Congenital dysgranulopoietic neutropenia
Abstract
We investigated a 15-year-old female with congenital dysgranulopoietic neutropenia (CDN) and her non-neutropenic mother who had recurrent stomatitis. In both patients, cells of the neutrophilic, eosinophilic, monocytic, megakaryocytic, and basophilic series were dysmorphic. Plasmacytoid lymphocytes and mild megaloblastic erythroid precursors were present. Bleeding times of both patients were prolonged. The mother had a secondary aggregation defect; the number of the plasmacytoid lymphocytes, dense granules of platelets, and dysmorphic neutrophils, neutrophil chemotaxis, and myeloperoxidase content fluctuated according to the presence or not of aphthae. The daughter's karyotype revealed 46,XX/46,XX, t(1;8). No ELA2 or G-CSFR mutation was detected. These findings support stem cell involvement in CDN. Pediatr Blood Cancer 2008;50:115–119. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Congenital dysgranulopoietic neutropenia (CDN) is characterized predominantly by ineffective myelopoiesis and morphological abnormalities in the neutrophilic series. Nine cases have been reported 1-3. Either no or little change was observed in the non-neutrophilic series in CDN 1-3. In this report, we present a female patient with CDN, who displayed morphological changes in all non-neutrophilic cell lineages. Interestingly, her non-neutropenic mother who had recurrent aphthous ulcers also displayed some morphological and functional hematologic abnormalities.
PATIENTS AND METHODS
The Daughter
She was referred to our clinic at 4½ years of age with a four-year history of recurrent infections. Her parents were consanguineous. No family member had neutropenia, but the mother had suffered from recurrent aphthous stomatitis. Her physical examination was normal. White blood cell count (WBC) was 3.5 × 109/L, neutrophil count 0.16 × 109/L, and monocyte count 1.6 × 109/L; hemoglobin (Hb), mean corpuscular volume (MCV), platelet count, and HbF were normal. The neutropenia was not cyclic. Bone marrow aspirates revealed a promyelocytic arrest. Cold agglutinins, i antigen, and diepoxybutane tests were negative. Immunoglobulins G, M, E, and A were normal. Her IgA level proved low at 12 years of age [45 mg/dl (N: 67–433)]. Blood cells were dysmorphic and this was attributed to infections and granulocyte colony-stimulating factor (G-CSF) therapy 4,5.
She was diagnosed with chronic idiopathic neutropenia; after detecting similar dysmorphism in the mother's peripheral blood cells, she was considered to have CDN. During infections, she was treated with G-CSF. She developed gingival enlargement at 7 years of age 6. The laboratory tests were performed following a G-CSF therapy-free period of a minimum of 5 days.
The Mother
The 43-year-old mother was a product of a non-consanguineous marriage; she had five healthy siblings. She had experienced recurrent aphthae for 20 years. She had no other mucosal lesions, chronic disorders, susceptibility to infection or drug addiction. Her physical examination revealed only oral aphthae.
Her Hb, MCV, and platelet count were normal. Regular WBC follow-up did not reveal neutropenia. Her WBC and leukocyte differential count were normal during both aph-positive (A+) and aph-negative (A−) periods. Her bone marrow taken in A+ periods revealed increased mature neutrophils, yielding an M/E ratio of 4.1/1. Serum iron, ferritin, vitamin B12, folic acid, HbF, and IgG, A, M levels were normal. Skin pathergy tests performed to exclude Behçet's disease, antibodies to human immunodeficiency virus types 1 and 2, i antigen and cold agglutinins were negative. During these investigations she did not have any infections and had not taken any antihistaminics, anti-inflammatory agents, or antibiotics for 2 weeks.
Light Microscopic Examination
Hematologic dysmorphism was evaluated and scored as described previously 4,7. Lymphocytes were evaluated as to the presence of plasmacytoid cells, cytoplasmic granules, or vacuoles. Since the daughter used G-CSF frequently, we did not score her neutrophilic series 4,5.
Cytogenetic Analysis
Metaphase chromosome preparations were obtained from phytohemagglutinin (PHA)-stimulated lymphocyte cultures according to standard procedures.
Neutrophil Elastase (ELA2) Mutation Analysis
After DNA extraction 8, five fragments of DNA covering all five exons, the promoter region of ELA2 and at least 15 bases of the flanking regions were amplified by polymerase chain reaction (PCR) and sequenced.
G-CSF Receptor (G-CSFR) Mutation Analysis
A fragment encompassing the nucleotides 2,384–2,429 (Accession M59818) were amplified by PCR and sequenced after subcloning.
Other Laboratory Evaluations
Standard techniques were performed for transmission electron microscopic [(TEM) 906E] observation of the blood cells, staining of dense bodies of platelets with mepacrine 9, neutrophil chemotaxis of the mother 10, platelet aggregation tests 9, PFA-100 in vitro bleeding time determination 11 and myeloperoxidase (MPO) content 12. Two healthy volunteers served as controls.
RESULTS
Light Microscopic Findings
Morphological changes were detected in all cell lineages. Erythroid precursors displayed mild megaloblastic changes in both mother and daughter. The mother's neutrophilic cells were larger and more dysmorphic than controls (Fig. 1, Table I). Some neutrophilic abnormalities, present in the A+ period, decreased in the A− period (Table I). In the end of the A+ period, hypogranulation/agranulation and macropolycyte percentage were at their maximum level. The daughter's neutrophilic cells displayed most of these abnormalities.
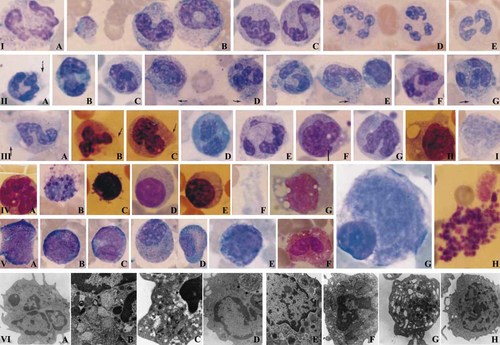
Dysmorphic hematologic findings from the mother's peripheral blood (A+ and A− periods) and bone marrow (A+ period) and from the daughter by light (1,000×) and transmission electron microscopy. Light microscopy: Macropolycytes (neutrophil with a diameter >14 µm 4) (IA–E; IID–G; IIIA,B,E), bizarre nuclei (IA–C, IIF), hypogranulation or agranulation (ID,E; IIIA), pseudo Pelger-Huet (PPH) or PPH-like cells (IE; IIE; IIIC,D), striking chromatin clumping (IA–D; IID,G; IIIA), cytoplasmic vacuoles (IB, IIG), irregular distribution of granules (IIA,C,D,F,G; IIIA,B), long chromatin (ID, IIIE), cytoplasmic protrusion with or without granules (IIA,D,E,G; IIIA–C with arrows), and cytoplasmic condensation (IIC,F). Neutrophil precursors with abnormal nuclei, irregular granule distribution and slight chromatin clumping (VA–C,E). Mature eosinophils: Irregular distribution of granules and cytoplasmic vacuoles (VF). Eosinophilic myelocytes with both eosinophilic and basophilic granules with slight cytoplasmic protrusion and striking chromatin clumping (VD). A basophil with reduced granules and increased cytoplasmic vacuoles (IVB). Plasmacytoid lymphocytes with or without vacuoles (IVD,E). Lymphocyte with cytoplasmic protrusions (IVC). Lymphocyte with cytoplasmic vacuoles (IIE). Monocytes: Dysmorphic nuclei with or without vacuoles (IIIF–I; IVA,G), cytoplasmic protrusions (IIIF,H; IVG). Macroplatelets (IB, IVF, VH). Mononuclear megakaryocytes (VG). Transmission electron microscopy: Reduced number of primary and secondary granules and condensed chromatin clumping in one nuclear lobe, from mother's peripheral blood (A+ period) (12,930×) (VIA). Giant granules with heterogeneous electron density, one being abnormally lucent (arrows), intragranular inclusion-like myeline figures and condensed chromatin clumping, from mother's peripheral blood (A+ period) (21,560×) (VIB). Primary and secondary granules of abnormal shape and irregular size, some of which included light, granular material with reduced electron density (arrows) and condensed chromatin clumping, from mother's peripheral blood (A+ period) (27,800×) (VIC). Reduced number of primary and secondary granules and an autophagic vacuole containing cellular material (arrows) from the daughter's peripheral blood (12,930×) (hypogranularity and neutrophils were few in the daughter's peripheral blood) (VID). An eosinophil from the daughter's peripheral blood. Granules with non-uniform size and their contents with heterogeneous electron density and intragranular atypical inclusions (12,930×) (VIE). A lymphocyte from the mother's peripheral blood (A+ period). Enlarged, active and condensed mitochondria with tubular cristae which were increased in number (16,700×) (VIF). A platelet with widely dilated open canalicular system, from the mother's peripheral blood (A+ period) (21,560×) (VIG). A monocyte with increased rough endoplasmic reticulum, from the mother's peripheral blood in A+ period (12,930×) (VIH). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Bone marrow neutrophilic series (Aph+) | Peripheral blood neutrophils | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Promyelocyte (mother) | Promyelocyte (control) | Myelocyte (mother) | Myelocyte (control) | Metamyelocyte (mother) | Metamyelocyte (control) | Band neutrophil (mother) | Band neutrophil (control) | Neutrophil (mother) | Neutrophil (control) | Mother Aph (+) | Mother Aph (−) | Control | |
| Diameter (µm) | 17.98 (13–29) | 17.8 (13–25) | 15.9 (11–21) | 14.88 (12–18) | 14.12 (12–18) | 13.1 (10–16) | 15.26 (12–18) | 11.63 (7–18) | 13.24 (10–18) | 10.58 (7–14) | 14.9 (14.4–15.4) | 13.27 (12.9–13.7) | 9.55 (9.2;9.8) |
| Macropolycytea (%) | 55 | 0 | 66 | 33 | 8.5 | ||||||||
| Bizarre nucleus (%) | 0 | 0 | 3 | 0 | 0 | 0 | 22 | 0 | 32 | 4 | 63.6 | 60.3 | 14 |
| Hypogranulation or agranulation (%) | 54 | 14 | 64 | 8 | 72 | 13.2 | 72 | 23.1 | 63 | 26 | 61.5 | 22.3 | 18 |
| Pseudo Pelger-Huet (PPH) and PPH-like cell | 17 | 7 | 22.6 | 29.9 | 4.5 | ||||||||
| Striking chromatin clumping (%) | 4 | 6 | 43 | 36 | 92 | 59.4 | 100 | 62.7 | 82 | 56 | 92 | 93.3 | 13 |
| Cytoplasmic vacuole (%) | 6 | 8 | 14 | 13 | 16 | 9.9 | 8 | 13.2 | 5 | 4 | 7.3 | 11.3 | 4.5 |
| Irregular distribution of granules (%) | 46 | 26 | 37 | 36 | 50 | 36.3 | 66 | 9.9 | 72 | 6 | 37 | 47 | 7.5 |
| Long chromatin strands between the nuclear lobes (%) | 8 | 3 | 15 | 8.3 | 1.5 | ||||||||
| Cytoplasmic protrusion with/without granules (%) | 2 | 1 | 6 | 2 | 0 | 8 | 0 | 8 | 0 | 20.6 | 31 | 9 | |
| Chemotaxis | Defective | Normal | Normal | ||||||||||
| MPO (%) | 38.4 | 98 | 95 | ||||||||||
- * The amount of dysplastic neutrophilic promyelocytes, myelocytes, metamyelocytes, band neutrophils, and neutrophils and the diameter of each neutrophil were expressed as a percentage of 50, 100, or 200 cells.
- a Macropolycyte: A neutrophil with a diameter >14 µm 4.
The eosinophilic series displayed both basophilic and eosinophilic granules, cytoplasmic vacuoles and irregular distribution of the cytoplasmic granules, large granules, and chromatin clumping (Fig. 1, VD), the latter increasing during maturation. Both patients' basophils also displayed similar findings (Fig. 1, IVB). Plasmacytoid lymphocytes were encountered at rates of 50.4% (mother, A+), 38.5% (mother, A−), and 29.4% (daughter) in the peripheral blood (control: 2.5%) and rates of 29% (mother, A+) and 6% (daughter) in the bone marrow (control: 4%). Some had cytoplasmic vacuoles and protrusions (Fig. 1, IIE; IVC–E). Both patients had dysmorphic megakaryocytes and macroplatelets of varying shape (Fig. 1, IB; IVF; VG,H). The monocytes displayed cytoplasmic vacuoles at rates of 34% (mother, A+), 29% (mother, A−), and 8% (daughter) in peripheral blood (control: 6%) and rates of 36% (mother, A+) and 24% (daughter) in the bone marrow (control: 10%). Cytoplasmic monocytic protrusions were encountered at rates of 23% (mother, A+), 25% (mother, A−), and 13% (daughter) in the peripheral blood (control: 0%), and at rates of 4% (mother, A+) and 8% (daughter) in the bone marrow (control: 2%; Fig. 1 IIIF–I; IVA,G).
Electron Microscopic Findings
Chromatin clumping (Fig. 1, VIA,B,C) was striking. Primary and secondary granules were reduced in number (Fig. 1, VIA,D) and showed heterogeneity in size, shape, and electron density (Fig. 1, VIB,C). The daughter's neutrophils and eosinophils showed autophagosomes (Fig. 1, VID) and large granules (Fig. 1, VIE), respectively. Enlarged and increased numbers of mitochondria in lymphocytes (Fig. 1, VIF), increased and enlarged rough endoplasmic reticulum (RER) cisternae in monocytes (Fig. 1, VIH) and widely dilated open canalicular system in platelets (Fig. 1, VIG) of the mother were observed.
Further Evaluation of the Neutrophils and Platelets
The mother's MPO, which was low, and neutrophil chemotaxis, which was abnormal, in the A+ period were normal in the A− period (Table I). MPO of the daughter was normal.
In vitro bleeding times were 218 (mother, A+), 168 (mother, A−), 162 (daughter) sec with collagen-epinephrine (N < 150); and 158 (mother, A+), 138 (mother, A−), and 130 (daughter) sec with collagen-adenosine 5′-diphosphate (ADP) (N < 114). The numbers of dense granules per platelet were 0.04 (mother, A+), 4.4 (mother, A−), and 3.8 (daughter) (control: 3.6). Platelet aggregation tests of the daughter with collagen, ristocetin, epinephrine, and ADP were normal. Those of the mother revealed an absent secondary wave with ADP (2 µM) in both A+ and A− periods. ADP aggregation test done with 6 µM ADP in the A+ period was normal.
Chromosomal Analysis
Whereas the mother's cytogenetic analysis revealed 46 XX, the daughter's karyotype from lymphocytes revealed 46, XX [83%]/46, XX, t(1;8) (p22; q13) [17%].
ELA2 Mutation and G-CSFR Mutation Analysis
No mutation in the gene for ELA2 nor in the region of the G-CSFR gene, where point mutations that lead to a premature stop codon are most frequently detected, could be determined in the DNA of the daughter.
DISCUSSION
The findings of the mother and the daughter showed partial and complete consistency with CDN, respectively. We believe that larger size, hyperdiploidy [unpublished data] and the segmentation defects of the nuclei in the neutrophilic lineage of the mother may be due to defects involving centrioles and microtubuli as reported in CDN 2. Hypogranularity may be due to granule lysis through autophagia, and hypogranular/agranular cells of the daughter may be destroyed in the bone marrow during promyelocytic arrest. Rare hypergranularity has also been reported in CDN 2. The qualitative abnormalities in the neutrophilic lineage of the mother started at the promyelocyte stage, although no maturation arrest was evident. Our observations indicate that morphological abnormalities in non-neutrophilic series can also be observed in CDN. No studies of lymphocytes, basophils, and platelets were done in any of the previous studies 1-3.
Evaluation of the mother's platelets revealed a coexistent storage pool disease. The daughter only had prolonged bleeding time. It is striking that neither the mother nor the daughter had bleeding symptoms, like other reported cases with platelet aggregation defects 9. The widely dilated open canalicular system of the platelets of the mother were probably non-specific 13. The abundance of plasmacytoid lymphocytes especially in PB may be due to B cell activation.
Haurie et al. 14 reported oscillations in the number of hematopoietic stem cells, platelets, monocytes, and lymphocytes in chronic neutropenia. Strikingly, chemotaxis, MPO level and some of the morphologic features of the mother's cells also fluctuated, in conjunction with the presence or not of aphthae. Our cases may have a stem cell defect which manifested as qualitative and quantitative abnormalities in the mother and the daughter, respectively. Variability in the mother's findings may reflect variable expression of a common defect by different clones, like in Pearson syndrome 15.
There are two cases reported in the literature of CDN from another family, both of whom displayed neutropenia 3. In our report, the mother displayed characteristics of CDN with some additional findings but not neutropenia. The mother may be heterozygous for CDN and cytopenias, as in a report of a patient with Fanconi anemia 16. We conclude that chronic familial neutropenia may be a variant of CDN and that family members other than the initially identified patient should be examined for recurrent aphthae.
Acknowledgements
We are grateful to Prof Şinasi Özsoylu, in reviewing the manuscript; Hüseyin Solmaz for his technical assistance in preparation of ultrastructural samples; and Alp Olcay, M.D., for his valuable help in providing related literature.




