tumor within the septa. The cystic spaces were lined by hobnail epithelium with subepithelial nodules, cambium-like layers of blastema and stroma and papillary projections by nodules of blastema into the cysts (Fig. 2A). The tumor showed focal positivity when stained with antisera to WT1. All of the resected nodes were negative for malignancy.
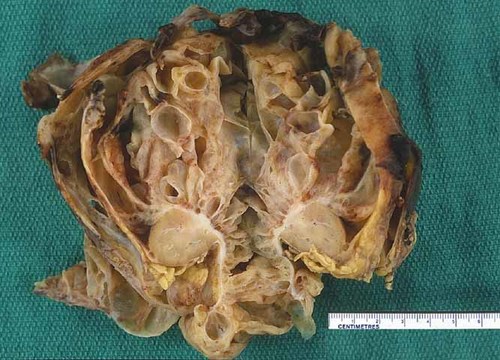
The tumor measures 12.5 cm × 12 cm × 5.5 cm and weighs 421.9 g. The cut surface reveals multiple fluid-filled, smooth-lined cystic structures. The septa are thickened but there is no expansile solid tumor. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
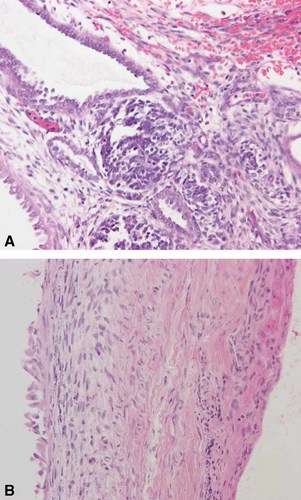
This H&E stain of the initial tumor demonstrates cystic structures. The cysts are lined by “hobnail” epithelium, subepithelial nodules and cambium-like layers of blastema and stroma as seen in triphasic Wilms tumor (A). This H&E stain of the recurrent tumor demonstrates flattened epithelium and loss of blastemal elements (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The patient's post-operative course was uneventful with the exception of intermittent hypertension. The patient was started on chemotherapy using NWTSG regimen EE-4A consisting of dactinomycin (0.045 mg/kg/dose IV push) once every 3 weeks and weekly vincristine (0.05 mg/kg/dose IV push). The patient was not treated with radiation. While on chemotherapy the patient had intermittent viral illnesses and fevers without neutropenia, requiring two dose reductions of actinomycin but no hospital admissions. After 6 weeks of treatment, a follow-up U/S showed a small 7 × 7 mm cyst along the anterior abdominal wall in the region of the falciform ligament, thought to be on the anterior peritoneal lining. This lesion was followed radiologically over the subsequent 8 weeks and noted to be increasing in size. Ultrasound was able to identify the cyst that was not initially seen on CT scan until it measured 9 × 14 mm and additional cystic lesions were seen. The patient remained on chemotherapy until week 14 of the protocol, when a second surgery was planned.
At the time of second laparotomy, multiple small cysts were removed and complete gross removal was achieved. Three of the cysts were in the peritoneum, right lower quadrant, and the omentum, respectively, with none arising from the renal bed itself. The pathology of three of the cysts showed flattened epithelial lining and stroma identical to that of the original tumor; however, these recurrent cysts had the appearance of differentiated Wilms tumor with no blastema seen (Fig. 2B). Two additional cysts were removed that were negative for malignancy.
As these recurrent lesions had arisen while the patient was being treated with chemotherapy, no further chemotherapy was given post-operatively. The patient is well and free of disease 27 months after the second surgery. Regular follow-up ultrasounds were done to monitor for recurrent disease every 3 months for the first 2 years, and every 6 months thereafter.
DISCUSSION
CPDN is a rare variant of Wilms tumor, comprising less than 1% of all nephroblastomas. CPDN is to be distinguished from cystic Wilms that includes a solid component and cystic nephroma (CN) which is similar to CPDN in gross appearance but contains only differentiated tissues but without embryonal elements 1,2.
The revised SIOP working classification of renal tumors of childhood classifies CPDN as a low risk tumor, not to be treated with post-operative chemotherapy 4. Our case is unique in that our patient had recurrent disease despite treatment with chemotherapy. A series published by the NWSTG reports 100% survival and no recurrence in 21 cases of CPDN 3. Eight patients were treated with nephrectomy alone, and thirteen were given adjuvant chemotherapy (NWTSG protocol EE-4A or DD-4A). There were two patients with stage II disease (with spillage) and one with stage V disease, all treated with adjuvant chemotherapy. All of the patients in this report survived and none had recurrence of their disease 3. The current therapy for CPDN is undefined; however, if our patient had been considered a stage III Wilms tumor, doxorubicin and whole abdominal radiation would have been added to the treatment of this patient. We chose to use a minimal treatment approach based on discussion with colleagues and reports of favorable outcome of patients with CPDN compared to children with standard Wilms tumor 3.
In a pathological review of CPDN 2, 18 cases were reviewed including 7 patients with stage II disease, and none with stages III–V. In this subgroup, five were treated with nephrectomy alone, and two were treated with chemotherapy. At publication, they were either well or lost to follow-up with the exception of one recurrence. This patient had two local recurrences at 1 and 2 years after surgery and was, at least initially, treated with nephrectomy alone. No further information is given about this case which is the only report of CPDN recurrence to date, albeit in a case treated with nephrectomy alone. These authors caution that the presence of poorly differentiated tissues and blastemal cells suggests a degree of malignant potential exists 2.
Given the rarity of CPDN, we conducted a search of the hematology-oncology database for other cases seen at the Hospital for Sick Children from 1985 to present. In addition to our case, only two other cases of CPDN were diagnosed at our institution and both had stage I disease. These patients were treated with nephrectomy alone and are well with no recurrent disease 8 and 12 years after surgery.
Given the friable nature of CPDN, it is surprising that the number of reports of local contamination is rare. Our case demonstrates the risk of local contamination and emphasizes the need for careful surgical extirpation of the tumor. We do not know whether the presence of implanted lesions suggests our patient's tumor was a more aggressive variant. The infrequent reporting of CPDN in the literature makes it very difficult to conclude whether or not the outcome was altered by our treatment approach. However, our case provides additional experience in the treatment of a rare tumor.




