Metabolite signature of diabetes remission in individuals with obesity undergoing weight loss interventions
Corresponding Author
Vidhu V. Thaker
Department of Pediatrics, Columbia University Irving Medical Center, New York, New York, USA
Correspondence
Vidhu V. Thaker, Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA.
Email: [email protected]
Blandine Laferrère, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, Russ Berrie Medical Science Pavilion, R-121-G, 1150 St. Nicholas Ave, New York, NY, USA.
Email: [email protected]
Search for more papers by this authorLydia Coulter Kwee
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Search for more papers by this authorHaiying Chen
Department of Biostatistics and Data Science, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA
Search for more papers by this authorJudy Bahnson
Department of Biostatistics and Data Science, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA
Search for more papers by this authorOlga Ilkayeva
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Sarah W. Stedman Nutrition and Metabolism Center, Durham, North Carolina, USA
Division of Endocrinology, Metabolism, and Nutrition, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA
Search for more papers by this authorMichael J. Muehlbauer
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Sarah W. Stedman Nutrition and Metabolism Center, Durham, North Carolina, USA
Search for more papers by this authorBruce Wolfe
Departments of Surgery and Medicine, Oregon Health & Science University, Portland, Oregon, USA
Search for more papers by this authorJonathan Q. Purnell
Departments of Surgery and Medicine, Oregon Health & Science University, Portland, Oregon, USA
Search for more papers by this authorXavier Pi-Sunyer
New York Obesity Research Center, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, New York, New York, USA
Search for more papers by this authorChristopher B. Newgard
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Sarah W. Stedman Nutrition and Metabolism Center, Durham, North Carolina, USA
Division of Endocrinology, Metabolism, and Nutrition, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA
Department of Pharmacology & Cancer Biology, Duke University School of Medicine, Durham, North Carolina, USA
Search for more papers by this authorSvati H. Shah
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Division of Cardiology, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA
Search for more papers by this authorCorresponding Author
Blandine Laferrère
New York Obesity Research Center, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, New York, New York, USA
Correspondence
Vidhu V. Thaker, Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA.
Email: [email protected]
Blandine Laferrère, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, Russ Berrie Medical Science Pavilion, R-121-G, 1150 St. Nicholas Ave, New York, NY, USA.
Email: [email protected]
Search for more papers by this authorThe Look AHEAD Research Group
Search for more papers by this authorCorresponding Author
Vidhu V. Thaker
Department of Pediatrics, Columbia University Irving Medical Center, New York, New York, USA
Correspondence
Vidhu V. Thaker, Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA.
Email: [email protected]
Blandine Laferrère, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, Russ Berrie Medical Science Pavilion, R-121-G, 1150 St. Nicholas Ave, New York, NY, USA.
Email: [email protected]
Search for more papers by this authorLydia Coulter Kwee
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Search for more papers by this authorHaiying Chen
Department of Biostatistics and Data Science, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA
Search for more papers by this authorJudy Bahnson
Department of Biostatistics and Data Science, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA
Search for more papers by this authorOlga Ilkayeva
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Sarah W. Stedman Nutrition and Metabolism Center, Durham, North Carolina, USA
Division of Endocrinology, Metabolism, and Nutrition, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA
Search for more papers by this authorMichael J. Muehlbauer
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Sarah W. Stedman Nutrition and Metabolism Center, Durham, North Carolina, USA
Search for more papers by this authorBruce Wolfe
Departments of Surgery and Medicine, Oregon Health & Science University, Portland, Oregon, USA
Search for more papers by this authorJonathan Q. Purnell
Departments of Surgery and Medicine, Oregon Health & Science University, Portland, Oregon, USA
Search for more papers by this authorXavier Pi-Sunyer
New York Obesity Research Center, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, New York, New York, USA
Search for more papers by this authorChristopher B. Newgard
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Sarah W. Stedman Nutrition and Metabolism Center, Durham, North Carolina, USA
Division of Endocrinology, Metabolism, and Nutrition, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA
Department of Pharmacology & Cancer Biology, Duke University School of Medicine, Durham, North Carolina, USA
Search for more papers by this authorSvati H. Shah
Duke Molecular Physiology Institute, Durham, North Carolina, USA
Division of Cardiology, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA
Search for more papers by this authorCorresponding Author
Blandine Laferrère
New York Obesity Research Center, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, New York, New York, USA
Correspondence
Vidhu V. Thaker, Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA.
Email: [email protected]
Blandine Laferrère, Division of Endocrinology, Department of Medicine, Columbia University Irving Medical Center, Russ Berrie Medical Science Pavilion, R-121-G, 1150 St. Nicholas Ave, New York, NY, USA.
Email: [email protected]
Search for more papers by this authorThe Look AHEAD Research Group
Search for more papers by this authorSvati H. Shah and Blandine Laferrère contributed equally.
Abstract
Objective
This observational study investigated metabolomic changes in individuals with type 2 diabetes (T2D) after weight loss. We hypothesized that metabolite changes associated with T2D-relevant phenotypes are signatures of improved health.
Methods
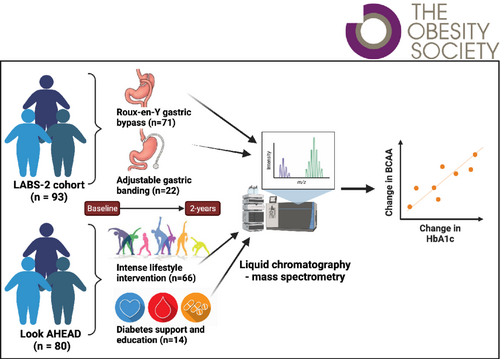
Fasting plasma samples from individuals undergoing bariatric surgery (n = 71 Roux-en-Y gastric bypass [RYGB], n = 22 gastric banding), lifestyle intervention (n = 66), or usual care (n = 14) were profiled for 139 metabolites before and 2 years after weight loss. Principal component analysis grouped correlated metabolites into factors. Association of preintervention metabolites was tested with preintervention clinical features and changes in T2D markers. Association between change in metabolites/metabolite factors and change in T2D remission markers, homeostasis model assessment of β-cell function, homeostasis model assessment of insulin resistance, and glycated hemoglobin (HbA1c) was assessed.
Results
Branched-chain amino acids (BCAAs) were associated with preintervention adiposity. Changes in BCAAs (valine, leucine/isoleucine) and branched-chain ketoacids were positively associated with change in HbA1c (false discovery rate q value ≤ 0.001) that persisted after adjustment for percentage weight change and RYGB (p ≤ 0.02). In analyses stratified by RYGB or other weight loss method, some metabolites showed association with non-RYGB weight loss.
Conclusions
This study confirmed known metabolite associations with obesity/T2D and showed an association of BCAAs with HbA1c change after weight loss, independent of the method or magnitude of weight loss.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
Supporting Information
| Filename | Description |
|---|---|
| oby23943-sup-0001-supinfo.pdfPDF document, 144.6 KB | Data S1. Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Long J, Yang Z, Wang L, et al. Metabolite biomarkers of type 2 diabetes mellitus and pre-diabetes: a systematic review and meta-analysis. BMC Endocr Disord. 2020; 20(1): 174.
- 2Ahola-Olli AV, Mustelin L, Kalimeri M, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019; 62(12): 2298-2309.
- 3Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009; 9(4): 311-326.
- 4Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011; 17(4): 448-453.
- 5Wittenbecher C, Guasch-Ferre M, Haslam DE, et al. Changes in metabolomics profiles over ten years and subsequent risk of developing type 2 diabetes: results from the Nurses' health study. EBioMedicine. 2021; 75: 103799.
- 6Zhu C, Liang QL, Hu P, Wang YM, Luo GA. Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta. 2011; 85(4): 1711-1720.
- 7Safai N, Suvitaival T, Ali A, et al. Effect of metformin on plasma metabolite profile in the Copenhagen insulin and metformin therapy (CIMT) trial. Diabet Med. 2018; 35(7): 944-953.
- 8Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012; 308(23):2489-2496.
- 9Purnell JQ, Dewey EN, Laferrere B, et al. Diabetes remission status during seven-year follow-up of the Longitudinal Assessment of Bariatric Surgery Study. J Clin Endocrinol Metab. 2020; 106: 774-788.
- 10Laferrère B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011; 3(80):80re2.
- 11Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008; 93(7): 2479-2485.
- 12Kwee LC, Ilkayeva O, Muehlbauer MJ, et al. Metabolites and diabetes remission after weight loss. Nutr Diabetes. 2021; 11(1): 10.
- 13Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012; 55(2): 321-330.
- 14Belle S; LABS Consortium. The NIDDK Bariatric Surgery clinical Research Consortium (LABS). Surg Obes Relat Dis. 2005; 1(2): 145-147.
- 15Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg. 2018; 153(5): 427-434.
- 16Purnell JQ, Selzer F, Wahed AS, et al. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the Longitudinal Assessment of Bariatric Surgery. Diabetes Care. 2016; 39(7): 1101-1107.
- 17 Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2014; 22(1): 5-13.
- 18Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012; 308(23): 2489-2496.
- 19White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB. Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab. 2021; 52:101261.
- 20White PJ, Newgard CB. Branched-chain amino acids in disease. Science. 2019; 363(6427): 582-583.
- 21Sun Y, Gao HY, Fan ZY, He Y, Yan YX. Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J Clin Endocrinol Metab. 2020; 105(4): 1000-1008.
- 22Ng ML, Wadham C, Sukocheva OA. The role of sphingolipid signalling in diabetes-associated pathologies (review). Int J Mol Med. 2017; 39(2): 243-252.
- 23Kolb H, Kempf K, Rohling M, Lenzen-Schulte M, Schloot NC, Martin S. Ketone bodies: from enemy to friend and guardian angel. BMC Med. 2021; 19(1): 313.
- 24Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a Meta-analysis of randomized controlled trials. Nutrients. 2020; 12(7):2005.
- 25Daly ME, Paisey R, Paisey R, et al. Short-term effects of severe dietary carbohydrate-restriction advice in type 2 diabetes-a randomized controlled trial. Diabet Med. 2006; 23(1): 15-20.
- 26Rondanelli M, Gasparri C, Peroni G, et al. The potential roles of very low calorie, very low calorie ketogenic diets and very low carbohydrate diets on the gut microbiota composition. Front Endocrinol (Lausanne). 2021; 12: 662591.
- 27Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012; 125(18): 2222-2231.
- 28She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007; 293(6): E1552-E1563.
- 29Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016; 535(7612): 376-381.
- 30Mahendran Y, Jonsson A, Have CT, et al. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia. 2017; 60(5): 873-878.
- 31Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care. 2017; 40(12): 1779-1786. PMC5701741.
- 32Boden G, Chen X. Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes. 1999; 48(3): 577-583.
- 33Batchuluun B, Al Rijjal D, Prentice KJ, et al. Elevated medium-chain acylcarnitines are associated with gestational diabetes mellitus and early progression to type 2 diabetes and induce pancreatic β-cell dysfunction. Diabetes. 2018; 67(5): 885-897. PMC5910003.
- 34Nowak C, Hetty S, Salihovic S, et al. Glucose challenge metabolomics implicates medium-chain acylcarnitines in insulin resistance. Sci Rep. 2018; 8(1): 8691.
- 35Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013; 62(12): 4184-4191.
- 36Bishay RH, Tonks KT, George J, et al. Plasma bile acids more closely align with insulin resistance, visceral and hepatic adiposity than total adiposity. J Clin Endocrinol Metab. 2021; 106(3): e1131-e1139.
- 37Chen Y, Lu J, Nemati R, Plank LD, Murphy R. Acute changes of bile acids and FGF19 after sleeve gastrectomy and roux-en-Y gastric bypass. Obes Surg. 2019; 29(11): 3605-3621.