Synthesis and Properties of a Shape Memory–Assisted Self-Healing Supramolecular Polyurethane Coating Based on Quadrupole Hydrogen Bonding
ABSTRACT
In this research, the synthesis and characterization of a shape memory–assisted self-healing polyurethane (SHSMPU) coating based on strong quadrupole hydrogen bonding interaction have been investigated. The coating was fabricated with an acrylic polyol, an isocyanate functionalized ureidopyrimidinone (UPy-NCO) as a hydrogen bonding provider, and methylene diphenyl diisocyanate (MDI) as chain extender. Fourier-transform infrared spectroscopy (FT-IR) confirmed that by incorporating the UPy-NCO dimer in the polyurethane (PU) structure, strong hydrogen bonding appears, which can provide the healing function of the polymer. In addition, differential scanning calorimetry (DSC) showed that by increasing the UPy-NCO amount, the overall Tg decreased and the material becomes more flexible. A rheometric investigation revealed that a sample containing 45% of UPy-NCO as total diisocyanate shows good shape recovery and an healing efficiency of macroscale cracks of around 94.2%, which is much higher than the corresponding sample without UPy-NCO (50.4%). Even better performances were obtained with microcracks, where tested samples were significantly healed at around 80°C in 10 min. Furthermore, the introduction of polyethylene glycol 400 (PEG400) into the polymer's backbone led to a reduced Tg and thus higher flexibility at room temperature. The combination of the observed properties makes these materials promising for application as durable and flexible polyurethane coatings.
1 Introduction
Over the past decades, due to sustainability awareness, there has been an increasing market demand for long-life materials, particularly in the field of polymers and coatings. Self-healing polymers (SHPs) are able to satisfy these demands, by decreasing the cost of maintenance and protection, also known as damage management [1, 2]. SHPs, either based on extrinsic or intrinsic mechanisms [3], are among the best candidates to make coatings more durable, especially intrinsic healing that can heal cracks several times since it does not consume chemicals [4-6]. Recently, among the numerous intrinsic self-healing approaches, thermal-reversible non-covalent interactions such as strong hydrogen bonding have been proposed for use in not only SHPs but also for shape memory polymers (SMPs) [7-10], as these two properties often come together in the material. In the systems containing reversible quadrupole hydrogen bonding as a stabilizer of mechanical strain, shape recovery is achieved upon heating, due to the dissociation of hydrogen (non-covalent) bonds [11-13]. It is important to note that both chemical and physical factors play a role in effective self-healing [14]. Apart from a few exceptions, self-healing materials are currently limited to micrometer-sized scratches, which require physical reattachment of broken portions in order to complete the healing process [15]. In line with crack size importance, Deflorian et al. [16], in their research focused on the intrinsic self-healing capability of polyurethane (PU) coating, showed some partial recovery occurred even by replacing 25% of covalent bonds to non-covalent bonds like hydrogen bonding of 2-amino-4-hydroxy-6-methylpyrimidine. This method did not result sufficient for large cracks (50 μm in diameter). In another research, Gao et al. synthesized a series of UV-curable self-healing PU containing 2-ureido-4[1H]-pyrimidinone and polycaprolactone diol (PCDL). The results revealed that by adding a proper active diluent, not only gloss, adhesion, and hardness, but also the self-healing ability and thermal stability were improved, while self-healing and better mechanical properties by the introduction of 20 wt.% of triethoxylated trimethylolpropane triacrylate were obtained, and healing efficiency at a depth of 4 μm was 95.1% [17]. Zhang et al. in their work mainly prepared adhesive composites, by utilizing a feasible method to improve the mechanical and self-healing properties, based on introducing a dual dynamic bond into hydroxyl-terminated polybutadiene (HTPB), showing an efficiency of self-healing at 25°C of 74%, in 6 h, which can improve up to 93% in 2 h at 60°C. Moreover, the elongation at break of this material was significantly higher than that of HTPB [18].
Regarding the “shape memory–assisted self-healing” concept, PUs with some other brilliant abilities such as chemical and weather resistance in the case of top-coating have gotten more attention [19]; it is suggested that the use of non-covalent interactions such as hydrogen bonds for the production of SMPs can offer substantial improvements. Moreover, researchers have shown that supramolecular structures such as pyridine-containing polyurethanes (PUPys) have great potential for use in both SHPs and SMPs [20]. However, the structure and morphology of PUPys have not been widely investigated.
The research presented here intends to evaluate and investigate the effect of pyrimidine content in the side chain and the hydrogen bonding effect on both shape memory and self-healing function of polyurethanes. According to the literature [21], self-healing through hydrogen bonding reassociation can take place via heating, light, or physical reattachment. So, by introducing the shape memory function into the PU, the two edges of cracked surfaces could come close to each other upon heating up; therefore, the network can be repaired and healed. To achieve these goals, MDI was used as a chain extender and hard domain in semi-network PU, while hexamethylene diisocyanate (HDI)-functionalized 6-methylcytosine was used to fabricate supramolecular structures based on quadrupole hydrogen bonding in SHSMPU, to reinforce the existing hydrogen bonding of normal urethane group.
2 Experimental
2.1 Material
Acrylic polyol resin 1210N as the hydroxylated component of PU was donated by Taak Resin Kaveh Chemical Co (Table 1). Hexamethylene diisocyanate (HDI) 98%, 2-amino-4-hydroxy-6-methylpyrimidine 99% (6-methylisocytosine), methylene diphenyl diisocyanate (MDI) 98%, used as the isocyanate component of PU, and dibutyltin dilaurate (DBTL) as a catalyst and polyethylene glycol Mw~400 (PEG400) as a flexibilizer were purchased from Sigma-Aldrich. Anhydrous pentane ≥ 99% used for washing, isopropyl alcohol ≥ 99% for cleaning the substrate, and chloroform (CHCl3) ≥ 99.5% as a solvent were also purchased from Sigma-Aldrich; chloroform was dried over molecular sieve 4 Å before use. All other chemicals were used as they were received.
| Appearance | Clear liquid |
|---|---|
| Molecular weight | 10000 g/mol |
| Solid content | 60 wt.% |
| Solvent | Xylene/MPA (1:1) |
| Measured Tg | 9°C |
| Hydroxyl content | 4.5% |
| Acid value | 5–10 mg KOH/g |
| Viscosity at 25°C | 2500–5000 cP |
2.2 Methods
2.2.1 Functionalization of 2-Amino-4-hydroxy-6-methylpyrimidine
An excess amount of HDI (4.75 mol) was functionalized with 2-Amino-4-hydroxy-6-methylpyrimidine (1 mol) in a three-neck flask under N2 atmosphere, in suspension at the temperature of 100°C for 16 h (Scheme 1, Scheme 2). Then, the crude mixture was washed with anhydrous pentane to remove the unreacted amount of HDI, and a solid white powder precipitated, which was isolated and dried overnight under vacuum at a temperature of 100°C. FT-IR and NMR were carried out to confirm the reaction [16, 22].
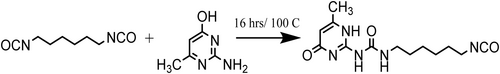
2.2.2 Supramolecular Polyurethane Film Formation
The product obtained from the previous step was introduced into a 3-neck flask with chloroform as the solvent, based on the formulation in Table 2, to react with acrylic polyol 1210N under Nitrogen at 60°C for 16 h; then, MDI, as chain extender (in addition to PEG400 for some formulations), was added to the reaction, to react with the OH groups at 60°C. This step is monitored by FTIR to make sure all the NCO groups reacted, and the shift disappeared. The molar ratio of NCO/OH was set to be at 1.05:1. To increase the viscosity of the solution, some chloroform was stripped off by applying a vacuum. In the final step, to make a film out of the obtained resin, the solution was poured on aluminum foil and the film was formed by a film applicator to reach the final film thickness of 50 μm. In addition, films were prepared with the casting process into a petri dish for rheological analysis. Also, samples were prepared for dynamic mechanical thermal analysis (DMTA) measurement the way of pouring a resin into a glass petri dish which was cleaned with isopropyl alcohol just before pouring the solution. All samples were aged for a week at ambient temperature (23 ± 2°C) to be fully cured. They were also post-cured at 80°C for 4 h. Thereafter, they were ground to a very fine powder to be molded (into disks 8 mm in diameter and 1 mm in thickness) under a hot press machine at 100°C at 4 MPa pressure for 20 min.
| Samples | UPy (wt. %) | MDI (wt. %) | PEG400 (g) | Acrylic Polyol(g) | CHCl3 (g) | DBTL (ppm) |
|---|---|---|---|---|---|---|
| SHSMPU-AC-UPy0 (ctrl)a | 0 | 100 (0.407 g) | — | 2 | 20 | 50 |
| SHSMPU-ACP-Upy0b | 0 | 100 (1.23 g) | 0.5 | 2 | 20 | 50 |
| SHSMPU-AC-Upy25 | 25 (0.227 g) | 75 (0.306 g) | — | 2 | 54 | 50 |
| SHSMPU-AC-Upy45 | 45 (0.409 g) | 55 (0.224 g) | — | 2 | 120 | 50 |
| SHSMPU-ACP-Upy45 | 45 (1.35 g) | 55 (0.67 g) | 0.5 | 2 | 120 | 50 |
| SHSMPU-AC-Upy75 | 75 (0.683 g) | 25 (0.102 g) | — | 2 | 200 | 50 |
| SHSMPU-ACP-Upy75 | 75 (2.25 g) | 25 (0.31 g) | 0.5 | 2 | 200 | 50 |
| SHSMPU-AC-Upy100 | 100 (0.954 g) | 0 | — | 2 | 300 | 50 |
- a AC is standing for acrylic polyol.
- b ACP for the formulation containing PEG400.
2.3 Characterizations
H1 NMR (400 MHz) and C13 NMR (100 MHz) spectra were recorded on an NMR Oxford AS400 (Oxford Instruments, Concord, MA, USA) in d6-DMSO, and the residual DMSO (signal 2.5 ppm) in H1 NMR and C13 NMR (signal 39.52 ppm) were used as a reference to interpret chemical shifts. Differential scanning calorimetry (DSC) measurements were conducted with a heating/cooling rate of 10°C/min under N2 on the TA 250 (TA Instruments, New Castle, DE, USA) system. The glass transition temperatures (Tg) correspond to the maximum of the first derivative of the second heating cycle. FT-IR spectra were recorded on an infrared spectrometer (Shimadzu IRTracer, Shimadzu Corporation, Kyoto, Japan) with attenuated total reflection and temperature control (Specac), which was used to study the prepared PU films (either ground into fine powder or thin film).
The final products obtained from casting were ground and molded in a hot press (TP400 Fontune Holland) at 130°C and four bar for 30 min. Two types of samples were prepared: disk-shaped samples for the rheology experiments (8 mm diameter–1 mm thickness) for self-healing tests and bar-shaped samples for the shape memory (40 × 10 × 1 mm).
The equipment was heated to a temperature that was roughly 40° over the transition temperature of each sample for a period of 60 s in order to examine the shape memory property of these materials. Following the application of 10% torsional strain, the temperature is then lowered to below the glass transition point (Tg) of about 40°, without any additional stress, for 1000 s (stress relaxation step), before being heated once more to determine whether the sample can recover to its initial shape (creep step) [23].
The thermo-mechanical properties of the disks were studied using a rheometer (Discovery HR-2, TA Instruments). The experiments to evaluate the change in modulus affected by cracking and healing were performed in oscillation mode (1 Hz) with 0.01% strain, 5 N of axial force, and temperature ramps of 3 K/min, and 8 mm parallel plate clamps are used. To make a crack, a razor blade that is heated is used to cut through in a size of 4 mm which is shown in Figure 9. In order to erase the polymer thermal history, the measurements were started at 120°C (or 150°C depending on the Tg of the polymer) and followed by a cooling ramp down to 60°C (below this temperature, the quality of measurements gets compromised as the samples start to slip from the measuring geometry).
3 Results and Discussion
3.1 Chemical Structure Characterization
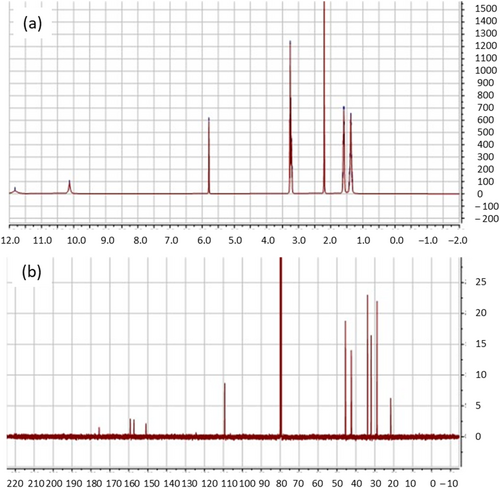
In order to make supramolecular polyurethanes (Scheme 3) based on quadrupole hydrogen bonding, 6-methylisocytosine was functionalized with isocyanate groups which resulted in UPy-NCO. Moreover, as explained earlier, polyurethanes in both forms of thin film as a coating and thicker film for further sample preparation were made. The success of the reaction of functionalization of 6-methylisocytosine with isocyanate (Scheme 1) was assessed by nuclear magnetic resonance (NMR) analysis at a constant temperature of 298 K. In Figure 1, the 1H-NMR and 13C-NMR spectra are reported. The result aligned with the data available in literature [16].
In order to prove the final structure of the polymer, the FT-IR spectra of acrylic polyol, NCO-functionalized 6-methylisocytosine, pure 6-methylisocytosine, and supramolecular polyurethane film with 45% content of UPy known as SMSMPU-AC-UPY45 are shown in Figure 2. The absorption of the NCO group (2270 cm−1) in the spectra of UPy-NCO confirms the functionalizing of 6-methylisocytosine with HDI.
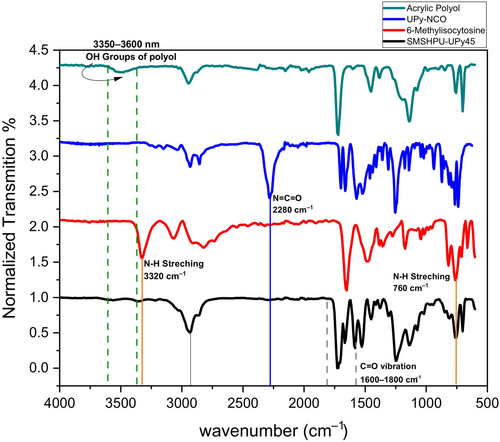
The absence of an amine peak at 3300 cm−1 in the spectra of UPy-NCO is attributed to the reaction of NCO and NH2. In the later step, the reaction between OH groups of acrylic polyols and isocyanates, the absence of absorption peaks of NCO group (2280 cm−1), either by MDI or UPy-NCO, and OH group broad peak (3350–3600 cm−1), is a proof of fabrication of polyurethane film (Figure 2). In the spectrum of SHSMPU-AC-UPy45, there are characteristic peaks of NH (3320 cm−1) and C=O (1600–1800 cm−1) and the CH aliphatic stretch bands (2945, 2858 cm−1).
3.2 Thermal analysis
In Figure 3a, reporting DSC curves of the prepared materials, the glass transition of annealed UPy-containing polyurethane gives information on the morphology after erasing the thermal history on all samples. The results of the second heating curve are presented, which reveal that by increasing the amount of UPy-NCO (UPy0 to UPy75 in Table 2), the glass transition of the soft phase drastically decreased from 115.5°C to 52.4°C for UPy75, which is most likely related to partial replacement of covalent bonds by non-covalent hydrogen bonding of UPy, increasing chain mobility [24].
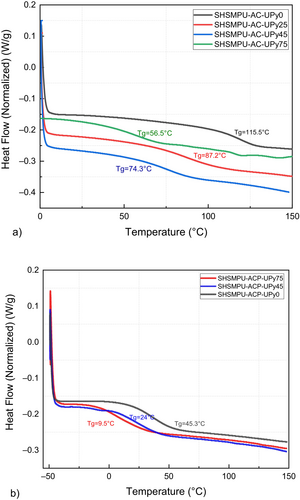
Additionally, it is evident that there is no discernible melting point. It can be inferred that, even in a reaction involving the OH functional group of polyols without a short-chain extender (as seen in the control sample), the hard segments may form a phase-separated dispersion resembling droplets within the continuous amorphous soft phase [25]. In traditional polyurethanes, it is expected to have both hard segments (isocyanate and short-chain extender) and soft segments (polyol). Since these two phases are not compatible, microphase separation can take place which results in the hard segment and soft segment rich phases, but this molecular structure is also dependent on several parameters, including the molecular weight of polyol, chemical composition, type, and amount of hydrogen bonding [26]. On the other hand, since the UPy-moieties attached to the polyol backbone as a pendant group, even by making strong quadruple hydrogen bonding due to the dimer, polymer aggregation is prevented and the soft phase makes a matrix for the hard-phase droplets, which is in accordance with results reported in the literature [27].
On the other hand, achieving optimal mobility for the healing components (hydrogen-bonding donators and acceptors) is crucial for effective room-temperature self-healing [28]. To address this, the formulation incorporates polyethylene glycol as a flexibilizer, causing a substantial reduction in the glass transition temperature (Figure 3b). In samples modified with PEG400, Tg has experienced significant drops of approximately 100° and 65°, yielding Tg values of 45°C and 10°C for SHSMPU-ACP-UPy0 and SHSMPU-ACP-UPy75, respectively. Nonetheless, this alteration may come at the cost of diminished mechanical properties, potentially imposing constraints on the material's applicability, particularly in terms of thickness.
3.3 Hydrogen-Bonding Evaluation of the Supramolecular Structure
According to the result of the thermal analysis earlier, the structures tend to phase mix by decreasing the amount of MDI. Since the hydrogen bonding is much stronger in hard segments than in soft segments, they tend to show the vibration at lower wavelengths [29]. For instance, CO number 3 (CO #3, Scheme 3) in the FT-IR spectra (Figure 4) shows absorption at a wavelength of 1660 cm−1 for both SHSMPU-AC-UPy45 and SHSMPU-75, shifted to 1664 cm−1 for SHSMPU-AC-UPy0. In contrast, the CO vibration of the urethane band which is indicated by number 1 (CO #1) appears at 1721 cm−1 as more strongly hydrogen-bonded. As discussed earlier, due to the symmetric structure of both dimers of UPy and MDI, packing of the polymer chain is much easier in hard segments which promotes phase separation but as the amount of MDI decreases and is replaced by UPy moieties, the number of hard segments in a matrix of soft segments declined and phase mixing is more likely [30]. Nevertheless, hydrogen bonding is a driving force for phase separation, the composition can be also distinguished by the evaluation of the NH and CO stretching band of FT-IR [31].

3.4 Shape Memory Evaluation of Self-Healing Behavior of Supramolecular SHSMPU Films
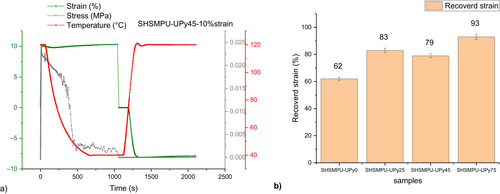
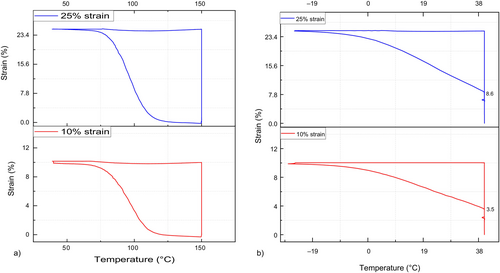
The results for the following samples of shape fixity are presented in Figure 5b.
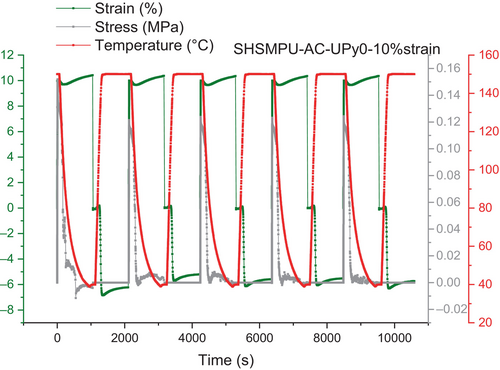
As the results revealed, by increasing the amount of hydrogen bonding of UPy-NCO dimer in 0%, 25%, 45%, and 75%, shape fixity is respectively shown enhancing from 62%, 83%, 73%, and 93%. Whereas results have shown that shape fixity will increase by UPy component incorporation, it is also important that this function has to be repeatable after some cycles. So, this experiment has been repeated five times continuously and the results are summarized in Figures 6-8, for SHSMPU-UPy0 at 10% strain, SHSMPU-AC-UPy45 at 10%, and SHSMPU-UPy45 at 25%, respectively. As it is shown in Figure 6, the fully covalent bonded (SHSMPU-AC-UPy0) not only did not present any repeatability at 10% strain but also its shape fixity dropped from 62% to 57% at the end indicating that during repeated deformation some of the covalent bonds are broken and as they are not reversible the function of shape recovery declined as well. On the other hand, Figure 7 illustrates that not only does SHSMPU-AC-UPy45 have some potential for shape recovery over repeated cycles, but its shape retention is also tremendously increasing up to 95%. It can be observed by comparing stress values in Figures 6 and 7, that by addition of UPy the maximum stress decreases. This is an obvious consequence of replacing covalent bonds with hydrogen bonds. However, this can be considered a potential advantage, as the material becomes more flexible. Since the results of SHSMPU-AC-UPy45 at 10% strain were quite interesting, the applied strain was increased to 25%, and the outcome in Figure 8 demonstrated a sharp increase in shape retention from 52.8% to 90.4% from cycle 1st to the 5th cycle. It is important to note that 1st cycle has been considered as a deleting thermal history and the results were evaluated by excluding the 1st cycle. On the other hand, for a strain of 25%, in every cycle from the 2nd to the 5th, the shape retention was slightly increased. In this respect, it can be concluded that even though for larger strain the shape retention might decrease, it still improves with repetition cycles. When it comes to material durability, it is abundantly clear that, in addition to intrinsically self-healing properties, the shape memory function of the polymer must be repeatable, which means that even if a surface is cracked multiple times, it can still be closed and healed [32]. Moreover, the results proved by the replacement of some covalent bonding with reversible non-covalent hydrogen bonding, regarding more movement of soft segments and better accessibility of hydrogen moieties shape recovery can improve during cycles. As previously stated, the reversibility of hydrogen bonding as a potential for shape memory could have assisted self-healing of cracked surfaces, though the depth and width of the crack have a significant effect on healing efficiency. A certain amount of covalent bonds are always required to provide the shape memory function to the polymer, according to some literature and theory [22, 33], as confirmed by the results of the cyclic test. Mechanical strength provided by covalent bonds should also be sufficient, depends on the end-use of the polymer [34]. Considering the trade-off among shape memory and mechanical strength, SHSMPU-AC-UPy45 has been chosen as an optimum to be compared with the control sample SHSMPU-AC-UPy0 for further evaluation and healing efficiency experiments.
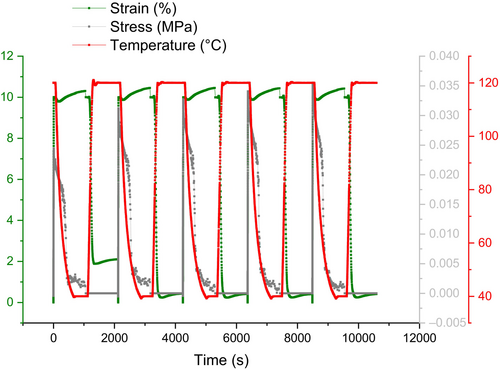
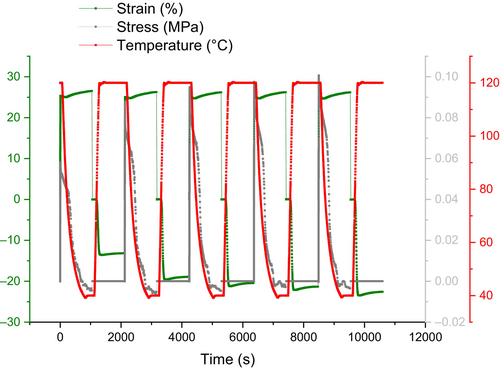
It has been proposed that hydrogen bonding-based self-healing may also support shape memory function and return a polymer's shape and crack to their initial states, even though the main focus of this study was on the role that shape memory might play in a material's capacity for self-healing [31]. For example, when there is significant deformation and many covalent bonds break as a result, soft segments containing hydrogen bonding moieties can move easily and contribute to the healing process by partially forming a bond that is not as strong as a covalent bond but is still strong enough to restore the deformed polymer network and improve the polymer's shape fixity [35]. In fact, in SHSMPU-AC-UPy45, weaker bonds replace 45% of the net points that the covalent bond originally created.
As mentioned earlier in this study, PEG400 has been incorporated into the structure of SHSMPU to enhance mobility at room temperature. Figure 9 presents a creep test at elevated temperature, comparing SHSMPU-AC-UPy45 with SHSMPU-ACP-UPy45. The results illustrate that while SHSMPU-AC-UPy45 can recover 100% of its original shape at strains of 10% and 25%, SHSMPU-ACP-UPy45 only achieves about 65% recovery under the same conditions. The significant difference lies in the recovery rate, which is notably faster in SHSMPU-ACP-UPy45 which is aligned with what Wang et al. [36] reported in their study of self-healing PEG gels. Examining the recovery curve at each sample's respective Tg, it is evident that SHSMPU-AC-UPy45 begins recovery at a Tg of 75°C as expected, whereas SHSMPU-ACP-UPy45 had approximately 40% recovery efficiency at its Tg of 24°C.
Even though both samples undergo urethanization with an identical ratio of hydrogen bonding to covalent bonding, their responses to deformations can vary due to differences in the Tg and the network morphology of the polymers [37]. While the non-covalent to covalent bond ratio remains consistent in both samples, the arrangement of hard segments is influenced by the presence of PEG400 in soft segments. In simpler terms, the hard segments in SHSMPU-ACP-UPy45 become more accessible but lose some of their dominance, impacting the overall healing efficiency and preventing an equivalent performance [38].
3.5 Self-healing Behavior of Supramolecular SHSMPU Film
This calculation was based on the measured complex modulus of each sample in different stages of original, cracked, and healed at 20° above its Tg, which is shown in Figure 10 for two chosen samples of SHSMPU-AC-UPy0 and SHSMPU-AC-UPy45. As shown in Figure 11, there is a remarkable improvement in healing due to the induced hydrogen bonding in sample SHSMPU-AC-UPy45.
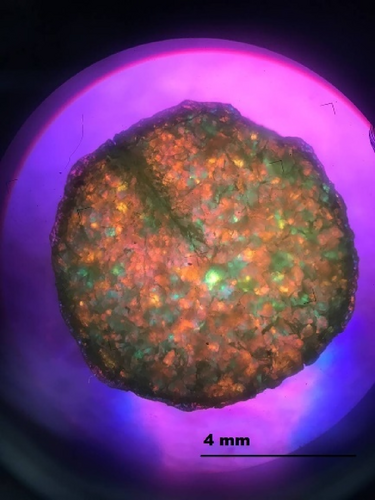
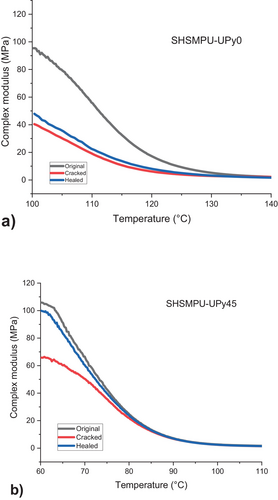
A general advantage of using hydrogen bonding as a self-healing mechanism is that it can heal without enforcement at ambient temperature [39], although this is also reliant on the Tg of the polymer. Because of the high Tg of this polymer, room-temperature healing was nearly impossible in this situation [40]. As a stimulus for both shape memory and healing performance, each sample was heated to 20° over its Tg between two clamps of a rheometer without applying extra force for 10 min and then complex modulus was recorded. In previous research, Xu et al. [41] claimed that the contribution of hydrogen bonding into the polymer backbone can enhance the strength and toughness of the PU tremendously.
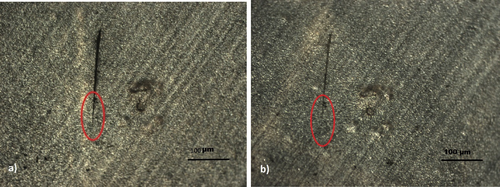
In the case of a microcrack in the coating, a cut less than 20 μm wide was made with a sharp razor blade as seen in Figure 12. It was then exposed to a hair dryer flow with a temperature of roughly 80° at a distance of 10 cm from the film. As a result, even if the applied temperature is close to its Tg (not above), some closure of the departing surface occurs [42], which is not only a proof of shape memory behavior, but it also begins to heal itself from the thinner cracked edge (red circle), where the cracked surfaces were already much closer together, which is in line with the result reported by Yu et al. [43]. Therefore, it is clear that the most important requirement for showing effective intrinsic healing is the closure of cracks [44]. Moreover, it will be facilitated even more if the coating has got a Tg below room temperature, then hydrogen bonding moieties can reconnect and heal the cracks at room temperature [45]. As a matter of fact, Ma et al. [46] in their research showed that by modifying a hydroxyl-terminated silicon oil with UPy, coatings with scratches up to 200 μm can be healed within first 2 min, as the molecular structures have good mobility at room temperature.
As indicated, the efficiency of healing is closely tied not only to the dimensions of the crack but also to the glass transition temperature (Tg) of the polymer. To explore this further, the same procedure outlined above was replicated with three samples of SHSMPU-ACP-UPy (0-75), where the Tg was lower. Heat treatments were conducted at a lower temperature (40°C, with a distance of 20 cm using a hair dryer).
The results depicted in Figure 13 reveal that self-healing in SHSMPU-ACP-UPy0, even within a thin film of 30 μm, is scarcely achievable. Notably, increasing the UPy content from 0% to 75% once again leads to a notable improvement in healing performance. Zhang et al. [47] in an interesting research evaluated unmodified and modified nano-SiO2 with UPy filled in PU which resulted in a self-healable coating at 25°C after 180 min. In another research, Kazazi et al. [48] by modification of the hydroxy-terminated polybutadiene (HTPB) with UPy and curing isophorone diisocyanate (IPDI) created a coating that can be self-healable with scratches with depth of 100 μm and the width of 200 μm at ambient temperature after 24 h after cross-cut. Although these two researches showed good healing efficiency, the healing time can be improved by temperature compensation as in this research.
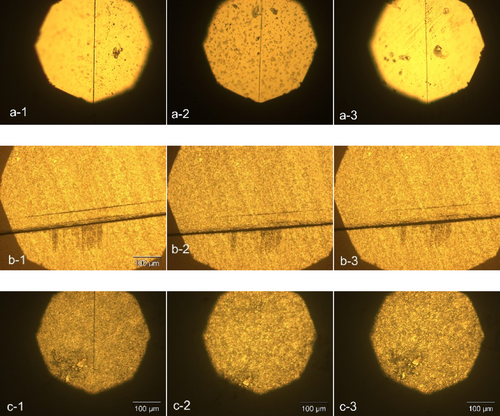
While the dimensions and depth of the crack continue to exert a significant influence on performance, it can be inferred that microcracks in thin films are self-healable if they possess a low Tg at room temperature or below.
4 Conclusions
In this research, we developed a shape memory–assisted self-healing PU coatings (SHSMPU) with good shape memory capabilities and self-healing in micro- and macrocracks based on supramolecular quadrupole hydrogen bonding. The latter is provided by incorporation of a pyrimidine-functionalized isocyanate (UPy-NCO), synthesized in this work. FT-IR showed the occurrence of strong hydrogen bonding, by significant shift of representative carbonyl peaks. The Tg of the materials, as measured by DSC, was dependent on the relative amount of the pyrimidine functional molecules (that was varied from 0% to 100% of isocyanate groups). With the increasing substitution of covalent bonds with hydrogen bonds, the materials became more flexible, as proved by the decreasing applied stress.
As a result of the rheological examination of the polymers, SHSMPU-AC-UPy45, containing 45% of UPy moieties as the diisocyanate component, showed an improvement in shape fixity, compared to the material without UPy-NCO, particularly when 25% deformation was applied upon repeated cycles. This material resulted the best candidate in terms of stability and a good compromise between shape retention and stiffness.
The polymers exhibited enhanced healing performance through the reassociation of hydrogen bonding. The assistance of shape memory in the polymer resulted in a substantial increase in the healing performance of macrocracks, rising from 50.2% to 94.2% as the UPy-NCO amount went from 0% to 45%.
Intrinsic healing still required a thermal stimulus; therefore, the healing process was performed above the measured Tg. The healing process was monitored via the complex modulus G*.
Finally, in order to increase flexibility of the material, and decrease the healing temperature, the incorporation of PEG400 in the formulation was tested. This lowers the glass transition temperature, which is beneficial for room-temperature self-healing and self-healing of microcracks seems to be achievable, but it also results in lowered mechanical properties, potentially limiting the range of applications.
In conclusion, this work demonstrated the effectiveness of incorporating quadrupole hydrogen bonding groups within the isocyanate moiety of PU materials, in order to introduce self-healing, and therefore improve the durability of such materials, assisted by the shape memory behavior of said material.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




