Effects of exercise on sleep in children with overweight/obesity: a randomized clinical trial
Abstract
Objective
The objective of this study was to examine the chronic effects of a 20-week exercise training program on device-assessed sleep and sleep-disordered breathing; and to determine whether participating in a session of the exercise program had effects on device-assessed sleep the subsequent night in children with overweight/obesity.
Methods
A randomized clinical trial was conducted from November 2014 to June 2016. A total of 109 children (age 8–11 years) with overweight/obesity were randomized into an exercise training or control group. The exercise program included aerobic and resistance training 3 to 5 days/week. The control group participants continued their usual lifestyle. Device-assessed sleep outcomes were measured using wrist-worn actigraphy at baseline, in the middle of the exercise program (10th week), and at postintervention for seven consecutive days (24 h/day), and sleep-disordered breathing was measured via the Pediatric Sleep Questionnaire.
Results
The exercise training program had a statistically significant effect on wake after sleep onset time (−10.8 min/day, −0.5 SDs, p = 0.040). No other chronic or acute effects (i.e., the subsequent night of attending a session of the exercise training program) were observed on the remaining sleep outcomes.
Conclusions
A 20-week exercise training program reduced wake after sleep onset time in children with overweight/obesity. Future randomized trials that include a sample of children with poor sleep health at baseline are needed to better appreciate the role of exercise in sleep health.
Study Importance
What is already known?
- Childhood obesity is highly prevalent worldwide, with implications on poorer sleep health.
- Exercise interventions have been shown to offer clear and substantial benefits for children's health.
- Positive effects of exercise on sleep have been found in adolescents with obesity, but the literature in childhood obesity is lacking.
What does this study add?
- An aerobic plus resistance exercise training program showed benefits on sleep fragmentation in children with overweight/obesity but had no effect on sleep-disordered breathing.
- Exercise programs should be promoted in children to improve their sleep health, especially in those with overweight/obesity.
How might these results change the direction of research or the focus of clinical practice?
- The results of the study provide new insights on the potential benefits of exercise training programs on sleep health in the pediatric obesity population.
INTRODUCTION
Sleep is essential for children's health and well-being [(1)]. The current lifestyles (e.g., late-night screen time, energy drinks, and delayed bedtimes) are compromising the sleep habits in children [(2)]. As a result, the attainment of sleep duration recommendations (uninterrupted 9–11 h of sleep/night) is decreasing in children [(3)]. The estimated prevalence of 6- to 13-year-old children sleeping less than 9 to 11 h/day is 98.1% as measured with accelerometers and 18.1% as reported by diaries [(4)]; the prevalence of sleep-disordered breathing (SDB) is around 4% to 11% in children [(5)]. Shorter sleep duration has been related to worse physical and mental health in children and adolescents [(6)], and poorer sleep outcomes (i.e., sleep duration and sleep patterns) appear to be associated with childhood obesity [(7-9)]. As an example, we have previously observed a link between device-assessed sleep outcomes [(10)] and SDB [(11)] with brain health (i.e., gray matter volume and academic performance) in children with overweight/obesity. Given the well-contrasted multiorgan benefits of physical exercise [(12)], it is of interest to investigate whether physical exercise improves sleep in children with overweight/obesity.
A large number of studies have supported the positive chronic and acute effects of exercise on device-assessed sleep in adults [(13)]. However, the literature that focused on the effects of exercise on sleep in the pediatric population is scarce. To date, only one randomized clinical trial (RCT), to our knowledge, has investigated the effect of exercise intervention on device-assessed sleep in children with autism spectrum disorders [(14)]. In obesity, the available trials on exercise and sleep are limited to adolescents [(15)], whereas the studies in childhood are all observational and have focused on lifestyle physical activity and sleep [(16, 17)]. In regard to SDB, few studies have shown that engaging in at least 20 to 40 min/day of vigorous aerobic exercise appeared to be beneficial in palliating symptoms of SDB in children who were 7 to 11 years old with overweight [(18)], but not in children with obesity and with SDB [(19)]. There is a need for RCTs on the effects of exercise on sleep in pediatric obesity.
Few studies have examined the chronic and acute effects of exercise on sleep outcomes (i.e., sleep time and quality) in adolescents with obesity [(15, 20)]. This information would inform public health recommendations and practice. Therefore, the aims of this study were to examine the following: 1) the chronic effects of a 20-week exercise training program on device-assessed sleep and on SDB in children with overweight/obesity; and 2) whether participating in a single bout of the exercise program had effects on device-assessed sleep the subsequent night in children with overweight/obesity.
METHODS
Study design and participants
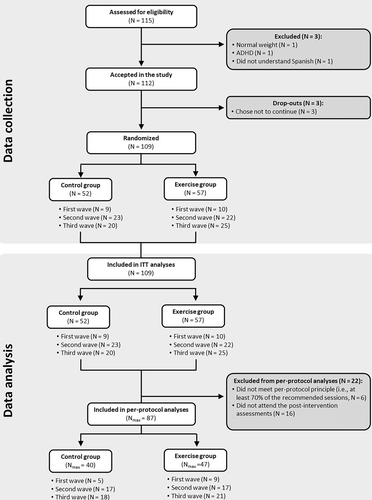
The ActiveBrains project was a parallel-group RCT (ClinicalTrials.gov registration no. NCT02295072) with the primary aim to investigate the effect of a 20-week exercise program on brain health indicators in children with overweight/obesity [(21, 22)]. This study examined the ActiveBrains RCT effects on sleep (secondary outcomes). Details on the design, eligibility, characteristics, and effects on the primary outcomes can be found elsewhere [(21, 22)]. Briefly, 109 children (age 8–11 years) took part in this RCT [(21, 22)]. All participants met the following criteria: 1) had overweight/obesity (age- and sex-specific cut-points equivalent to adult body mass index ≥ 25 kg/m2) based on the sex- and age-specific international body mass index cutoff points proposed by the World Obesity Federation [(23, 24)]; 2) had no physical disabilities or neurological disorders that could affect physical performance; 3) had prepubertal status according to the Tanner stages; and 4) for the girls, had no menarche. After baseline data collection, participants were randomly allocated to either the wait list control group (n = 52) or exercise program (n = 57) using a computer-based simple randomization procedure in a ratio of 1:1 by a person not involved in the assessments. Participants in the wait list control group continued with their usual routines. Figure 1 depicts the participant flowchart. Data were collected from November 2014 to June 2016 in Granada, Spain. The ActiveBrains RCT [(21, 22)] was conducted according to the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee on Human Research (CEIH) of the University of Granada. Written informed consent was obtained by the legal guardians or parents of all participants, who were informed of the purpose of the study and provided informed assent. Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed for the reporting of trial results [(25)].
Exercise training program
The ActiveBrains exercise training program had a duration of 20 weeks and was based on the international guidelines on physical activity for children [(26)]. Participants were recommended to attend a minimum of three out of five sessions/week. The exercise training program was based on aerobic exercise and resistance exercise using body weight, fitballs, and/or resistance bands, organized by active games and playing with peers with the intention of generating adherence to exercise. Each session lasted 90 min (i.e., 5-min warm-up consisting of 1–2 physically active games; 60-min aerobic exercise consisting of around 4–5 physical multigames requiring moderate-to-vigorous intensities; 20-min resistance exercise consisting of muscle- and bone-strengthening game-based activities; and 5-min cool-down consisting of stretching and relaxation exercise).
The intensity of the ActiveBrains exercise training program was controlled in every session for both aerobic and resistance training. Every child wore a heart rate monitor (POLAR RS300X, Polar Electro Oy Inc., Kempele, Finland) that was programmed for each child based on their baseline sex, age, weight, height, and maximum heart rate achieved in a maximal incremental test. Heart rate monitoring was used by trainers to track exercise intensity of the participants in all sessions. Children trained at an average intensity of 70% of the maximum heart rate and spent an average of 38 min/session above 80% of their maximum heart rate [(21)].
Measurements
Both control and exercise groups were assessed at baseline and post-intervention. Sleep and physical activity were additionally monitored in the middle of the exercise program (10th week) in both groups. Assessors were not fully blinded to the participant's group allocation due to practical reasons. More information about the measurements can be found elsewhere [(21)].
SDB
The children's legal guardians completed the Spanish version of the Pediatric Sleep Questionnaire (PSQ) to evaluate SDB, which has shown high reliability and internal consistency [(27)] as well as validity for the identification of SDB [(28, 29)]. The SDB scale (range: 0–1) was used to quantify SDB, with higher scores indicating greater breathing severity [(29)]. This scale consists of 22 closed-response items of the reduced version of the PSQ, which were divided into the following three subscales [(28, 29)]: snoring; sleepiness; and inattention/hyperactivity. Further details on the questionnaire can be found elsewhere [(11, 30)].
Device-assessed sleep
Sleep was monitored with nondominant wrist-worn accelerometers (ActiGraph GT3X+, ActiGraph LLC, Pensacola, Florida) for 7 consecutive days (24 h/day). Participants reported information on the time they went to bed and woke up every day through diaries. Accelerometers were initialized to record accelerations at 100 Hz, and the files were processed in the R package GGIR (version 1.5.12, https://www.cran.r-project.org/, R, Vienna, Austria) [(31)]. More detailed information on accelerometer data processing for device-assessed sleep outcomes analysis can be found elsewhere [(32)]. The algorithm developed by Sadeh et al [(33)] was applied within the total time-in-bed frame to classify every minute as “sleep” or “wake” time. Device-assessed sleep outcomes included indicators of sleep timing, sleep duration (i.e., total sleep time and total time in bed), and sleep quality (i.e., sleep efficiency and wake after sleep onset [WASO] time). A valid week was considered if the participant registered at least 4 days (≥ 16 h/day), including a minimum of three weekdays and one weekend day. Moreover, a valid day was considered if the participants accumulated as wear time the equivalent of two-thirds of night hours and two-thirds of the waking hours.
Statistical analysis
Descriptive characteristics of the study sample are shown as mean and SDs or percentages and frequencies.
Chronic effect of the exercise training program on sleep
The chronic effects of the ActiveBrains exercise program on each sleep-related outcome were conducted according to the per-protocol principle (i.e., attending at least 70% of the sessions) using an analysis of covariance (ANCOVA). Per-protocol analyses were the main analyses in this trial to investigate the efficacy rather than effectiveness of our intervention (i.e., effects of the preconceived intervention). Post-exercise sleep-related outcome values were included as dependent variables, group (i.e., exercise vs. control) as fixed factor, and baseline outcome data as covariates [(34)]. Raw and standardized (z scores) estimates are provided, with the post-exercise z scores calculated relative to the baseline mean and SD as an indicative of the change [(21, 34)]. A difference of 0.2, 0.5, and 0.8 SDs were considered small, medium, and large effect sizes, respectively [(35)]. Differences exceeding 0.2 Cohen's d were interpreted as minimum meaningful effect [(36)].
Exploratory analyses were conducted following the intention-to-treat principle, including all the recruited participants, and imputing the missing values using predictive mean-matching multiple imputations [(37)]. We checked whether missing data were missing at random prior to performing the multiple imputation.
Acute effect of the exercise training program on sleep
The acute effects of the ActiveBrains exercise program were investigated over the sleep outcomes from the subsequent nights (at maximum of 4 nights) to the sessions the participants attended versus the sleep outcomes from the nights after the days in which they did not attend to the sessions (at 10th week, in the middle of the intervention). This analysis only included participants allocated to the exercise group. We excluded Fridays, Saturdays, and Sundays from the data set given the expected changes in the sleep patterns during weekends. Linear regression mixed models were used with the attendance indicator (0 = nonattendance to exercise program, 1 = attendance) as fixed factor, the identification as random factor, and sleep indicators as outcomes. Random and fixed intercepts were defined to investigate the within-group (attendance and nonattendance) and within-participant effect of the exercise session, respectively.
Analyses were performed using the Statistical Package for Social Science (IBM SPSS Statistics for Windows, version 22.0, IBM Corp., Armonk, New York) and R version 3.4.1 (https://cran.r-project.org/, R). The level of significance was set at p < 0.05.
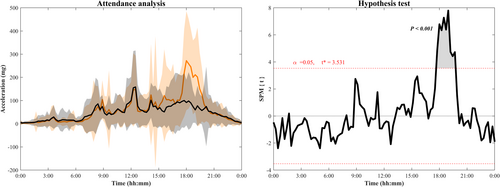
To better understand the acute effects of the exercise program on sleep, we investigated whether the children compensated the amount of exercise performed in the ActiveBrains session with activities that they usually perform in their daily life. For such purpose, we performed a one-dimension curve analysis using statistical parametric mapping software (SPM1D; Wellcome Department of Cognitive Neurology, London, United Kingdom) to verify whether acceleration values identified a significant physical activity increment (higher acceleration, i.e., movement) in the days attending the exercise sessions compared with the days not attending. From Monday to Thursday, mean acceleration curves were presented from midnight to the following midnight (i.e., 24-h curves). Student t tests were used to determine significant differences among the curves for attendance and nonattendance exercise group throughout the day.
RESULTS
A total of 87 children with overweight/obesity (n = 47 in the exercise group) were included in the per-protocol analysis (90% of the participants initially enrolled); the children who did not adhere to the protocol were not different in baseline characteristics from the included children (Figure 1). Descriptive baseline characteristics are shown in Table 1.
| All | Exercise group | Control group | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) or % | n | Mean (SD) or % | n | Mean (SD) or % | |
| Sex | ||||||
| Girls | 41 | 41.4 | 16 | 34 | 25 | 48.1 |
| Boys | 58 | 58.6 | 31 | 66 | 27 | 51.9 |
| Age (y) | 99 | 10.1 (1.1) | 47 | 10.0 (1.1) | 52 | 10.1 (1.1) |
| Weight (kg) | 99 | 56.5 (11.0) | 47 | 57.4 (12.7) | 52 | 55.7 (9.4) |
| Height (cm) | 99 | 144.6 (8.3) | 47 | 143.9 (8.9) | 52 | 145.2 (7.9) |
| Parent education level | ||||||
| Primary school | 65 | 65.7 | 33 | 70.2 | 32 | 61.5 |
| Secondary school | 16 | 16.2 | 6 | 12.8 | 10 | 19.2 |
| University degree | 18 | 18.2 | 8 | 17.0 | 10 | 19.2 |
| Peak height velocity (y) | 99 | −2.2 (1.0) | 47 | −2.4 (0.9) | 52 | −2.1 (1.1) |
| SDB | ||||||
| SDB scale (0–1) | 99 | 0.19 (0.13) | 47 | 0.19 (0.13) | 52 | 0.19 (0.14) |
| SDB presence (%) | 16.2 | 12.8 | 19.2 | |||
| Device-assessed sleep | ||||||
| Total sleep time (min/day) | 97 | 462.5 (36.0) | 47 | 462 (33.3) | 50 | 463 (38.7) |
| Total time in bed (min/day) | 97 | 529 (31.0) | 47 | 524.6 (35.1) | 50 | 533.1 (26.4) |
| Sleep efficiency (%) | 97 | 85 (4.6) | 47 | 85.3 (4.0) | 50 | 84.8 (5.2) |
| WASO time (min/day) | 97 | 74.9 (22.0) | 47 | 73.9 (19.1) | 50 | 75.8 (24.5) |
- Note: Data analyses were primarily conducted under the per-protocol principle, i.e., attending 70% of the sessions or keeping the usual lifestyle for exercise and control groups, respectively. SDB was measured using the Spanish version of the Pediatric Sleep Questionnaire. Device-assessed sleep was measured using wrist-worn accelerometers.
- Abbreviations: SDB, sleep-disordered breathing; WASO, wake after sleep onset.
Chronic effect of the exercise training program on sleep
The effects of the ActiveBrains exercise training program on sleep outcomes are presented in Table 2. The largest effect of the ActiveBrains exercise training program was found on WASO time, with an intervention versus control difference of −10.79 min/day (95% confidence interval [CI]: −21.09 to −0.51; p = 0.040), showing a difference of medium effect size in the exercise group compared with the control group (SD = −0.507 [95% CI: −0.989 to −0.024]). Furthermore, a smaller and nonsignificant effect was observed for sleep efficiency (+2% [95% CI: −0.330 to 3.671]; SD = 0.398). We found no evidence of exercise effects either on the remaining device-assessed sleep outcomes (i.e., total sleep time, sleep efficiency, and total time in bed) or on SDB (all p > 0.05). The intention-to-treat analyses showed similar results, but they were of smaller magnitude than per-protocol analyses (Table S1). More participants in the exercise group showed meaningful changes (i.e., reduction of ≥0.2 SDs) compared with the control group in WASO time (31% vs. 19%, p = 0.405; Figure S1).
| Nall | Mean (95% CI) | p | |||||
|---|---|---|---|---|---|---|---|
| n | Exercise group | n | Control group | Difference between groups (exercise minus control) | |||
| SDB scale | 87 | 47 | 40 | ||||
| Raw score | 0.195 (0.165 to 0.225) | 0.177 (0.145 to 0.210) | 0.017 (−0.027 to 0.062) | 0.438 | |||
| z score | 0.050 (−0.182 to 0.281) | −0.084 (−0.335 to 0.167) | 0.134 (−0.208 to 0.476) | ||||
| Total sleep time (min/day) | 66 | 39 | 27 | ||||
| Raw score | 435.231 (422.518 to 447.944) | 431.078 (415.799 to 446.357) | 4.152 (−15.725 to 24.029) | 0.678 | |||
| z score | −0.793 (−1.137 to −0.448) | −0.905 (−1.319 to −0.491) | 0.113 (−0.426 to 0.651) | ||||
| Total time in bed (min/day) | 66 | 39 | 27 | ||||
| Raw score | 504.153 (491.353 to 516.952) | 506.982 (491.594 to 522.370) | −2.829 (−22.860 to 17.202) | 0.779 | |||
| z score | −0.799 (−1.202 to −0.395) | −0.709 (−1.195 to −0.224) | −0.089 (−0.721 to 0.542) | ||||
| Sleep efficiency (%) | 66 | 39 | 27 | ||||
| Raw score | 84.021 (82.744 to 85.298) | 82.351 (80.815 to 83.886) | 1.670 (−0.330 to 3.671) | 0.100 | |||
| z score | −0.338 (−0.642 to −0.033) | −0.736 (−1.102 to −0.370) | 0.398 (−0.079 to 0.875) | ||||
| WASO time (min/day) | 66 | 39 | 27 | ||||
| Raw score | 77.798 (71.222 to 84.374) | 88.594 (80.689 to 96.499) | −10.796 (−21.085 to −0.508) | 0.040 | |||
| z score | 0.212 (−0.096 to 0.521) | 0.719 (0.348 to 1.090) | −0.507 (−0.989 to −0.024) | ||||
- Note: z score values indicate how many SDs the post-exercise program values changed with respect to the baseline mean and SD, e.g., a 0.50 z score means that the mean value at post-exercise is 0.50 SDs higher than the mean value at baseline, indicating a positive change, with negative values indicating the opposite. All data presented were adjusted for baseline values. SDB was assessed by the Pediatric Sleep Questionnaire.
- Abbreviations: SDB, sleep-disordered breathing; WASO, wake after sleep onset.
Acute effect of the exercise training program on sleep
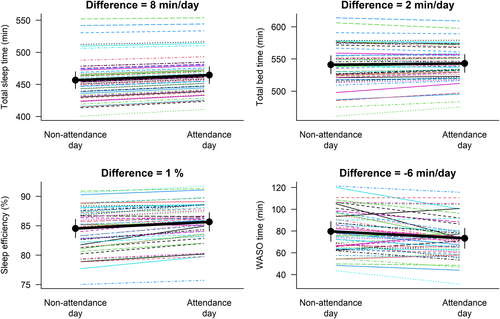
The acute effects on device-assessed sleep outcomes of attending (n = 127) versus not attending (n = 73) the ActiveBrains exercise sessions are presented in Figure 2. Attending the exercise sessions resulted in a longer sleeping time by 8 min/day (p = 0.166), total time in bed by 2 min/day (p = 0.750), and better sleep efficiency by 1% (p = 0.146), as well as a reduced −6 min/day WASO time (p = 0.185) compared with nonattendance days, although all of these associations did not reach statistical significance.
The statistical parametric mapping analysis of the 24-h activity curve shows how attendance days to exercise intervention significantly increased their activity levels compared with the nonattendance days (threshold = 3.531; p < 0.001) at week 10 of the intervention (excluding Friday, Saturday, and Sunday; Figure 3).
DISCUSSION
The ActiveBrains RCT contributes to the existing literature with the following findings: 1) a 20-week exercise training program reduces the WASO by around 10 min/day in children with overweight or obesity; and 2) all device-assessed sleep indicators, especially sleep time and WASO time, were improved in those days in which the participants attended the sessions, although they did not reach statistical significance and should be taken with caution.
This is one of the few RCTs in the literature examining both chronic and acute effects of an exercise training program on device-assessed sleep habits in childhood overweight/obesity. This study's findings provide valuable insights, suggesting that exercise may have chronic and certain acute benefits (but nonsignificant) on sleep habits, specifically on WASO. On the other hand, we did not observe a statistically significant effect on SDB.
To date, only one RCT investigated the effect of physical intervention on device-assessed sleep in children, but this was done in children with autism spectrum disorders [(14)]. Similar to our study, Tse et al. found that a 12-week exercise intervention (45 min/session and 2 sessions/week) had a positive effect on WASO and sleep efficiency [(14)]. They also found a significant effect on sleep duration measured by nondominant wrist-worn accelerometry [(14)]. Moreover, in consonance with our results, but using a questionnaire to measure sleep quality, an RCT in children and adolescents reported that 12 weeks of both judo or ball games benefited sleep quality (i.e., aspects related to sleep pattern and the frequency with which these may occur) [(38)]. In adolescents with obesity, and in contrast with our findings, a 12-week exercise intervention (180 min/week) significantly increased sleep efficiency (0.7 SDs) and total sleep time (0.8 SDs) [(15)]. In our study, the effect of the intervention was significant only on WASO time; however, certain evidence of effects on sleep efficiency was observed. A null effect was observed on sleep duration in contrast with the significant effects observed in both previous studies [(14, 15)]. A possible explanation of these inconsistencies could be the following: 1) our participants had a healthy sleep at baseline and, therefore, there was little room for improvement; 2) children with autism spectrum disorder might have unhealthier sleep habits than children with normal development [(39)], which could enhance the exercise effects; and 3) the age groups (adolescents vs. children age 8–11 years) and the methods used to measure sleep (polysomnography vs. accelerometry) [(15)] could lead to inconsistent findings [(40)]. Additionally, the type of aerobic exercise (cycling/treadmill/rower vs. running games) or the inclusion or not of resistance exercise might partially explain the differences. A systematic review and meta-analysis in healthy participants concluded that running exercise was related to greater WASO reduction compared with cycling [(41)]; therefore, it could be that inflammation and/or muscular damage caused by this type of aerobic exercise (i.e., running) may positively affect WASO time. However, further investigations are required to elucidate the role of inflammation or muscular damage on sleep.
In regard to sleep disorders, the ActiveBrains exercise program did not improve SDB in children with overweight/obesity. Similarly, in a recent study in children with obesity and SDB, neither 8 weeks nor 16 weeks of exercise (24 min of high-intensity interval training plus 20 min of resistance training) influenced the SDB scale or subscales [(19)]. Consistent with these findings, a previous study in adolescents with obesity observed that apnea-hypopnea index (measured with polysomnography), an index used to indicate the severity of sleep apnea, was not changed after a 12-week exercise program (180 min/week) [(15)]. Likewise, Roche et al. found that a combination of 9 months of aerobic exercise and diet had no effect on obstructive sleep apnea measured with polysomnography in adolescents with obesity [(42)]. However, Davis et al. found that 15 weeks of aerobic exercise (i.e., low-dose exercise of 20 min/day or high dose of 40 min/day) could improve SDB scale, specifically snoring, in 7- to 11-year-old children with overweight [(18)]. The lack of significant effects in our study might be due to the lower SDB severity in our sample at baseline (score of ~0.19 in a scale range of 0–1 and 16.2% of our participants presented SDB) compared with that of the study by Davis et al. (score of ~0.24 and 25% of participants presented SDB). Further studies in a sample with higher SDB severity at baseline should confirm or contrast our findings. Furthermore, a current meta-analysis highlights the importance of future well-designed RCTs to understand the independent effects of exercise on obstructive sleep apnea and sleep health in children and adolescents with obesity [(43)].
Our results complement the existing literature in children (44)] by suggesting that acute exercise during the afternoon/evening could improve sleep in children with overweight/obesity. It seems that acute aerobic exercise performed at high intensity have positive effects on sleep efficiency in children [(44)]. Our findings are in line with the previous literature but cannot confirm this acute effect (because it was nonsignificant) in children with overweight/obesity enrolled in a concurrent aerobic plus strength exercise program. Likewise, in a study of adolescent girls with obesity, the authors showed that acute exercise in the morning has a positive effect on sleep duration and quality measured with accelerometers [(20)]. Furthermore, it seems that acute exercise performed in the evening does not have an adverse effect on sleep but rather a small positive effect in healthy adults [(41)]. It could be that children sleep better due to the exhaustion caused by the exercise, independent of the time of the day when it is performed. However, a previous study showed that exercise was beneficial to improve device-assessed sleep (i.e., decreased WASO time and increased sleep efficiency) only when it was completed 4 h before bedtime and not 2 h (i.e., late evening) in healthy young adults [(45)]. We did not have the opportunity to investigate the timing effect of the exercise on sleep because all participants performed the ActiveBrains exercise sessions at the same time (i.e., during the afternoon), and we encourage future studies to address that gap in the literature. So far, the literature in children and youth has examined whether physical activity was associated with sleep the subsequent nights, finding limited evidence to support this relationship [(17)].
The limitations of this study include the following: 1) our findings apply to children with overweight/obesity and should not be extrapolated to other populations (e.g., other age groups, individuals with normal weight); 2) we used questionnaires instead of the gold-standard (polysomnography) to measure SDB; and 3) the accelerometer estimates of sleep are based on movement patterns, not purely sleep; however, the accelerometers provide a noninvasive, objective, and valid assessment of sleep in free-living conditions [(33)].
On the other hand, there are some strengths that should be noted. These include the randomized design, the use of device-assessed sleep behavior with accelerometers across an entire week, the advanced processing on accelerometer raw data, and the focus on children with overweight/obesity, who often show poorer sleep patterns than children with normal weight. The novel analyses of accelerometer-measured physical activity all throughout the day (24 h) to accurately investigate whether changes in overall physical activity levels took place in the attendance days of the exercise training program and at what time of the day are also a key strength.
CONCLUSION
Our findings suggest that exercise can have positive effects on device-assessed sleep habits, particularly chronic effects with certain changes also being observed acutely, by producing ~6 to 10 min lower WASO time per night. However, no effects were observed on SDB in children with overweight/obesity. These findings support public health initiatives that promote exercise training programs in children with overweight/obesity in their potential to improve sleep quality. Further RCTs, especially in children with sleep difficulties at baseline, are needed to better appreciate the role of exercise in sleep health.
FUNDING INFORMATION
This work is part of a doctorate thesis conducted in the Official Doctoral Program in Biomedicine of the University of Granada, Spain. The ActiveBrains project was funded by the Spanish Ministry of Economy and Competitiveness and the European Regional Development Fund (ERDF/FEDER) (DEP2013-47540, DEP2016-79512-R, DEP2017-91544-EXP, and RYC-2011-09011). Lucia V. Torres-Lopez is supported by a grant from the Spanish Ministry of Science, Innovation and Universities (FPU17/04802). Cristina Cadenas-Sanchez is supported by the Spanish Ministry of Science and Innovation (FJC2018-037925-I). Additional support was obtained from the Alicia Koplowitz Foundation (ALICIAK-2018), the University of Granada, Proper Research Plan 2016; excellence actions: Units of Excellence, Scientific Excellence Unit on Exercise and Health (UCEES), the Junta of Andalusia, Ministry of Knowledge, Research and Universities; and European Regional Development Fund (ref. SOMM17/6107/UGR). In addition, funding was provided by the Mother-Child Health and Development Network (Red SAMID) III network; Redes temáticasa de Investigación Cooperativa en Salud (RETICS), funded by the PN I+D+I 2017 to 2021 (Spain), the EXERNET Research Network on Exercise and Health (DEP2005-00046/ACTI; 09/UPB/19; 45/UPB/20; 27/UPB/21); and the European Union's 2020 research and innovation program under grant agreement no. 667302. Additional funding was obtained from the Andalusian Operational Program, supported by ERDF/FEDER (project reference: B-CTS-355-UGR18). Funding for open access charge: Universidad de Granada/CBUA.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT02295072.
Open Research
DATA AVAILABILITY STATEMENT
Deidentified individual participant data will not be made available. We did not obtain parental consent to widely share the data, nor was it included in the institutional review board protocol.





