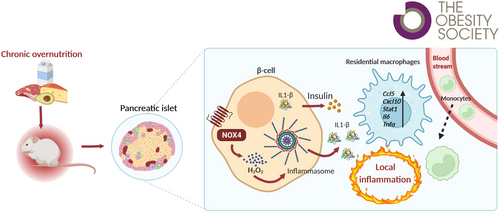
NADPH oxidase 4 in mouse β cells participates in inflammation on chronic nutrient overload
Abstract
Objective
By exposing mice carrying a deletion of NADPH oxidase isoform 4, NOX4, specifically in pancreatic β cells (βNOX4−/−) to nutrient excess stimulated by a high-fat diet (HFD), this study aimed to elucidate the role of β-cell redox status in the development of meta-inflammation within the diabetic phenotype.
Methods
The authors performed basic phenotyping of βNOX4−/− mice on HFD involving insulin and glycemic analyses, histochemistry of adipocytes, indirect calorimetry, and cytokine analyses. To characterize local inflammation, the study used caspase-1 activity assay, interleukin-1β immunochemistry, and real-time polymerase chain reaction during coculturing of β cells with macrophages.
Results
The phenotype of βNOX4−/− mice on HFD was not associated with hyperinsulinemia and hyperglycemia but showed accumulation of excessive lipids in epididymal fat and β cells. Surprisingly, mice showed significantly reduced systemic inflammation. Decreased interleukin-1β protein levels and downregulated NLRP3-inflammasome activity were observed on chronic glucose overload in βNOX4−/− isolated islets and NOX4-silenced INS1-E cells resulting in attenuated proinflammatory polarization of macrophages/monocytes in vitro and in situ and reduced local islet inflammation.
Conclusions
Experimental evidence suggests that NOX4 pro-oxidant activity in β cells is involved in NLRP3-inflammasome activation during chronic nutrient overload and participates in local inflammatory signaling and perhaps toward peripheral tissues, contributing to a diabetic inflammatory phenotype.
Study Importance
What is already known?
- Redox signaling is involved in insulin secretion upon glucose induction in pancreatic β cells (NADPH oxidase 4 [NOX4] is the major player), whereas oxidative stress in β cells leads to the development of type 2 diabetes.
What does this study add?
- Sustained activation of NOX4 in β cells by chronic overnutrition induces the inflammasome and IL-1β production, leading to macrophage activation and local inflammation. Thus, βNOX4-/- mice do not develop chronic inflammation on high-fat diet, suggesting the role of sustained NOX4 activation in diabetes development.
How might these results change the direction of research or the focus of clinical practice?
- Redox regulation in β cells is an important player in their physiology and NOX4 might be a potential pharmacological target during chronic nutritional overload.
INTRODUCTION
Pancreatic β cells require redox signaling for proper function, including embryonic development, postnatal maturation [(1, 2)], and insulin secretion [(3, 4)]. We have recently shown that cytosolic NADPH oxidase 4 (NOX4)-derived H2O2 signaling is essential for efficient insulin secretion during glucose stimulation [(5)]. Moreover, glucose induction decreases mitochondrial matrix reactive oxygen species (ROS) [(6)]. This indicates the important regulatory role of redox signaling in β cells. In chronic nutrient overload, redox homeostasis enters a more pro-oxidant deleterious state, leading to activation of stress-induced signaling pathways and stress of endoplasmic reticulum, resulting in β-cell dedifferentiation and dysfunction and the development of insulin resistance in peripheral tissues, all leading to the development of type 2 diabetes (T2D) [(7, 8)]. NOX4 is constitutively expressed in β cells, and its activity is induced by glucose metabolism [(5)]. However, its role in the development of T2D, that is, chronic metabolic overload of β cells, is unknown.
In addition, β cells also express NOX2, which is activated under pathological conditions by increased saturated fatty acids, calcium influx, hypoxia, or protein kinase C (PKC) signaling and further impairs insulin secretion [(9, 10)]. Note that NOX2 has no effect on immediate glucose-stimulated insulin secretion (GSIS) [(5)]. The progression of the diabetic phenotype can be mimicked in C57BL/6J mice by feeding them a high-fat diet (HFD) for at least 8 weeks, during which features such as hyperinsulinemia, hyperglycemia, hypertension, insulin resistance, and inflammation develop [(11)]. Insulin resistance then primarily affects white adipose tissue (WAT), muscle, and liver, accelerating lipolysis and hyperglycemia with excessive release of glucose from the liver. The altered metabolic state of peripheral tissues activates resident and infiltrated immune cells and creates a chronic low-grade pro-inflammatory environment known as meta-inflammation [(12)].
The destruction of β cells is associated with internal oxidative stress [(7, 8)]. β cells contain relatively low levels of redox buffers, such as glutathione, making them vulnerable in the pro-oxidant environment [(13)]. Consequently, concomitant chronic inflammation is the major player in the pathology of T2D. Proinflammatory cytokines, interleukin (IL)-1β, IL-6, monocyte chemoattractant protein1 (MCP1), and tumor necrosis factor α (TNFα), were detected in the blood of HFD-treated mice and patients with T2D, suggesting meta-inflammation [(14)]. c-Jun-N terminal kinase (JNK) and nuclear factor-κB (NF-κB) are involved in the expression of proinflammatory cytokines [(15)]. The β cells themselves are involved along with WAT as the main source and target of inflammatory processes along with an altered gut microbiota [(16)]. Glucolipotoxicity leads to proinflammatory cytokine/chemokine production inside and outside the islets through activation of resident macrophages and infiltration of islets by circulating monocytes that differentiate into M1-like proinflammatory macrophages [(17)]. Moreover, the depletion of macrophages with clodronate improved glucose tolerance and insulin secretion [(18)]. Certain cytokines, such as IL-1β and IL-18, require processing by an inflammasome, the assembly of which is associated with a pro-oxidant intracellular redox status in macrophages. ROS activates the mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 (MAPK/ERK1/2) pathway for inflammasome assembly and caspase-1 activity, which then enables the maturation of the previous cytokines [(19)]. Thioredoxin-interacting protein may be involved in this signaling to activate the inflammasome [(20)]. In addition to immune cells, β cells and their pro-oxidative status induced by chronic stress conditions could also serve as triggers for local proinflammatory development, which could eventually contribute to diabetic meta-inflammation. The question arises as to the role of NOX4 in β cells in this redox switch, that is, in the transition from physiological transient redox signaling to the permanent pro-oxidative state underlying oxidative stress.
We are interested in the role of NOX4 in β cells in the development of the diabetic phenotype after nutrient overload and its possible involvement in the formation of the NLR family pyrin domain containing 3 (NLRP3)-inflammasome. By exposing βNOX4−/− mice, that is, mice with specifically ablated NOX4 in β cells, to nutrient excess simulated by HFD, we aimed to experimentally distinguish which scenario has a greater impact: (i) an impaired GSIS that accelerates the establishment of the diabetic phenotype or (ii) a more reduced intracellular status of β cells that protects mice from the diabetic phenotype and inflammasome formation. The first option is supported by evidence that NOX4-full knockout mice in HFD tend to have diet-induced obesity and insulin resistance [(21)]. The effect of NOX4 is attributed to hypertrophy, hypoxia, and apoptosis of adipocytes. The latter option has been favored based on recent results using NOX inhibitors showing that NOX4 may be involved in stress-induced islet cell death in human islets in vivo and that a NOX2/4 inhibitor can counteract glucose intolerance in HFD-treated mice [(22)]. However, the interpretation of these results is complicated by the insufficient specificity of the inhibitors and their global effects. Several isoforms of NOX, including NOX4, have been shown to be involved in the activation of the effective immune cell response [(23)]. Therefore, NOX inhibitors could suppress the immune system, and the effect on β cells would therefore be indirect.
Here, we present results characterizing the role of NOX4 in pancreatic β cells in inflammation as a part of the diabetic phenotype on HFD treatment. Our results show that NOX4 in pancreatic β cells is involved in the development of chronic local inflammation caused by excessive nutrient intake.
METHODS
Unless specified otherwise, chemicals were purchased from Sigma-Aldrich (St Louis, Missouri).
Cell cultures and transfection
Rat insulinoma cell line-1E (INS-1E) cells (AddexBio, San Diego, California) were cultured as described in [(5)]. Rat NR8383 alveolar macrophages (ATCC CRL-2192) were cultured according to the manufacturer's instructions. Treatment with lipopolysaccharide (LPS) (2 μg/mL) and nigericin (20 μM) was performed for 6 and 3 h, respectively. Peripheral blood mononuclear blood cells (PBMC) were isolated with SepMate tubes according to the instructions (STEMCELL Technologies, Vancouver, Canada), and adherent monocytes were used for analyses. The macrophage line or isolated monocytes were cocultured with INS-1E cells for 6 h after hyperglycemic treatment. INS-1E cells were transfected with Nox4 short interfering RNA and CMV-CAT/SV40-CAT vectors (kindly provided by Professor S. Lenzen and Dr. C. Glorieux, Professor J.B. Verrax) for catalase overproduction as in [(5)] and transfection efficiency was checked for each experiment (Figure S4A). For NOX4 inhibition, INS-1E cells were incubated with 10 μM GKT137831 (Cayman Chemicals, Michigan) for 72 h.
Mouse model
Adult mice (12–16 weeks) from control mice expressing either NOX4 floxed alleles (designated wild-type [WT]) and RIP-Cre alleles (B6.Cg-Tg(Ins2-cre)25Mgn/J) (designated RIP-Cre) versus mice with ablated NOX4 specifically in β cells (B6.Cg-Nox4<tm1Ams> Tg[Ins2-cre]25Mgn) (designated βNOX4−/−) as described in [(6)] were used for experiments. Mice were kept under standard conditions (12/12 light/dark cycle, 22°C, 55% humidity) with free access to water and chow (Altromin 1314 Forti, Lage, Germany) or HFD (Sniff, E15742-34, Soest, Germany). Chow/normal diet (ND) facilitates 59% carbohydrates, 27% protein, and 14% fat with metabolized energy of 13.7 MJ/kg whereas HFD facilitates 22% carbohydrates, 24% protein, and 54% fat with metabolized energy of 24 MJ/kg. Unless otherwise stated, both sexes were used in all experiments. All animal experiments were ethically reviewed and performed in accordance with European Directive 86/609/EEC and approved by the Czech Central Commission for Animal Welfare.
Insulin and glycemic analysis
For the intraperitoneal glucose tolerance test, animals were fasted overnight (16–18 h) and given water ad libitum. Fasting blood glucose levels were measured (T = 0) using a glucometer (Roche, Basel, Switzerland) in blood collected from the tail vein. Two g of glucose in phosphate-buffered saline (PBS)/kg body weight was injected intraperitoneally. Blood glucose levels were measured at 5, 10, 15, 20, 60, and 120 min after glucose administration. Insulin was quantified from blood serum using an ultrasensitive enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden) as described in [(5)].
Biochemistry of blood serum
Blood samples were collected from isoflurane-anesthetized mice by retro-bulbar sinus puncture into ethylenediaminetetraacetic acid (EDTA)-coated tubes. Plasma biochemical parameters (serum amyloid P component (SAP), high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides) were measured using a Beckman AU480 analyzer (Beckman Coulter, Brea). Triglycerides from islet lysates (PBS + TritonX100) were determined using a kit (TRO100, Sigma) according to the manufacturer's instructions.
Histochemistry of adipocytes and immunohistochemistry of pancreatic slices
Mouse white adipose tissue (WAT) and pancreas were fixed overnight in formalin (4°C) and converted to paraffin. They were sliced (40 μm) onto glass slides using a microtome. After deparaffinization and rehydration, visceral WAT sections were stained with Gill's hematoxylin and eosin. The slides were preserved with Pertex embedding medium. Adipocyte size was analyzed in ImageJ (Rasband, W.S., NIH, USA). Pancreatic slices were stained with primary F4/80 and secondary anti-rat donkey Alexa-488 antibodies (both Invitrogen, Waltham, Massachusetts). The pancreas of mice treated with streptozotocin served as a positive control for pancreatic islet (PI) inflammation. Visualization was performed using the Leica SP8 AOBS WLL MP confocal microscope (Wetzlar, Germany) and Leica LAS AF Lite software.
Isolation of islets and real-time polymerase chain reaction analysis from islets and cells
Pancreatic islets (PIs) were isolated as described in [(22)]. Messenger RNA (mRNA) was immediately isolated using a RNeasy Kit (Qiagen, Hilden, Germany). Five hundred ng of total RNA was transcribed using a GrandScript cDNA Synthesis KIT (Tataabiocenter, Göteborg, Sweden). Real-time polymerase chain reaction (RT-PCR) was performed in a CFX Real-Time PCR Detection System (Bio-Rad, Hercules) using EvaGreen Mastermix (Biotium, Feromont). The amount of relative expression was calculated from the crossing points of each run using the Livak method. All sequences of the primers are listed in Tables S2 and S3.
Quantification of specific cytokines, nuclear factor erythroid 2-related factor 2, and IL-1β
Selected cytokines were analyzed from blood plasma. A Bio-Plex 200 suspension array system (Bio-Rad) was used for multiplex analysis. In parallel, cytokines were detected by immunochemistry with specific antibodies, namely IL-10 (sc-7888), IL-1β (sc-7884), TNFα (sc-52,746), IL-6 (sc-7920), and nuclear factor erythroid 2-related factor 2 (NRF2) (sc-722, all from Santa Cruz Biotechnology, Dallas, Texas). IL-1β protein was quantified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot, using anti-IL-1β (ab9722, Abcam, Cambridge, UK). Coomassie staining intensity and β-actin immunochemistry (A1978, Sigma-Aldrich, St Louis, Missouri) were used for normalization.
Analysis of inflammasome activity
Inflammasome activity was expressed by caspase-1 activity analysis using the Caspase-Glo 1 Inflammasome Assay (Promega, Madison, Wisconsin) and performed according to the manufacturer's protocol.
Statistical analysis
All experiments were performed at least in duplicate and repeated at least three times. Data are expressed as means with SDs. ANOVA with Tukey or Sidak tests on the prevalidated data by a normality test or Student t tests (two samples) were used for statistical analyses with GraphPad Prism (San Diego, California). Differences were accepted as statistically significant for p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
RESULTS
βNOX4−/− mice exhibit impaired insulin secretion, ectopic fat accumulation, and reduced innate immune response under HFD
We have previously shown that βNOX4−/− mice have impaired GSIS and develop impaired glucose tolerance and insulin resistance irrespective of gender in adults [(5)]. When HFD was administered for 8 weeks, WT adult (16 weeks) mice showed significant upregulation of insulin secretion, whereas βNOX4−/− mice showed suppressed insulin secretion, similar to the results of ND (Figure 1A). The low insulin secretion of βNOX4−/− mice during glucose induction corresponded to high glycemic values at 60 min (Figure 1B). Interestingly, fasting insulin levels in adult βNOX4−/− mice were comparable to WT under both nutrient conditions (Figure S1A). As expected, WT mice became hyperinsulinemic under HFD (Figure S1A). Fasting glycemia of WT and βNOX4−/− mice was higher under HFD than that of ND, but without statistical significance between strains (Figure S1B). Eight weeks of HFD also induced fat accumulation in both strains, with increased fat-to-muscle volume ratio (Figure S1D), epidydimal fat weight (Figure 1C), and adipocyte hypertrophy (Figure 1C) observed in βNOX4−/− mice on HFD and ND compared with WT mice and RIP-Cre mice. Increased ectopic fat accumulation was observed in PIs of βNOX4−/− mice under both diets, as represented by quantification of triglycerides and increased expression of acetyl-CoA synthetase long-chain family member 3 (Acsl3) in β cells, indicating enhanced de novo lipogenesis (Figure 1D). Because of the inadequate GSIS of βNOX4−/− mice and their impaired ability to respond to a nutrient stimulus, they were unable to reduce blood glucose to normal levels within the time period studied under either dietary condition [(5)], which corresponded with the reduced food intake (Figure S1E) and feeding time observed by indirect calorimetry (Figure S1G). These data were also in hand with serum leptin levels, which were significantly increased in βNOX4−/− mice under both dietary conditions as well as in WT mice under HFD (Figure S1F). Meta-inflammation is commonly associated with HFD-induced obesity. Indeed, analysis of WT and RIP-Cre mice on HFD showed a significantly increased acute proinflammatory marker SAP, whereas no increase was observed in βNOX4−/− mice (Figure 1E). There were no significant differences between genders (Figure S1C).
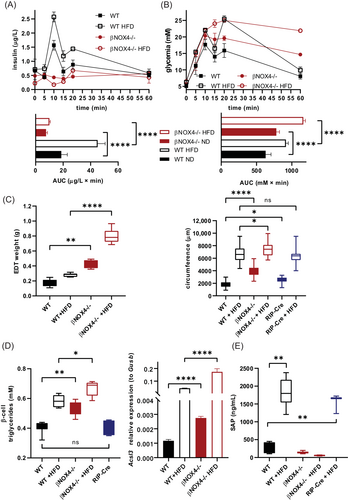
βNOX4−/− mice are less proinflammatory on HFD
Detailed analysis of selective proinflammatory markers in blood serum (IL-6, IL-17, interferon [IFN]γ, TNFα, and IL-1β) confirmed reduced inflammation in βNOX4−/− mice after 8 weeks of HFD (Figure 2A). In contrast, the anti-inflammatory IL-10 was increased in βNOX4−/− mice on HFD (Figure 2A). Immunochemical analysis (not shown) was validated by multiplex ELISA (Figure 2A) using different sets of antibodies. We also quantified NRF2 in blood serum as another inflammatory marker. In addition to its role in supporting the expression of antioxidant defense proteins in cells, NRF2 promoted the production of anti-inflammatory Th2 cytokines (IL-4, IL-13) compared with proinflammatory Th1 cytokines [(24)]. Indeed, the expression of NRF2 increased in βNOX4−/− mice after 8 weeks of HFD (Figure 2B). The lower inflammatory state of βNOX4−/− mice on HFD might be indirectly supported by the significantly increased HDL/LDL ratio (Figure 2C) because HDL has been shown to have anti-inflammatory properties [(25)].
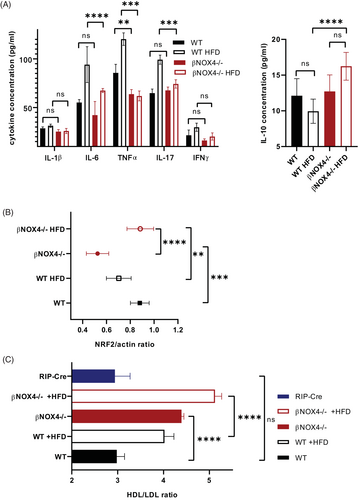
NOX4 elimination in β cells reduces inflammasome formation and production of IL-1β under chronic hyperglycemia
Because systemic inflammation and PI inflammation are closely related, we focused our attention on pancreatic β cells. β cells produce the proinflammatory cytokine IL-1β [(26)], which requires the formation of the NLRP3-inflammasome for its maturation. Moreover, β cells express exceptionally high levels of its receptor, making IL-1β particularly important in PI inflammation [(26)]. We found lower IL-1β intracellular protein levels in INS-1E cells with silenced NOX4 and PIs from βNOX4−/− mice under simulated hyperglycemic conditions (Figure 3A,B). Note that the number of α/β cells in PIs is not significantly different between WT and βNOX4−/− islets (Figure S2A). HFD causes increased number of apoptotic cells both in WT and βNOX4−/− islets (Figure S2B), and proliferation is minor (not shown). Hyperglycemia is relevant to mimic HFD conditions, because glucose is the main energy and regulatory stimulator (Figure S3A,B) whereas fatty acids enhance insulin secretion (Figure S3C). There is no significant change in IL-1β protein content when treated with fatty acids compared with the hyperglycemic condition only (Figure S3D). Moreover, glucose stimulates NOX4 expression for up to 120 min, whereas no further increase in gene expression was observed under hyperglycemic cultivation (Figure S2C). NOX2 expression also does not change under long-term hyperglycemic conditions (Figure S2C). However, cultivation of PIs/INS1E in hyperglycemic conditions induces ROS production but not in NOX4 ablated cells (Figure S2D). This corresponded to increased expression of selected antioxidant genes in PIs on hyperglycemic treatment (Figure S2E) and increased activity of NOX4 (Figure S2F). By applying the NOX1/4 inhibitor GKT137831 to control INS-1E cells, we also observed a reduction in IL-1β protein compared with positive control (LPS/nigericin treated monocytes/macrophages) (Figure 3A). Quantification of the expression of Nlrp3, a key protein unit of the inflammasome, showed that its expression was increased in control/scrambled INS-1E cells in the presence of high glucose compared with NOX4-silenced cells (Figure 3D). In addition, determination of NLRP3-inflammasome function by caspase-1 specific activity on chronic hyperglycemia showed its reduction in NOX4-silenced INS-1E cells and PIs of βNOX4−/− mice (Figure 3E,F). Pro-oxidative intracellular status is important for inflammasome assembly. Therefore, we investigated whether the lack of H2O2 production by NOX4 is the factor for the reduced NLRP3-inflammasome assembly in NOX4-silenced cells. Overexpression of catalase in INS-1E cells showed the decreasing trend of IL-1β protein levels with increasing catalase expression under chronic hyperglycemia (Figure 3C) and the low activity of the NLRP3-inflammasome represented by caspase-1 activity (Figure 3E).
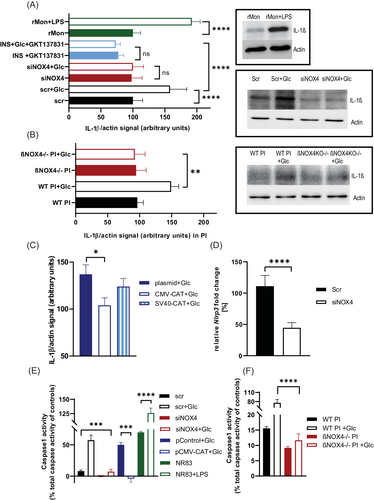
Cocultivation of NOX4-silenced INS-1E cells with rat monocytes/macrophages in hyperglycemic conditions suppresses their polarization toward inflammation
To understand whether IL-1β secreted by β cells during chronic nutrient overload can polarize macrophages toward proinflammatory phenotype, we cocultured control/scrambled and NOX4-silenced INS-1E cells incubated at high glucose (25 mM) for 72 h with either alveolar rat macrophages (NR8383) or monocytes isolated from rat blood. After 6 h of coincubation, we examined the expression profile (mRNA) of macrophage/monocyte differentiation using markers typical of an M1-like phenotype (Tnfα, Il-6, C-C motif chemokine ligand 5/Ccl5, C-X-C motif chemokine ligand 10 10/Cxcl10, signal transducer and activator of transcription 1/Stat1) or an M2-like phenotype (Il-10, Il-4, transforming growth factor beta/Tgfβ, arginase 1/Arg1, Ccl17, Stat6). We confirmed that NOX4-silenced INS-1E cells cocultured with alveolar rat macrophages or rat blood monocytes suppressed their expression of proinflammatory markers compared with control/scrambled cells (Figure 4A,B). As a positive control of proinflammatory polarization, we used rat alveolar macrophages/blood monocytes activated by LPS/nigericin for 6 h, and indeed, we observed a significant induction of proinflammatory gene expression (Figure 4C and Figure S4B). Involvement of the pro-oxidative intracellular status of β cells on hyperglycemic conditions (48 h) in the polarization of macrophages was documented by suppression of their proinflammatory differentiation on reduction of ROS production by catalase overexpression (Figure 4D). Analysis of coculturing NOX4-silenced INS-1E cells with alveolar rat macrophages under nonstimulating glucose conditions, that is, normoglycemia (11 mM glucose), showed no significant changes in mRNA expression of M1-like proinflammatory genes compared with control/scrambled cells, whereas expression of M2-like anti-inflammatory genes was slightly increased but not significant, compared with hyperglycemic conditions (Figure 4E). Note that overall expression of proinflammatory genes was lower in normoglycemic conditions in control and NOX4-silenced INS-1E cells. We confirmed that LPS activation also induced Nlrp3 expression in rat alveolar macrophages (Figure 4F), whereas activation of alveolar rat macrophages or rat blood monocytes was partially suppressed when cocultured with NOX4-silenced INS-1E cells (Figure 4F). INS-1E cells are thus able to polarize immune cells in their immediate environment toward inflammation under hyperglycemic conditions.
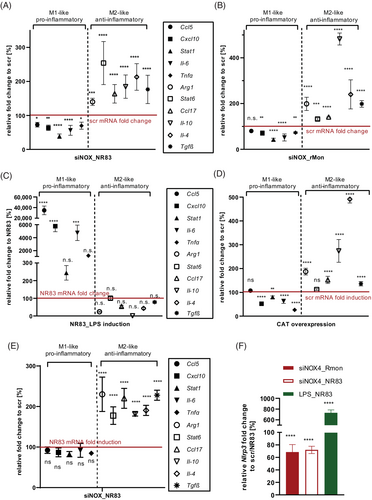
To analyze the presence of resident macrophages in PIs in vivo, we performed immunohistochemistry on pancreatic sections from WT and βNOX4−/− mice (16 weeks) after 8 weeks of HFD treatment with F4/80 antibody, the marker for mature resident macrophages. Interestingly, we observed macrophages mainly in WT PIs on HFD, whereas βNOX4−/− PIs on HFD had few of them, just like both strains on ND (Figure 5A). These data suggest that pancreatic β cells are capable of triggering local inflammation during chronic overnutrition and that the redox status of β cells is involved in this signaling.
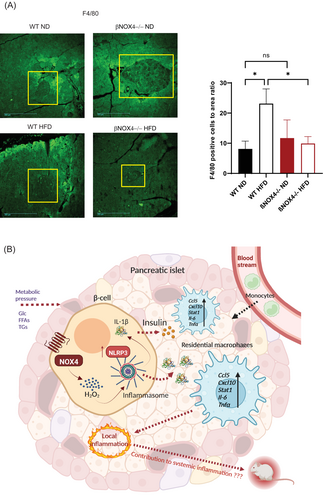
DISCUSSION
Our results show that chronic nutrient overload/hyperglycemia causes a redox shift toward pro-oxidation through sustained NOX4 activity, leading to long-term production and secretion of the mature cytokine IL-1β, which can enhance a proinflammatory environment in PIs (Figure 5B). Thus, β cells are directly involved in local inflammation when overloaded by diet, that is, the condition associated with the development of T2D.
Phenotypic characterization of the βNOX4−/− mouse model on HFD revealed an altered phenotype of diabetic features compared with WT animals (summary in Table S1). Exposure of βNOX4−/− mice to HFD impaired GSIS, similar to ND [(5)], and suppressed the hyperinsulinemia that otherwise developed in WT mice on HFD. Surprisingly, low insulin secretion in βNOX4−/− mice did not result in hyperglycemia similar to ND conditions. Apparently, βNOX4−/− mice adjusted their feeding regimen to maintain normoglycemia and consequently consumed less food on both normal and high-fat feeding regimens.
One of the main features of the diabetic phenotype under HFD is fat accumulation caused by dysregulated lipid metabolism (more in [(27)]). βNOX4−/− mice showed significantly increased lipid accumulation, not only in the epidydimal region but also ectopically in β cells, compared with WT animals. However, lipid accumulation might be directly related to NOX4 ablation, as it has already been observed in the conditions of ND. This lipogenic dysregulation could be the consequence of impaired insulin signaling and peripheral tissue resistance observed previously on ND [(5)] and exacerbated by HFD feeding. An altered redox environment caused by NOX4 deficiency in β cells and altered glucose metabolism and sensing could affect triglyceride metabolism, because the expression of specific genes and transcription factors driving triglyceride synthesis is regulated by glucose-responsive signaling factors [(28)]. Furthermore, triglyceride accumulation typically impairs GSIS [(29)]. Feeding behavior leading to increased fat accumulation may also be regulated by autonomic signaling in the brain of βNOX4−/− mice because NOX4 ablation was generated using RIP-Cre mice expressing Cre recombinase downstream of the Ins2 promoter and both Ins2 and Nox4 are expressed in certain brain cells [(30, 31)]. However, dysregulation of NOX in the central nervous system has been implicated in some neurodegenerative disorders and neurovascular diseases (more in [(32)]). Such results raise the question of whether the used mouse model is appropriate for adiposity studies. Nevertheless, the results of fat accumulation in RIP-Cre mice showed no significant effect of the RIP-Cre construct on fat accumulation and, most importantly, no effect on inflammation on HFD. Nevertheless, the issue of increased adiposity in βNOX4−/− mice requires further investigation involving neuronal activity. Similarly, further studies are required to show that an increased HDL/LDL ratio in βNOX4−/− mice under both dietary conditions might have a beneficial effect on cardiovascular disease risk.
Another important feature of the diabetic phenotype, low-grade chronic inflammation, was significantly suppressed in βNOX4−/− mice, as documented by decreased serum SAP, IL-6, IL-17, TNFα, IL-1β, and IFNγ, along with slightly increased anti-inflammatory IL-10 and upregulated NRF2. SAP expression was shown to be regulated by IL-1, TNFα, and IL-6 [(33)]. Low-grade inflammation was indirectly supported by an increased serum HDL/LDL ratio, because HDL has potent anti-inflammatory properties [(34)]. It upregulates activating transcription factor 2 (ATF3) in macrophages, thereby downregulating the expression of TLR-induced proinflammatory cytokines (IL-6, IL-12b, IFNγ, etc.). Systemic inflammation often accompanies increased adiposity and altered gut microbiota, but it is obvious that in βNOX4−/− mice this is not the case. Further investigation is required to explain these features. As the relationship between systemic inflammation and local tissue inflammation is complex and unknown, we focused on the effects of NOX4 deletion in β cells on local inflammation. We analyzed IL-1β production in β cells under nutritional stress conditions, as this cytokine has been shown to trigger the initiation and propagation of the inflammatory signal through the recruitment and reprogramming of myeloid cells [(35)]. IL-1β has been identified as an important regulator of β-cell physiology, as β cells have the highest expression of the IL-1 receptor of all tissues [(36)]. Treatment with an IL-1β-specific antibody or with a IL-1 receptor antagonist reduced islet infiltration by CD45+ immune cells or CD68+, MHCII+, and CD53+ immune cells [(37)]. In contrast, postprandial induction of circulating IL-1β potentiates insulin secretion [(38)]. Therefore, IL-1β is thought to be a master regulator and enhancer of immunological responses in addition to its effect on glucose homeostasis [(38)].
Our data quantifying IL-1β production in INS-1E cells and PIs under chronic hyperglycemia-mediated nutrient overload showed that a NOX4-induced oxidative environment is critical for NLRP3-inflammasome formation and IL-1β maturation. This may recruit monocytes/macrophages, as we showed in vitro and in situ on HFD-treated tissue samples, and other myeloid cells or activate resident macrophages to propagate further proinflammatory signals (Figure 5B) [(39)]. Enhancement of a proinflammatory environment in islet cells leads to deterioration of β-cell function in a long-term process. The deleterious role of IL-1β emerges from the findings that a downregulated antagonist of the IL-1 receptor discovered in individuals with diabetes, together with overproduction of IL-1β in chronic overeating, damages β cells [(38)].
On the other hand, acute exposure of β cells to IL-1β has recently been shown to have an insulinotropic effect, acting as a postprandial stimulation of insulin secretion [(38, 40)]. The enhancement of local inflammation is mainly mediated by macrophages. These are either tissue-resident macrophages that develop prenatally and reprogram in response to external stimuli in islets or circulating monocytes from the blood that differentiate once they enter the islets. Both types of macrophages differentiate in response to their changing microenvironment, ranging from the proinflammatory M1 to the reparative M2 type. β cells and macrophages exchange a variety of signals and constantly adapt to the systemic metabolic state and the resulting insulin demand. β cells communicate with their environment mainly via exosomes and the content of secreted insulin granules. The exosomes carry microRNAs that can then influence post-transcriptional gene expression of immune cells [(41)] and immunostimulatory chaperones that may play a role in triggering autoimmune responses as in type 1 diabetes [(42)]. Insulin granules release ATP along with insulin. Tissue-resident macrophages sense the microenvironment through ATP-activated purinergic receptors, facilitating their perception of β-cell secretory activity [(43)]. β cells also contain lipid droplets whose lipolytic machinery provides fatty acid precursors for inflammatory lipid mediators [(44)]. All those external stimuli might impact macrophage polarization.
Still, the NOX4 isoform could be the pro-oxidant trigger in a long-term action because it is constitutively expressed and its activity is induced by glucose metabolism, that is, under chronic nutrient stress. Whether IL-1β alone or other molecules secreted by β cells, are required for activation of macrophage inflammatory polarization remains to be elucidated, as does its relationship to the low-grade systemic inflammation observed in patients with T2D.
In summary, we found that NOX4 activity in β cells behaves like a double-edged sword. Transient activation is required for efficient GSIS, as we have described previously [(5)], whereas sustained NOX4 activation, triggered, for example, by chronic nutrient overload, promotes persistent oxidative stress signaling that, in combination with low antioxidant capacity, leads to activation of local inflammation and other features of the diabetic phenotype, further impairing β-cell function.
AUTHOR CONTRIBUTIONS
Conceptualization: Lydie Plecitá-Hlavatá and Blanka Holendová. Investigation and experiments: Lydie Plecitá-Hlavatá, Blanka Holendová, Štěpánka Benáková, Monika Křivonosková, Vojtěch Pavluch, Jan Tauber, and Eva Gabrielová. Data analysis: Lydie Plecitá-Hlavatá and Blanka Holendová. Writing the original manuscript: Lydie Plecitá-Hlavatá. Manuscript review and editing: Lydie Plecitá-Hlavatá, Petr Ježek, and Blanka Holendová. Funding acquisition: Lydie Plecitá-Hlavatá, Petr Ježek, and Štěpánka Benáková. Lydie Plecitá-Hlavatá is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGMENTS
We thank C. Glorieux and Professor J.B. Verrax, Universite Catholique de Louvain, Belgium, and Professor S. Lenzen, Hannover Medical School, Germany, for plasmid donation; Jana Vaicová for technical assistance with islet isolation and immunochemistry, and RNDr. A. Vojtíšková of the Institute of Clinical and Experimental Medicine (IKEM), Prague, Czech Republic, for help with histochemistry. We thank the Czech Centre for Phenogenomics at BIOCEV for assistance in obtaining calorimetry data and the Animal Facility supported by LM 2018126 awarded to the Czech Centre for Phenogenomics by MEYS and OP RDE CZ.02.1.01/0.0/0.0/18_046/0015861 CCP Infrastructure Upgrade II by MEYS and ESIF.
FUNDING INFORMATION
This work was supported by the Grant Agency of the Czech Republic, grant No. 22-11439S to Lydie Plecitá-Hlavatá and No. 21-01205S to Petr Ježek, Charles University Grant Agency, grant No. 110120 to Štěpánka Benáková, and the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104) - Funded by the European Union Next Generation EU [Correction added on 4 April 2024, after first online publication: The funding information has been updated.]
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request. No applicable sources were created or analyzed during the current study.