Nutritional considerations with antiobesity medications
Abstract
The improved efficacy and generally favorable safety profile of recently approved and emerging antiobesity medications (AOMs), which result in an average weight reduction of ≥15%, represent significant advancement in the treatment of obesity. This narrative review aims to provide practical evidence-based recommendations for nutritional assessment, management, and monitoring of patients treated with AOMs. Prior to treatment, clinicians can identify preexisting nutritional risk factors and counsel their patients on recommended intakes of protein, dietary fiber, micronutrients, and fluids. During treatment with AOMs, ongoing monitoring can facilitate early recognition and management of gastrointestinal symptoms or inadequate nutrient or fluid intake. Attention should also be paid to other factors that can impact response to treatment and quality of life, such as physical activity and social and emotional health. In the context of treatment with AOMs, clinicians can play an active role in supporting their patients with obesity to improve their health and well-being and promote optimal nutritional and medical outcomes.
Study Importance
What is already known?
- No comprehensive evidence-based reviews, to our knowledge, have addressed the topic of nutritional recommendations for patients treated with newly available and emerging antiobesity medications (AOMs), which result in an average weight reduction of ≥15%.
What does this review add?
- This review provides clinicians with evidence-based nutritional recommendations; guidance on identifying, monitoring, and managing patients at risk of nutritional deficiency; and practical tips to support patients' efforts to achieve a healthier lifestyle and promote optimal nutritional and medical outcomes during treatment with AOMs.
How might these results change the direction of research or the focus of clinical practice?
- The availability of AOMs with greater efficacy and a generally favorable safety profile may enable more clinicians to play an active role in the treatment of their patients with obesity. This review provides practical nutritional recommendations and tips for patient monitoring and management to empower clinicians and promote optimal health outcomes among patients treated with AOMs.
INTRODUCTION
Newly approved and emerging antiobesity medications (AOMs) can facilitate average body weight reductions of ≥15%, which were previously seen only with intensive interventions such as bariatric surgery or very low-calorie diets (VLCDs). These intensive interventions are typically provided in specialized obesity treatment centers in the context of structured nutritional assessment, recommendations, and monitoring. However, with the availability of additional effective treatment options and a better understanding of obesity as a chronic disease, more clinicians will be empowered to provide obesity care in addition to those who practice in specialized centers. This narrative review aims to provide practical evidence-based recommendations for nutritional assessment, management, and monitoring of patients treated with AOMs to support patients' efforts to achieve a healthier lifestyle and to promote optimal nutritional and medical outcomes through maintenance of reduced body weight.
BACKGROUND
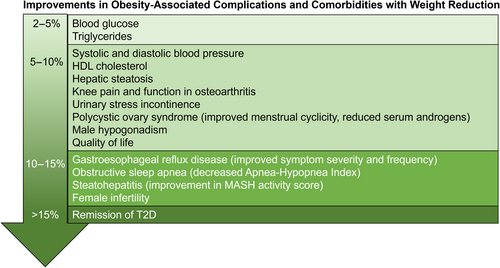
Obesity is a chronic, relapsing, and progressive disease associated with over 200 complications and an increased risk of premature mortality [(1-4)]. Complications are improved with weight reduction, and proportionally greater benefit is observed with greater weight reductions (Figure 1) [(5-8)]. Lifestyle modification is a cornerstone of treatment for obesity and can induce clinically meaningful weight reductions of ≥3% to 5%, as well as improvements in obesity-related complications [(4)]. However, long-term efficacy is often limited [(9, 10)], and the patient or the clinician may incorrectly interpret this as failure on the part of the patient rather than failure of treatment. Successful obesity treatment involves a fundamental shift in the balance between energy expenditure, energy intake, and energy storage, each of which has multiple contributing factors, including both modifiable and nonmodifiable determinants [(11, 12)].
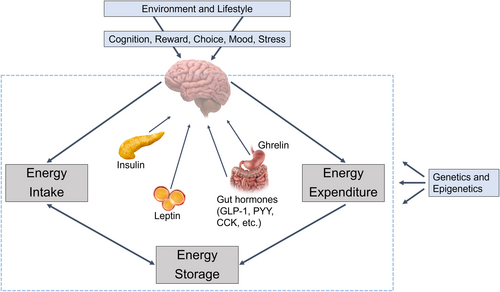
The central nervous system plays a major role in regulating food intake, energy expenditure, and body weight in response to signals from peripheral organs and tissues (Figure 2). Hormonal and metabolic changes induced by weight reduction result in elevated appetite and reduced metabolic rate [(13-15)]. These changes, along with environmental and psychosocial factors that drive food intake, may make maintenance of reduced body weight more difficult when lifestyle modification is employed in isolation.
Recent advances in AOMs may offer improved treatment outcomes for people with obesity. AOMs are indicated as an adjunct to lifestyle modification for individuals with a body mass index (BMI) of 27 kg/m2 or greater with at least one weight-related complication or those with a BMI of 30 kg/m2 or greater [(16)], although only 2% of eligible patients are currently receiving therapy [(17)]. Since 2021, a new generation of AOMs has emerged, offering improved efficacy, including a mean weight reduction of approximately 15% or more [(18)], along with a generally favorable safety profile. These new AOMs include semaglutide and tirzepatide; other therapies are in various stages of development.
Semaglutide (approved in 2021 for chronic weight management) and tirzepatide (approved in 2023 for chronic weight management) are AOMs that act in the central nervous system to reduce food intake and body weight [(19)]. In clinical trials, semaglutide and tirzepatide have been demonstrated to improve factors related to the control of food intake, such as hunger, fullness, and food cravings [(20, 21)]. In the Semaglutide Treatment Effect in People with Obesity (STEP)-1 randomized controlled trial (ClinicalTrials.gov identifier: NCT03548935) of semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, mean change in body weight was −14.9% in patients treated with semaglutide 2.4 mg once weekly compared with −2.4% in placebo-treated control individuals at 68 weeks of treatment [(22)]. Weight reduction of ≥15% was achieved by 50.5% of participants at 68 weeks [(22)]. Improvements in cardiometabolic risk factors, including waist circumference and systolic blood pressure, and patient-reported physical function have also been observed with semaglutide treatment [(22, 23)]. A large cardiovascular outcomes trial (Semaglutide Effects on Heart Disease and Stroke in People with Overweight or Obesity [SELECT]; NCT03574597) demonstrated a 20% reduction in major adverse cardiovascular events with semaglutide treatment in people with obesity or overweight and established cardiovascular disease (hazard ratio, 0.80; 95% confidence interval [CI]: 0.72–0.90; p < 0.001) [(24)].
Tirzepatide is a long-acting glucose-dependent insulinotropic polypeptide (GIP) receptor and GLP-1 receptor agonist. In the SURMOUNT-1 randomized controlled trial (NCT04184622) of tirzepatide for obesity, mean change in body weight was −15.0% (5 mg), −19.5% (10 mg), and −20.9% (15 mg) in tirzepatide-treated patients relative to −3.1% in placebo-treated control individuals at 72 weeks of treatment. With the 15 mg dose, 70.6% of participants achieved ≥15% weight reduction, and 56.7% of participants achieved ≥20% weight reduction at 72 weeks [(25)]. Improvements in cardiometabolic risk factors, including waist circumference, systolic blood pressure, triglycerides, high-density lipoprotein and non-high–density lipoprotein cholesterol, and fasting insulin, and patient-reported physical function were also demonstrated with tirzepatide treatment [(25)]. An ongoing outcomes trial (SURMOUNT-MMO; NCT05556512) is evaluating the effect of tirzepatide on morbidity (including major adverse cardiovascular events) and mortality in adults with obesity.
The most common adverse events associated with semaglutide and tirzepatide treatment include gastrointestinal events such as nausea, diarrhea, and constipation [(22, 25)]. In registration trials, most events were mild or moderate and generally transient, infrequently leading to treatment discontinuation [(22, 25)]. Evidence has suggested that the experience of adverse gastrointestinal events is not significantly related to the amount of weight reduction achieved [(26, 27)]. Additional therapies such as retatrutide (a single molecule that activates the receptors for GIP, GLP-1, and glucagon) and a combination of semaglutide and cagrilintide (an amylin analog) are currently in clinical development for the treatment of obesity, with mean efficacy predicted to be greater than that of current medications [(28, 29)].
Owing to the recent emergence of AOMs with a mean weight reduction of ≥15%, nutritional recommendations for patients receiving these therapies are limited. However, studies of patients with obesity observed before and after treatment with VLCDs or bariatric surgery can provide evidence for anticipated nutritional deficiencies and target ranges of nutrient intake, as discussed later in this manuscript. Energy intake is expected to decrease with AOMs. Studies of ad libitum food intake during a buffet-style lunch have identified up to approximately 345-kcal greater reductions from baseline in mean energy intake in participants receiving AOMs compared with placebo [(21, 30, 31)]. In addition, Hall reported model-estimated population mean reductions in daily energy intake as large as 1200 kcal/day from baseline intake [(32)]. With decreased energy intake, the consumption of nutrient-dense foods becomes more important, and patients should receive appropriate counseling and monitoring to reduce the risk of nutritional deficiencies. Herein are recommendations for practitioners to manage clinical interactions, provide nutritional counseling, screen and monitor for nutrient deficiencies, and consider other factors that may influence treatment outcomes with AOMs.
APPROACH TO THE PATIENT WHEN CONSIDERING AOMs
Regardless of the treatment modality, people with obesity should receive evidence-based obesity care that is individualized, patient-centered, and focused on health outcomes rather than on weight loss alone (Figure 3) [(4)]. The “5 A's” model (Ask, Assess, Advise, Agree, Assist) can be used to guide clinical interactions with patients with obesity or other chronic diseases [(4, 33)]. Clinicians should ask for permission before starting a conversation about weight and then assess the patient. Assessment should include a comprehensive medical history (including psychosocial, weight, dietary, and other lifestyle history), physical examination, and appropriate laboratory or imaging assessments to evaluate potential root causes of obesity, identify any obesity-related complications, and assess nutritional status, including risk of malnutrition [(34)]. Laboratory assessment of nutritional status is recommended when there is a clinical suspicion of deficiency (detailed in later sections of this manuscript). Potential barriers to treatment, including barriers to dietary modification, should be identified. Clinicians should advise patients about treatment options and discuss expectations for treatment, including benefits relevant to the patient. Clinicians and patients should agree on goals related to health, dietary patterns and other lifestyle factors, and weight. An individualized approach to goal setting is important because patients' responses to treatment are heterogeneous. Clinicians should assist patients in addressing challenges and barriers to weight management, taking into consideration social determinants of health, including access to healthy food and opportunities for physical activity. Because obesity is a chronic disease that requires a long-term approach, clinicians should also arrange for follow-up and refer patients for additional support as needed. Referral to a registered dietitian, when available, is recommended for medical nutrition therapy to complement and support other obesity therapies, including treatment with AOMs [(4)]. Medical nutrition therapy delivered by registered dietitians has been associated with improvements in dietary quality, health outcomes, and weight-loss outcomes [(35, 36)].
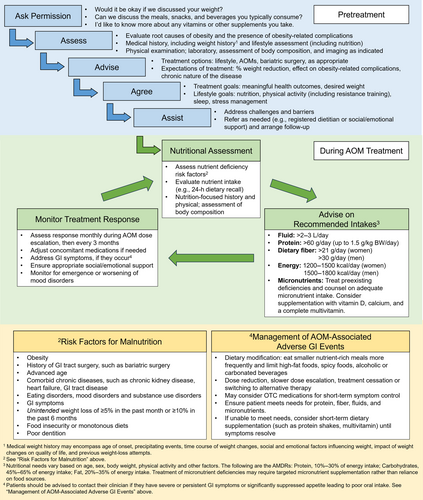
Prior to initiation of AOM therapy, it is important to establish expectations for treatment, including the potential magnitude of weight reduction with different agents and the heterogeneity of response [(4)]. Prior to AOM initiation, patients should understand the route (oral or subcutaneous) and frequency of administration, as well as the importance of meeting goals for nutrition and hydration, as discussed in the following section, even if appetite is significantly reduced. The patient should also be counseled about the potential impact of weight reduction on the management of existing obesity-related conditions such as type 2 diabetes and hypertension. With significant weight loss, dose reductions are often needed for antidiabetic medications, antihypertensives, and diuretics [(37)]. In some cases, proactive dose adjustments of these concomitant medications may be considered early in the course of AOM therapy to reduce the risk of hypoglycemia (in patients on insulin or sulfonylureas) or hypotension (e.g., in some patients with well-controlled hypertension or in older individuals). Weight-based therapies such as thyroid hormone replacement may also need monitoring and adjustment with weight reduction. Patients should be counseled about the importance of follow-up visits to monitor treatment response and guide dose escalation. Guidelines suggest regular assessment of treatment response, e.g., monthly during dose escalation and then at least every 3 months [(38)]. Less frequent assessments may be possible after achievement of goal weight during maintenance of reduced body weight on treatment. In general, if weight loss is <5% after 3 months on the highest therapeutic dose of an AOM, discontinuation of therapy should be considered, and an alternate treatment approach should be initiated [(38, 39)]. It is important to note that short-term use of pharmacotherapy for obesity is generally not recommended due to the potential for weight regain [(5)]. Across clinical trials, weight regain was typically observed when AOM treatment was stopped [(23, 40, 41)]. Long-term use of pharmacotherapy is recommended to assist with maintenance of weight loss over time, consistent with the chronic nature of obesity [(5, 38, 39)].
EVIDENCE-BASED NUTRITIONAL RECOMMENDATIONS FOR PATIENTS RECEIVING AOMs
This section provides targeted recommendations for patients receiving AOMs, with a focus on healthy dietary patterns enabling adequate intake of energy, macronutrients (proteins, fats, and carbohydrates), micronutrients, fiber, and fluids (Tables 1 and 2). Limited evidence exists to guide nutritional recommendations for patients receiving newly available AOMs that have an efficacy of ≥15% weight reduction on average, and additional research is needed to address this knowledge gap. In the meantime, nutritional recommendations for patients receiving AOMs can be based on evidence from the general population, low-calorie diets, and bariatric surgery, including observations of preoperative patients. Specifically, evidence for this review was identified via a PubMed search using keywords “dietary,” “nutritional,” “nutrition,” “weight loss,” “obesity,” “obese,” “very low-calorie diet,” “malnutrition,” “bariatric,” “guidelines,” and “reference.” Manuscript reference lists were also reviewed. Because this was a narrative review, searches were augmented with relevant research by expert consensus. All authors reviewed the references that were selected for inclusion.
| Dietary component | Recommended intake | Recommended sources | Signs and symptoms of deficiency | Additional considerations |
|---|---|---|---|---|
| Fluids | >2–3 L/d |
|
Hypotension, tachycardia, dizziness |
|
| Energy |
|
A healthy dietary pattern emphasizing vegetables, fruits, whole grains, lean protein foods, low-fat dairy or dairy alternatives, and healthy fats | Reduced fat and lean mass, decreased strength and functional capacity |
|
| Fiber |
|
|
Constipation |
|
| Protein |
|
|
Loss of lean body mass, weakness, edema, hair loss, skin changes |
|
| Carbohydrates |
|
|
Although there is no absolute dietary requirement for carbohydrates, very low-carbohydrate (ketogenic) diets may increase the risk of dehydration, fatigue, halitosis, and other adverse events |
|
| Fats |
|
|
Essential fatty acid deficiency: dry skin, hair loss, impaired wound healing |
|
- Abbreviations: AOMs, antiobesity medications; RDA, Recommended Dietary Allowance.
| Micronutrient | Dietary sourcesa | Symptoms and signs of deficiency | Additional considerations |
|---|---|---|---|
| Fat-soluble vitamins | |||
| Vitamin A |
|
Eye diseases including night blindness and xerophthalmia, susceptibility to infections, rough skin (follicular hyperkeratosis), impaired growth, impaired fertility |
|
| Vitamin D | Fatty fish, fortified foods (e.g., milk, cereals), egg yolk | Impaired bone mineralization (osteomalacia in adults; rickets in children), muscle weakness and pain, hypocalcemia |
|
| Vitamin E | Wheat germ, vegetable oils, nuts, avocado, green leafy vegetables, fish | Neurologic symptoms including ataxia, peripheral neuropathy, myopathy, retinopathy |
|
| Vitamin K |
|
Hemorrhage, bruising, impaired bone mineralization |
|
| Water-soluble vitamins (must be consumed daily) | |||
| Thiamin (vitamin B1) | Fortified cereals, whole-grain and enriched-grain products, pork, fish, beans, peas, lentils, nuts |
|
|
| Riboflavin (vitamin B2) | Dairy foods, meat, fish, poultry, eggs, nuts, green vegetables, mushrooms, fortified cereals, whole-grain or enriched breads | Dermatitis, cheilosis and angular stomatitis (cracks or lesions on the lips or at the corners of the mouth), sore throat, inflammation and redness of the tongue (“magenta tongue”) |
|
| Niacin (vitamin B3) | Meat, eggs, poultry, fish, milk, whole-grain and enriched breads, fortified cereals and grain products, nuts, beans, peas, and lentils | Pellagra: sun-sensitive dermatitis, diarrhea, and dementia (“the three Ds”), bright red tongue, vomiting, anorexia, depression, behavioral changes, memory loss |
|
| Pantothenic acid (vitamin B5) | Found in most plant and animal foods; meat, fish, poultry, dairy products, egg yolk, avocado, mushrooms, nuts, seeds, beans, peas, and lentils are good sources; also synthesized by gastrointestinal tract bacteria | Paresthesia (numbness, burning sensation) in the hands and feet, headache, fatigue, irritability, insomnia |
|
| Vitamin B6 (pyridoxine, pyridoxal, pyridoxamine) | Chickpeas, meat, poultry, fish, shellfish, fortified cereals, nuts, soy products, banana, avocado, potatoes, spinach | Neurologic changes, e.g., seizures, irritability, confusion; skin disorders such as cheilosis, glossitis, and stomatitis; microcytic anemia |
|
| Biotin (vitamin B7) | Egg yolk, meat, fish, seeds, nuts, avocado, sweet potato; also synthesized by gastrointestinal tract bacteria | Alopecia (hair loss); scaly dermatitis around the eyes, nose, mouth, and perineum; neurological changes, e.g., ataxia, paresthesia, depression, seizures |
|
| Folate (folic acid, vitamin B9) | Green leafy vegetables, beans, peas, lentils, nuts, seeds, avocado, citrus fruit, melon, enriched breads and grain products | Macrocytic anemia, hypersegmented neutrophils, fatigue, weakness, irritability, ulcerations on the tongue and oral mucosa |
|
| Vitamin B12 (cobalamin) | Animal products: meat, fish, poultry, shellfish, milk, cheese, eggs, fortified cereals | Macrocytic anemia, glossitis, fatigue, neurologic complications, including memory impairment, paresthesia, and peripheral neuropathy |
|
| Vitamin C (L-ascorbic acid) | Citrus fruits, berries, red pepper, cruciferous vegetables, tomatoes, potatoes | Scurvy: bleeding gums, loose teeth, bruising, perifollicular hemorrhage, poor wound healing, fatigue |
|
| Minerals | |||
| Calcium | Milk and milk products, green leafy vegetables, sardines (with bones), tofu, beans |
|
|
| Copper | Organ meats, seafood, nuts, seeds, whole grains, cocoa | Anemia, neutropenia |
|
| Iron | Red meat, fish, poultry, shellfish, green leafy vegetables, beans, peas, lentils, nuts, dried fruits, fortified cereals | Microcytic and hypochromic anemia, fatigue, weakness, tachycardia, dyspnea, pallor, cold intolerance, koilonychia (brittle spoon-shaped nails), glossitis |
|
| Magnesium | Nuts, seeds, beans, peas, lentils, dairy products, green leafy vegetables, fruits (e.g., banana, avocado), fish | Hypomagnesemia can cause muscle weakness and cramps, anorexia, nausea, and vomiting; it can also contribute to the development of hypocalcemia and hypokalemia |
|
| Potassium | Fruits, vegetables, nuts, seeds, beans, peas, lentils, dairy products | Hypokalemia can cause muscle weakness and cramps, fatigue, cardiac arrhythmias |
|
| Zinc | Meats, seafood, poultry, whole grains, fortified cereals, beans, peas, lentils, nuts, dairy products, eggs | Dermatitis, impaired wound healing, impaired taste, decreased immune function |
|
- Note: Adapted from Wilson et al. [(74)]. Additional information from Parrott et al. [(83)], Astrup and Bugel [(84)], and the Linus Pauling Institute [(73)].
- Abbreviations: AOM, antiobesity medication.
- a Treatment of micronutrient deficiencies may require targeted micronutrient supplementation rather than reliance on food sources.
Individuals treated with AOMs such as semaglutide and tirzepatide generally experience reduced appetite, including reduced hunger and increased satiety, leading to decreased food intake [(21)]. As a result, dietary quality becomes even more important because nutritional needs must be met within the context of reduced food intake. In the United States, most individuals do not currently meet recommendations for a healthy dietary pattern [(42)]. Emerging evidence has suggested that AOMs such as semaglutide and tirzepatide may impact food preferences in ways that could support improved dietary quality, although additional research is needed [(20, 43, 44)]. In general, healthy dietary patterns consisting of nutrient-dense foods and beverages [(42)] are appropriate for any patient, including those treated with AOMs. Nutrient-dense foods are good sources of vitamins, minerals, or other health-promoting components and have little added sugars, saturated fat, and sodium. Examples of nutrient-dense foods include vegetables, fruits, whole grains, seafood, eggs, beans, peas and lentils, unsalted nuts and seeds, fat-free and low-fat dairy products, and lean meats and poultry [(42)].
No particular dietary pattern has demonstrated superiority in terms of inducing and sustaining weight loss [(45-48)]. However, a number of healthy dietary patterns such as the Mediterranean diet, a healthy plant-based diet, and other dietary patterns that meet key nutritional recommendations in the Dietary Guidelines for Americans [(42)] have been consistently associated with improved health outcomes, including a lower risk of cardiovascular disease and mortality [(49-52)]. Consideration of individual food preferences, cultural traditions, and budgetary requirements may aid in the adherence to healthy dietary patterns, a key predictor of success [(42, 45)]. The US Department of Agriculture (USDA) provides practical resources to help patients eat healthily and achieve nutritional goals on a budget [(53)].
Energy intake
Energy requirements vary based on an individual's age, sex, body weight, and physical activity level, as well as other factors. As a result, recommended minimum goals for energy intake during weight loss should be personalized. In general, energy intakes of 1200 to 1500 kcal/day for women and 1500 to 1800 kcal/day for men have been recommended as safe during weight reduction [(54)]. Patients with low energy intakes (<1200 kcal/day) may have difficulty meeting nutritional needs by diet alone, but it is important to remember that patients with higher energy intakes may also be at risk of deficiencies due to poor dietary quality. VLCDs of <800 kcal/day typically include meal-replacement products (i.e., formulated foods supplemented with protein, vitamins, and minerals) and should be used only under the supervision of a specially trained clinician owing to an increased risk of nutritional and medical complications. These risks include dehydration, electrolyte imbalance, and cholelithiasis [(55)]. Energy intake goals may need to be adjusted to achieve weight stability (e.g., when target weight is achieved).
Dietary protein
Protein is a key macronutrient, and insufficient intake of protein can lead to excessive loss of lean body mass, weakness, edema, hair loss, and skin changes. Recommendations for minimum protein intake vary by population. The Recommended Dietary Allowance for protein for healthy adults of normal weight is 0.8 g/kg body weight/day, and USDA guidelines suggest a minimum goal of 46 g/day for healthy women and 56 g/day for healthy men [(42, 56)]. In patients with obesity, however, protein intakes of at least 60 to 75 g/day and up to 1.5 g/kg body weight/day have been recommended during weight reduction with various treatments, including VLCDs and bariatric surgery [(55, 57-61)]. Higher protein intake (i.e., >1.5 g/kg body weight/day) may be considered for select individuals such as those who have undergone a malabsorptive bariatric procedure like the duodenal switch [(58)]. In the setting of a hypocaloric diet with adequate protein intake, physical activity (either resistance training or combined resistance and aerobic training) can increase muscle strength and physical function and may promote greater loss of fat mass, although the impact of exercise on the loss of lean mass during weight reduction is unclear [(62-64)]. For individuals treated with AOMs, meal-replacement products such as shakes, bars, or other formulated foods can be used to supplement dietary intake and help meet protein needs, especially early in treatment if appetite is significantly reduced. Use of liquid meal replacements typically containing 15 to 25 g of high-quality protein per serving has been shown to be effective for weight reduction and is commonly recommended after bariatric surgery [(61, 65-67)]. These considerations are even more important in older individuals seeking to lose weight because muscle mass decreases with age (potentially leading to sarcopenia); therefore, older individuals may be at higher risk of excessive muscle loss and risk of falls and fracture with weight loss [(68)]. Additional research on changes in body composition and functional outcomes with weight reduction should inform protein intake recommendations in older individuals with obesity [(69, 70)]. Patients should be instructed to consume high-protein foods first at each meal to ensure adequate protein intake.
Dietary carbohydrate
The acceptable macronutrient distribution range (AMDR) for carbohydrates for healthy adults is 45% to 65% of energy intake [(42, 56)], which corresponds to approximately 135 to 245 g/day for a 1200- to 1500-kcal/day diet or 170 to 290 g/day for a 1500- to 1800-kcal/day diet. Severe carbohydrate restriction is not necessary because it does not produce greater long-term weight reduction and may promote restricted intake of fruits, vegetables, and whole-grain foods, which are important sources of micronutrients and dietary fiber. Ketone body production with very low-carbohydrate (ketogenic) diets may promote increased urination, dehydration, and electrolyte imbalance [(55)]. For patients who prefer a low-carbohydrate dietary pattern, attention to hydration (with fluid intake of >2 L/day) is recommended, along with consumption of micronutrient-rich, high-fiber vegetables and fruits.
Dietary fat
Less evidence exists to guide recommended ranges of intake for dietary fats, and health outcomes can be met while taking patient preferences into account. The AMDR for fat for healthy adults is 20% to 35% of energy intake, corresponding to approximately 25 to 60 g/day for a 1200- to 1500-kcal/day diet or 35 to 70 g/day for a 1500- to 1800-kcal/day diet [(42)]. Dietary fats are important to aid in the absorption of fat-soluble vitamins (A, D, E, and K) and to stimulate gallbladder emptying during weight reduction, which may reduce the risk of gallstone formation [(55)]. Intake of foods rich in n-3 polyunsaturated fatty acids (e.g., flaxseed, soybean or canola oil, or fatty fish), n-6 polyunsaturated fatty acids (e.g., nuts, seeds, and vegetable oils), or monounsaturated fatty acids (e.g., olive oil) should be prioritized, whereas intake of sources of saturated fat (e.g., animal fats and tropical oils) should be limited. A balanced and judicious intake of healthy fats should be considered. It has been recommended that fried and high-fat foods be avoided to decrease gastrointestinal side effects associated with AOMs [(71, 72)].
Dietary fiber
Over 90% of American adults fail to meet recommended intakes of dietary fiber [(42)]. Given this shortfall and the importance of dietary fiber for good health, the Dietary Guidelines for Americans identified fiber as a major dietary component of public health concern in the United States [(42)]. The Adequate Intake level of dietary fiber is 21 to 25 g/day for women and 30 to 38 g/day for men, depending on age [(42, 56)]. Good sources of dietary fiber include fruits, vegetables, and whole grains. Adequate intake of dietary fiber is associated with lower cardiovascular disease risk and is important for gastrointestinal health. Certain soluble, nonfermentable, gel-forming fibers such as psyllium and insoluble fibers such as coarse wheat bran can increase stool water content and bulk, which may aid stool passage [(73-75)]. For patients receiving AOMs who do not currently meet recommended fiber intakes, gradual increases in fiber intake along with adequate fluid intake may reduce constipation-related adverse events. Use of a fiber supplement may be considered when patients are unable to meet fiber goals with food alone.
Micronutrients
Micronutrients of public health concern for adults in the United States include potassium, calcium, and vitamin D, and guidelines recommend increased intake of food sources of these nutrients (vegetables, fruits, low-fat dairy, and fortified soy alternatives) [(42)]. Additional nutrients of concern include iron (in women of childbearing age) and vitamin B12 (in older adults) [(42, 56)]. Individuals with obesity are at increased risk of additional micronutrient deficiencies, as discussed later in this manuscript. Therefore, consideration should be given to recommending supplementation with a complete multivitamin, calcium, and vitamin D as appropriate for patients receiving AOMs [(71)].
Fluid intake
The Adequate Intake level for fluids is 2.2 L/day for women and 3.0 L/day for men, and fluid requirements can increase further with physical activity or hot climates [(56)]. Emerging data suggest that GLP-1 receptor agonism may reduce thirst and fluid intake in rodents and in adults with primary polydipsia [(76, 77)]. Additional research is needed to understand whether this effect is also observed in adults with obesity who are treated with AOMs that activate the GLP-1 receptor. However, patients receiving AOMs should be encouraged to achieve target fluid intakes of >2 to 3 L/day. Ideally, target fluid intakes should be met with water, low-calorie beverages (such as unsweetened coffee or tea), or nutrient-dense beverages (such as low-fat dairy or soy alternatives). Limitation or avoidance of caffeine intake has been recommended during weight loss in light of the potential diuretic effect of high caffeine intake [(58, 71, 78)]. The Dietary Guidelines for Americans recommends that alcohol intake be limited to 1 drink/day for women or 2 drinks/day for men [(42)]. However, recent guidance suggests that carcinogenic effects and other risks associated with intake of alcohol occur with any amount, which suggests that there is no “safe” level of alcohol consumption [(79)].
OBESITY AND MALNUTRITION
Malnutrition is a broad term used to describe deficiencies, excesses, or imbalances in the intake of energy or nutrients along with the resulting physiological changes. Obesity increases the risk of certain forms of malnutrition. For example, individuals with obesity are frequently found to have biochemical evidence of deficiencies of micronutrients, including vitamin D, vitamin B12, folate, thiamine, iron, and zinc [(80-83)]. In addition, inadequate intakes of calcium, magnesium, and vitamins A, E, and C have been reported for patients with obesity [(73, 74, 83, 84)]. Data from bariatric surgical cohorts suggest that the prevalence of preexisting nutritional deficiencies is increasing in this patient population [(83)]. Micronutrient deficiencies can affect multiple organ systems, leading to fatigue, reduced physical function, impaired mood and cognition, immune dysfunction, and multiple other complications (Table 2).
Obesity can coexist not only with micronutrient deficiencies but also with sarcopenia, a reduction in skeletal muscle mass and strength most often associated with aging [(85)]. Sarcopenic obesity has been associated with reduced physical function and quality of life along with an increased risk of disability, as well as cardiometabolic complications [(86)]. In patients with sarcopenic obesity, weight-loss–associated reductions in muscle mass may exacerbate the condition. Incorporation of strategies to minimize the loss of muscle mass and to improve muscle function (such as adequate protein intake and resistance training) may improve outcomes for these patients during obesity treatment [(57)].
Micro- and macronutrient deficiencies may also occur with obesity treatments such as low-calorie diets, VLCDs, or bariatric surgery due to inadequate nutrient intake or impaired absorption, although dietary quality may drive nutrient intake more than the level of energy intake. Preventive actions and regular monitoring of patients can reduce the risk of medical and nutritional complications observed with treatment, and we can apply the knowledge gained in these settings to reduce these risks in patients who are treated with AOMs. For example, for patients considering bariatric surgery, nutritional assessment (including evaluation by a registered dietitian and screening laboratory tests) and treatment of preexisting micronutrient deficiencies are recommended [(58)]. Following bariatric surgery, routine vitamin and mineral supplementation, including a complete multivitamin, calcium, and vitamin D, is recommended along with regular assessment of nutritional status. Micronutrient malabsorption can occur after some types of bariatric surgery but is not expected during treatment with most AOMs. Therefore, the risk of developing a micronutrient deficiency may be lower in individuals treated with AOMs than in surgical patients, and appropriate monitoring, as discussed in the following section, may further reduce the risk. In adults, dietary supplementation with a complete multivitamin has been associated with a reduced risk of micronutrient deficiency, particularly for those with obesity [(87, 88)].
IDENTIFICATION OF PATIENTS AT INCREASED RISK OF MALNUTRITION PRIOR TO INITIATION OF AOM THERAPY
Given the frequent coexistence of obesity and malnutrition, an assessment of nutritional status should be performed prior to treatment with AOMs. Clinical assessment may include a 24-h or usual dietary recall and weight history. Evaluation of nutritional status may also include medical history and physical examination directed to an assessment of symptoms and signs of nutritional deficiencies (Tables 1 and 2), anthropometric measurements, body composition analysis, biochemical tests, and assessments of physical function such as grip strength. Increased risk of micronutrient deficiencies in obesity may be a result of consumption of nutrient-poor diets, including in the setting of food insecurity, and changes in the absorption, distribution, or metabolism of nutrients, resulting from obesity-associated systemic inflammation and disease [(84)].
Although obesity is generally associated with an increased risk of nutritional deficiencies, certain patients are at even greater risk of nutritional compromise. Identification of these individuals may help prevent more serious nutritional and medical complications that might occur with decreased food intake associated with AOMs. Patients with monotonous diets of poor quality are likely to be at higher risk, and dietary counseling by a registered dietitian may help improve dietary quality. Older adults are also at greater risk of nutritional complications [(42)]. Older adults may not meet recommended intakes of key nutrients such as protein and vitamin B12, and aging-associated changes in protein metabolism and vitamin B12 absorption may compound the issue [(42)]. In addition, older adults may have a greater risk of dehydration than younger individuals due to multiple factors, including aging-associated changes in thirst and decreased urinary concentrating capacity [(56, 89)]. Furthermore, older adults may be more prone to complications of dehydration, such as acute kidney injury [(89)]. Patients with comorbid diseases such as chronic kidney disease or heart failure or those on medications that impact nutrient metabolism or absorption (such as metformin or proton pump inhibitors) may also be at increased risk of nutritional complications during AOM therapy. Individuals who have undergone bariatric surgery are also a high-risk group. There is increasing attention on patients who experience poor postsurgical weight loss and/or weight regain, and treatment with AOMs may be considered in these situations [(58)]. However, given the impact of common bariatric procedures on micronutrient absorption and the high prevalence of postoperative nutritional deficiencies in bariatric surgery patients, regular nutritional monitoring is essential if treatment with AOMs is instituted. Additional research is needed to explore potential benefits and risks of AOMs in patients with binge-eating disorder [(90, 91)]. Treatment for binge-eating disorder should include behavioral counseling with a trained mental health professional and may also include approved pharmacotherapies such as lisdexamfetamine, which has demonstrated efficacy in this population [(92)]. AOMs are generally inappropriate for those with other eating disorders, including bulimia or anorexia nervosa [(71)].
Treatment of preexisting nutritional deficiencies prior to AOM initiation should be individualized based on the specific deficiencies identified, the severity of deficiencies, and the presence of comorbid diseases that may alter nutrient metabolism. For example, in one study documenting a high prevalence of micronutrient deficiencies among individuals with obesity prior to treatment, the consumption of a low-calorie formula diet containing >100% of the Dietary Reference Intake for micronutrients for 3 months was associated with inconsistent effects, including improvement in the status of some micronutrients but deterioration in others [(82)]. No studies, to our knowledge, have examined the impact of routine micronutrient supplementation on the risk of nutritional deficiencies with AOM therapy, but the study by Damms-Machado et al. from 2012 [82] suggests that supplementation with a complete multivitamin alone may not be adequate to correct all preexisting deficiencies, particularly under decreased food intake associated with AOMs. Therefore, for patients with micronutrient deficiencies identified before or during AOM therapy, correction of these deficiencies through targeted micronutrient supplementation may be necessary, even when a complete multivitamin is recommended.
IDENTIFICATION AND MANAGEMENT OF PATIENTS AT INCREASED RISK OF NUTRITIONAL COMPLICATIONS DURING AOM THERAPY
Treatment with AOMs typically leads to decreased appetite and food intake, resulting in weight reduction. Patients who experience gastrointestinal adverse events, greater appetite suppression, or excessive weight loss during AOM therapy may be at an increased risk of nutritional complications. This section includes recommendations for the identification and management of these specific issues during AOM therapy, which should be considered along with the nutritional recommendations proposed earlier in this manuscript and in Tables 1 and 2.
Therapy with AOMs that activate the GLP-1 receptor (such as liraglutide, semaglutide, and tirzepatide) is associated with an increased risk of gastrointestinal adverse events, including nausea, vomiting, diarrhea, dyspepsia, and constipation. Most of these events are mild or moderate, dose-dependent, and transient in nature [(22, 25)]. To limit these symptoms and reduce the risk of downstream nutritional complications, management strategies may include adjustments to medications and/or dietary intake. Slower AOM dose escalation or dose reduction can be employed on an individualized basis to mitigate these symptoms [(72)]. Attention should be paid to concomitant medications, such as metformin, which can increase the risk of gastrointestinal adverse events with AOMs [(93)]. Exacerbation of gastroesophageal reflux disease may be managed with short-term use of proton pump inhibitors or H2 receptor blockers, with the understanding that weight reduction often improves symptoms of gastroesophageal reflux disease. Dietary modification may also be helpful. Patients can be advised to consume smaller and more frequent meals, stop eating prior to feeling full, and avoid foods and beverages that may worsen gastrointestinal symptoms (e.g., high-fat foods, spicy foods, alcohol, and carbonated beverages) [(72)]. For constipation, strategies for management may include attention to fiber and fluid intake and use of osmotic or stimulant laxatives. Patients should be advised to reach out to their clinicians if gastrointestinal symptoms do not resolve with time, as expected, or if symptoms worsen.
Patients who experience greater appetite suppression or more severe gastrointestinal adverse events with treatment than is typical may be at increased risk of nutritional complications, as well as dehydration, acute kidney injury, and electrolyte imbalance. Individuals with significantly depressed appetite should be encouraged to consume fluids and small nutrient-rich meals more frequently, and dose adjustment should be considered if poor appetite prevents adequate intake of fluids or key nutrients such as protein or micronutrients. Early recognition and management of persistent or more severe gastrointestinal symptoms can enable clinicians to take preventive action to reduce the risk of complications. These actions may include AOM dose adjustment or cessation of AOM therapy, as well as medical management of gastrointestinal symptoms, regular reevaluation of fluid and nutritional status, short-term dietary supplementation (e.g., with a multivitamin, calcium, and vitamin D or micronutrient-enriched protein shakes), and referral to a registered dietitian for evaluation and dietary counseling. Consideration should also be given to adjustment of baseline medications such as antidiabetic medications, antihypertensives, and diuretics.
In rare cases, patients treated with AOMs may experience excessive weight loss [(18)]. Determination of whether a patient's weight loss is excessive should be individualized, patient-centered, and focused on health status. Clinically, weight loss may be excessive if it results in adverse health outcomes such as micronutrient deficiency; impairment of normal physiologic processes (e.g., amenorrhea), physical function, or psychological well-being; or a BMI < 18.5 kg/m2 or total body fat percentage < 10% for men and <15% for women [(18)]. In this situation, AOM dose reduction or temporary cessation of therapy may be indicated to allow for further evaluation and an appropriate amount of weight regain. Evaluation for secondary causes of weight loss (e.g., eating disorders, endocrine disease, gastrointestinal tract disorders, cancer, or other chronic disease) should be considered, particularly if cessation of AOM therapy does not result in weight stabilization and/or regain.
OTHER GENERAL CONSIDERATIONS FOR OBESITY MANAGEMENT WITH AOMs
A common misconception is that slower weight loss leads to greater long-term health benefits, including smaller loss of lean mass, better preservation of energy expenditure, and greater maintenance of reduced body weight over time, compared to more rapid weight loss. However, if individuals meet key lifestyle goals (such as sufficient protein intake and physical activity), the rate of weight loss may not significantly impact long-term changes in body composition, energy expenditure, or cardiometabolic factors [(94-97)]. Weight reduction, regardless of rate, typically leads to a reduction in lean mass as well as fat mass, and these effects are typically proportional to the amount of weight reduction achieved [(95, 98)]. However, the loss of muscle or bone mass may be particularly undesirable for some individuals such as adults with sarcopenic obesity or those with risk factors for osteoporosis [(97)]. In these individuals, body composition assessment and measures to limit muscle or bone loss should be considered during AOM therapy.
People with obesity frequently experience weight bias and stigma, which can negatively impact health behaviors and outcomes. The experience of weight stigma increases the risk of psychological distress (e.g., depression and anxiety), disordered eating patterns, avoidance of physical activity, and physiological stress (evidenced by elevated levels of cortisol and inflammatory markers), all of which can hinder weight-management efforts [(99)]. Individuals may internalize a message of weight bias, and this can negatively impact weight and health. Clinicians are encouraged to recognize weight bias in health care and work to reduce its impact in order to optimize clinical care for patients with obesity [(100)].
Patients should be monitored for the emergence or worsening of depression, suicidal ideation, or other psychopathologies. Patients who achieve significant weight reduction may experience unexpected changes in the dynamics of their relationships with others, which can sometimes be distressing [(18)]. For example, friends or family members may not be supportive of patients' lifestyle changes if they impact shared activities and time together. Patients may be uncomfortable with the attention that they receive after weight reduction, particularly if they have a history of trauma or abuse. Clinicians should be sensitive to patients' social and emotional needs and provide this support or refer patients for help with coping strategies [(18)]. Opportunities for patient support include support groups, mobile applications (apps), or referral to a qualified mental health professional.
Patients may report other concerns associated with dramatic weight reduction or lifestyle changes, such as excess skin, hair thinning, or perceptions of a healthy amount of weight reduction as excessive. If the patient has had good health outcomes (including improvement in the risk of obesity-associated complications, preserved or improved physical function, and good nutritional status), the clinician can provide reassurance and treatments aimed at addressing concerns. For example, a patient bothered by excess skin may benefit from a referral to a plastic surgeon for consideration of panniculectomy after a period of maintenance at goal weight. A patient concerned about hair loss may be reassured that increased hair shedding is common with sustained negative energy balance and usually resolves after weight stability. Appropriate evaluation should first be conducted to rule out reversible nutritional causes of hair loss, such as inadequate protein intake or iron deficiency. Ultimately, the weight target should be patient-centered, and it should be clear to the patient that the goal of treatment does not need to be achievement of a “normal” BMI (i.e., BMI < 25 kg/m2).
CONCLUSION
Recently approved and emerging AOMs represent a significant advancement in the treatment of obesity, facilitating improvement in cardiometabolic risk factors and physical function along with average body weight reductions of ≥15%, which were previously seen only with intensive interventions such as bariatric surgery. Nutritional assessment and counseling of patients treated with AOMs can facilitate the identification and management of preexisting risk factors for malnutrition and can provide patients with goals for the intake of key nutrients including protein, dietary fiber, micronutrients, and fluid. Ongoing monitoring during treatment can enable early recognition and management of any concerns that emerge, including inadequate nutrient intake, gastrointestinal symptoms, or mood disorders. Current data to guide nutritional recommendations for patients treated with AOMs are limited. For this reason, the nutritional recommendations proposed in this review include evidence from general population health, bariatric surgery, and VLCDs. Additional research can help inform best practices to promote optimal nutritional and medical outcomes in patients treated with AOMs.
AUTHOR CONTRIBUTIONS
Jaime P. Almandoz, Colleen Tewksbury, Caroline M. Apovian, Angela Fitch, Zhaoping Li, Jesse Richards, Kadie S. Vanderman, and Lisa M. Neff were involved with the design of the work. All authors were involved with data collection or interpretation. Irina Jouravskaya, Kadie S. Vanderman, and Lisa M. Neff drafted the manuscript, and all other authors provided critical revision of the manuscript for important intellectual content. All authors were involved in writing the manuscript and provided final approval of the submitted and published versions.
ACKNOWLEDGMENTS
The authors would like to thank Raena Fernandes, MSC, ELS, for editorial assistance.
FUNDING INFORMATION
This work was supported by Eli Lilly and Company. Writing assistance was provided by Syneos Health, supported by Eli Lilly and Company.
CONFLICT OF INTEREST STATEMENT
Jaime P. Almandoz has received consulting fees from Boehringer Ingelheim, Eli Lilly and Company, and Novo Nordisk A/S; received payment or honoraria for lectures from Clinical Care Options, the Institute for Medical and Nursing Education, and PeerView; and served in a leadership or fiduciary role with The Obesity Society Governing Board. Thomas A. Wadden has received consulting fees from Novo Nordisk A/S and WW International, Inc. (formerly Weight Watchers). Colleen Tewksbury has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from the Academy of Nutrition and Dietetics and the Commission on Dietetic Registration; received support for attending meetings and/or travel from the Academy of Nutrition and Dietetics; served in a leadership or fiduciary role for the Academy of Nutrition and Dietetics Weight Management Dietetic Practice Group Executive Committee; and served as a spokesperson for the Academy of Nutrition and Dietetics. Caroline M. Apovian has received institutional grants from GI Dynamics Inc. (now Morphic Medical), Novo Nordisk A/S, and the Patient-Centered Outcomes Research Institute; received consulting fees from Cowen and Company, LLC; received payment or honoraria for lectures from Rhythm Pharmaceuticals, Inc.; participated on advisory boards for Altimmune, CinFina Pharma, Currax Pharmaceuticals, EPG Communication Holdings, Form Health, L-Nutra, NeuroBo Pharmaceuticals, Inc., Novo Nordisk A/S, PainScript, Palatin Technologies, Inc., Pursuit By You, ReShape Lifesciences, Inc., Riverview School, and Roman Health Ventures Inc.; served in a leadership or fiduciary role with the World Obesity Federation; and received stock or stock options from Gelesis and Xeno Biosciences. Angela Fitch has received consulting fees from Eli Lilly and Company, Jenny Craig, Novo Nordisk A/S, Sidekick Health, and Vivus; received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Novo Nordisk A/S; received payment for expert testimony from the state of Massachusetts; received support for attending meetings and/or travel from the Obesity Medicine Association and Pfizer Inc.; served in a leadership or fiduciary role with the Obesity Medicine Association; and received stock or stock options from Eli Lilly and Company and Novo Nordisk A/S. Jamy D. Ard has received grants or contracts from Boehringer Ingelheim, Eli Lilly and Company, Epitomee, KVK Tech, Nestlé Health Science, UnitedHealth Group R&D, and WW International Inc.; received consulting fees from Brightseed, Eli Lilly and Company, Intuitive, Level2, Nestlé Health Science, Novo Nordisk A/S, OptumLabs R&D, Regeneron Pharmaceuticals, Inc., Spoke Health, and WW International Inc.; served in a leadership or fiduciary role for The Obesity Society and American Society for Nutrition Foundation; and received equipment, materials, drugs, medical writing, gifts, or other services from KVK Tech, Nestlé Health Science, and WW International Inc. Zhaoping Li has served on advisory boards for Abbott Laboratories. Jesse Richards has received grants or contracts from speakers bureaus for Eli Lilly and Company; received payment or honoraria for lectures from speakers bureaus for Novo Nordisk A/S and Rhythm Pharmaceuticals, Inc.; and served on an advisory board for Rhythm Pharmaceuticals, Inc. W. Scott Butsch has received grants from Eli Lilly and Company; consulting fees from Novo Nordisk A/S; payment from Med Learning Group and Potomac Center for Medical Education; and served on advisory boards for Abbott Laboratories, Eli Lilly and Company, Medscape, and Alfie Health. Irina Jouravskaya is an employee of Eli Lilly and Company. Kadie S. Vanderman is an employee of Syneos Health. Lisa M. Neff is an employee and stockholder of Eli Lilly and Company; has received grants or contracts from Aegerion Pharmaceuticals Inc.; and has served in a leadership or fiduciary role with Current Developments in Nutrition (journal) and the National Board of Physician Nutrition Specialists.





