The angiotensin II type 1 receptor blocker valsartan in the battle against COVID-19
Abstract
Objective
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) uses the host’s angiotensin-converting enzyme 2 (ACE2) as a cellular entry point. Therefore, modulating ACE2 might impact SARS-CoV-2 viral replication, shedding, and coronavirus disease 2019 (COVID-19) severity. Here, it was investigated whether the angiotensin II type 1 receptor blocker valsartan alters the expression of renin-angiotensin system (RAS) components, including ACE2, in human adipose tissue (AT) and skeletal muscle.
Methods
A randomized, double-blind, placebo-controlled clinical trial was performed, in which 36 participants (BMI 31.0 ± 0.8 kg/m2) with impaired glucose metabolism received either valsartan or placebo for 26 weeks. Before and after 26 weeks’ treatment, abdominal subcutaneous AT and skeletal muscle biopsies were obtained, and gene expression of RAS components was measured by quantitative reverse transcription polymerase chain reaction.
Results
Valsartan treatment did not significantly impact the expression of RAS components, including ACE2, in AT and skeletal muscle.
Conclusions
Given the pivotal role of ACE2 in SARS-CoV-2 spread and the clinical outcomes in COVID-19 patients, the data suggest that the putative beneficial effects of angiotensin II type 1 receptor blockers on the clinical outcomes of patients with COVID-19 may not be mediated through altered ACE2 expression in abdominal subcutaneous AT.
Study Importance
What is already known?
- ► Renin-angiotensin system (RAS) blockade might prove advantageous for patients with coronavirus disease 2019 (COVID-19) because RAS blockade decreases detrimental effects of the angiotensin-converting enzyme (ACE)-angiotensin II (AngII)-AT1R axis and may promote beneficial effects through the ACE2-Ang(1-7)-mitochondrial assembly receptor axis.
What does this study add?
- ► Here, we found that treatment with the AngII type 1 receptor blocker valsartan for 26 weeks did not impact the expression of RAS components, including ACE2, in human adipose tissue and skeletal muscle.
How might these results change the direction of research or the focus of clinical practice?
- ► Given the pivotal role of ACE2 in severe acute respiratory syndrome-coronavirus 2 spread and AngII breakdown, and consequently the clinical outcomes of COVID-19 patients, our data suggest that the putative beneficial effects of AngII type 1 receptor blockers on the clinical outcomes of patients with COVID-19 might not be mediated through altered ACE2 expression in abdominal subcutaneous adipose tissue.
INTRODUCTION
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) uses the host’s angiotensin-converting enzyme 2 (ACE2) as a cellular entry point. Therefore, ACE2 is pivotal in SARS-CoV-2 replication and viral shedding. ACE2 is part of the renin-angiotensin system (RAS), which appears to be a central player in the clinical outcomes of patients with coronavirus disease 2019 (COVID-19) ((1, 2)). Angiotensin (Ang)II is the main effector peptide of the RAS, which is converted by cleavage of AngI into AngII by the angiotensin-converting enzyme (ACE) ((3)). AngII has a high binding affinity for the AngII type 1 receptor (AT1R), thus increasing blood pressure, inflammation, fibrosis, and oxidative stress. ACE2 is the rate-limiting enzyme in the degradation of AngII, counteracting deleterious effects of AngII by converting AngII into Ang(1-7). SARS-CoV-2 reduces ACE2 activity, resulting in elevated (tissue) AngII concentrations, which appear to be associated with poor clinical outcomes. Therefore, modulating ACE2 expression/activity might impact SARS-CoV-2 viral replication, shedding, and the severity of complications in COVID-19 patients ((2)).
At the beginning of the SARS-CoV-2 pandemic, it was suggested that pharmacological blockade of the RAS by commonly used ACE inhibitors or AT1R blockers (ARBs) might increase the risk of SARS-CoV-2 infection because ACE inhibition and AT1R blockade might increase ACE2 expression/activity ((1, 2)). However, results from large observational and experimental studies have suggested that ACE inhibitors or ARBs are not associated with the risk of SARS-CoV-2 infection ((2)). Rather, RAS blockade might prove advantageous in COVID-19 because RAS blockade decreases detrimental effects of the ACE-AngII-AT1R axis and it may be beneficial through the ACE2-Ang(1-7)-mitochondrial assembly receptor axis. ARB treatment might be a promising strategy to also prevent SARS-CoV-2 infection, since ACE2 can enter cells only by AT1R-mediated endocytosis ((2, 4)). Currently, RAS-modulating drugs are being investigated as a therapeutic strategy in several clinical trials to prevent SARS-CoV-2 infection and to alleviate disease severity ((5)). Notably, excess adipose tissue (AT) in people with obesity might serve as a reservoir for SARS-CoV-2 spread, thus providing a link between obesity and the susceptibility to and severity of COVID-19 ((2, 6)). Therefore, the effects of RAS modulators on AT are worth exploring since RAS components are highly expressed in human AT.
Since it is unknown whether RAS blockade alters tissue-specific expression of RAS components in humans, here we investigated whether treatment with the ARB valsartan for 26 weeks alters the expression of RAS components, including ACE2, in human AT and skeletal muscle.
METHODS
We previously performed a randomized, double-blind, placebo-controlled clinical trial in which participants with impaired glucose metabolism received either the ARB valsartan (160 mg for first 2 weeks and 320 mg for subsequent 24 weeks) or placebo for 26 weeks (ISRCTN Registry: ISRCTN42786336) ((7)). The study was approved by the Medical-Ethical Committee of Maastricht University Medical Centre+ and performed according to the Declaration of Helsinki. Before and after the 26-week treatment, abdominal subcutaneous AT and skeletal muscle (m. vastus lateralis) biopsies were collected after an overnight fast (n = 36; BMI: 31.0 ± 0.8 kg/m2; systolic blood pressure: 128 ± 2 mmHg; and diastolic blood pressure: 80 ± 1 mmHg). Total RNA was extracted and stored at −80°C.
Gene expression analyses
Here, we performed quantitative reverse transcription polymerase chain reaction (qRT-PCR) analyses to determine the effects of ARB treatment on gene expression of RAS components in abdominal subcutaneous AT and skeletal muscle. Total RNA was extracted from the biopsies and stored at −80°C until analyses. Next, total RNA was reverse transcribed using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, California), and qRT-PCR was performed in the CFX384 Real-Time System (Biorad, Hercules, California). For both tissues, the geometric mean of ribosomal protein, large, P0 (RPLP0), ribosomal protein L26 (RPL26), and 18S RNA was used as internal control. Both SensiMix SYBR Hi-ROX master mix (Bioline, London, UK) and HOTFIREPol Probe qPCR Mix Plus (ROX) (Solis Biodyne, Tartu, Estonia) were used for quantification of mRNA expression levels. TaqMan primers ACE (Hs001741179_m1), ACE2 (Hs01085333_m1), and AT1R (Hs00258938_m1) (Applied Biosystems) were used with HOTFIREPol Probe qPCR Mix Plus (ROX). Angiotensinogen (AGT), 18S, RPLP0, and RPL26 were amplified using SensiMix SYBR Hi-ROX master mix and gene-specific forward and reverse primers. Gene expression was defined using a derivative of the ΔΔCt method. The expression of the housekeeping genes was presented as 2 − ΔCt.
Statistical analyses
The effects of valsartan treatment were tested using repeated-measures ANOVA with time as the within-person factor and treatment as the between-person factor. Statistical analyses were performed using SPSS Statistics for Mac version 24.0 (IBM Corp., Armonk, New York), and p < 0.05 was considered statistically significant. Values are presented as mean ± SEM.
RESULTS AND DISCUSSION
We found no significant differences in the expression of AGT, ACE, ACE2, and AT1R in AT after 26 weeks of valsartan treatment compared with placebo (Figure 1A). In skeletal muscle also, no differences were found in AGT and ACE expression (Figure 1B). Unfortunately, expression of ACE2 and AT1R could not be reliably quantified in muscle. So far, data on plasma ACE2 activity and Ang(1-7) levels in patients without COVID-19 who have been treated with ACE inhibitors or ARBs are inconsistent; some studies have shown increased circulating ACE2 activity, whereas others have not ((1)). Our finding that ARB treatment did not alter AT ACE2 expression is in agreement with a recent report showing that ARB treatment has no significant effect on systemic ACE2 levels, either in COVID-19 patients or in controls ((8)). A limitation of the present study is the lack of data on systemic RAS components owing to unavailability of plasma samples. Notably, we cannot exclude that valsartan treatment alters RAS components, including ACE2, in people with (morbid) obesity, who usually present with higher RAS activity in AT ((2)). Likewise, it would be interesting to assess the effects of RAS blockade on AT expression of RAS components in patients with COVID-19.
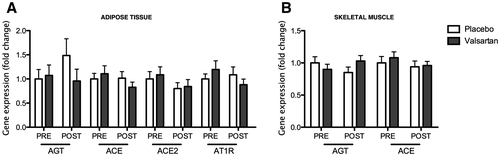
In conclusion, we provide the first evidence that the ARB valsartan does not affect the expression of RAS genes in AT and skeletal muscle in humans with overweight. Our findings may help us to better understand the results of ongoing clinical trials investigating the effects of RAS blockade on clinical outcomes in patients with confirmed COVID-19. Given the increased risk of worse clinical outcomes after SARS-CoV-2 infection in people with obesity, further studies are warranted to investigate the effects of RAS blockade on ACE2 in AT in people with obesity and COVID-19.
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of Professor Michaela Diamant (deceased), Dr. Chantalle Moors, and Dr. Nynke van der Zijl to the execution of the original study.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
ISRCTN Registry, ISRCTN42786336.





