Attrition Bias in an Observational Study of Very Low-Energy Diet: A Cohort Study
Abstract
Objective
Obesity treatment is plagued by attrition. Estimates of attrition bias are needed. Thus, in this study, percent change from baseline BMI at 1, 2, and 3 years following enrollment in a 2-year weight management program using a very low-energy diet was calculated. Program data were supplemented with information from medical records.
Methods
Attrition was classified as occurring early (<6 months), late (6-21 months), at program completion (22-28 months), and after program completion (>28 months). Stepwise multivariable regression examined attrition and other covariates.
Results
A total of 881 subjects had ≥3 years of follow-up. BMI decreased by a mean (SD) of 11.8 (9.2), 8.6 (9.3), and 5.2 (10.0) kg/m2 at 1, 2, and 3 years after enrollment, respectively. At year 1, every 10-kg/m2 increase in baseline BMI was associated with a 2% (95% CI: 1%-3%) decrease in BMI. Individuals with early attrition decreased their mean BMI by 13% (11%-15%) less than program completers and by 9% (7%-11%) at 2 years. At 3 years, there was no significant difference in BMI between individuals with early attrition and program completers. However, BMI decreased 5% (3%- 8%) more in individuals who extended participation compared with program completers.
Conclusions
Reported outcomes of weight management programs must account for program attrition.
Study importance
What is already known?
- ► Little is known about how weight management program attrition affects reported weight outcomes. One previous study attempted to measure this bias, but the unavailability of electronic medical records and the small sample size prohibited nuanced modeling of the effect.
What does this study add?
- ► We were able to quantify the bias because of early and late attrition in an intensive lifestyle program for people with moderate to severe obesity using a very low-energy diet and also to demonstrate the benefit of prolonged treatment.
How might your results change the direction of research or the focus of clinical practice?
- ► First, by highlighting the bias associated with attrition, policy models may be corrected to more accurately reflect weight loss outcomes in the real world. Second, our results may help both policy makers and payers to recognize the importance of ongoing treatment of obesity, similar to other chronic diseases.
Introduction
More than one-third of adults in the United States have obesity ((1)), and the worldwide prevalence of obesity is increasing ((2)). Obesity creates an economic burden on both individuals and health systems ((3-5)). Total per capita annual direct medical costs are, on average, $1,901 higher for individuals who have obesity compared with those who are normal weight ((6)). Effective and cost-effective treatments for obesity are needed.
Modest weight loss (5%-7%) has been shown to provide health benefits that include decreased risk of hypertension and diabetes ((7)). In addition, numerous programs have been shown to reduce weight among people with obesity, and meta-analysis suggests that the nadir of weight loss is 5% to 9% at 6 months ((8)). However, weight maintenance following initial weight loss is an ongoing challenge. For example, a data synthesis of 80 studies with over 26,000 subjects using six weight loss modalities showed a sustained weight loss of only 3% to 6% at 2 years ((8)).
Policy makers increasingly use simulation models to estimate the long-term effectiveness and cost-effectiveness of treatments for obesity. For example, several authors have developed models of intensive lifestyle intervention, very low-energy diet (VLED), and bariatric surgery ((9-11)). However, to our knowledge, no simulation model has taken into account the effect of program attrition on outcomes.
Understanding attrition is critical to correctly estimating the effectiveness of an intervention. Program attrition, defined as leaving a program before its designated completion, has been reported to be as high as 50% in weight management programs ((12-14)). When attrition occurs, especially during the intensive weight-reducing phase of a clinical program, the reported results are prone to bias because of data that are missing not at random. For example, a patient who is not adherent to the treatment, or for whom the treatment is not working, may be more likely to withdraw or be lost to follow-up. In both instances, weight loss estimates based on retained participants may overestimate treatment effects.
Meta-analyses have used the baseline value carried forward approach ((14)) to provide a conservative estimate for program effectiveness in the face of attrition, but this approach is equivalent to saying that individuals who do not complete the program had no change in weight. This assumption may be too conservative. Lantz and colleagues ((13)) are one of the very few groups who attempted to measure outcomes among missing individuals. Their study was performed in the era before the widespread availability of linked electronic health records (EHRs), and despite a moderately sized sample, they could not assess attrition at different stages of the intervention over time. They also could not account for potential confounding variables. Policy makers need greater clarity related to the effect of attrition bias on reported outcomes, and an understanding of the potential confounding between attrition and other predictors.
This paper presents a model of change in BMI following a 2-year intensive lifestyle intervention using VLED. We used data from the EHR to augment data collected in the program in order to estimate the bias due to attrition at three discrete points in time.
Methods
Participants
Eight hundred eighty-one participants with either BMI ≥35 kg/m2 or ≥32 kg/m2 with at least one weight-related health condition were recruited for the study at the time of enrollment in the University of Michigan Weight Management Program. To be included in the analysis, participants had to have enrolled at least 3 years before January 1, 2019. All participants provided written informed consent for the study, which was reviewed and approved by the University of Michigan Institutional Review Board and registered on ClincialTrials.gov (NCT02043457).
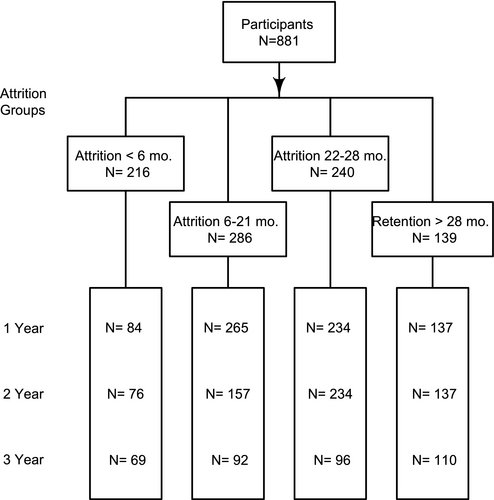
Of the 881 participants, 216 withdrew or were lost to follow-up at <6 months. Another 286 withdrew or were lost to follow-up between 6 and 21 months. Of the 379 individuals who completed the 2-year program, 139 extended treatment beyond 2 years, and 240 did not extend their engagement with the program. Data were collected from program records and supplemented with measurements from the EHR.
Nine individuals had bariatric surgery following their last visit to the program and were censored at the time of surgery. Eight of them had early attrition, and one had late attrition. Two individuals had surgery prior to year 1, one participant had surgery after year 1 but prior to year 2, and six were censored after year 2 and before year 3.
Treatment
The Weight Management Program is a 2-year intensive, multicomponent, multidisciplinary behavioral lifestyle intervention. During the first 3 months, participants incorporate a VLED in the form of liquid meal replacements followed by a 3-month period of transition to a low-calorie maintenance diet consisting of conventional food. Participants are encouraged to get at least 40 minutes of moderate physical activity per day for the first 3 months followed by an increase to 60 minutes of moderate to vigorous physical activity per day during the weight loss maintenance period. The program has been described in detail elsewhere ((15)). Individuals who completed at least 22 months were considered to have completed the program. All participants had the option to continue the program beyond 2 years.
Outcome variable
The primary outcome of interest was the percentage of change in BMI (%ΔBMI) from baseline. Height was obtained at the initial visit using a wall-mounted stadiometer (Easy-Glide Bearing Stadiometer; Perspective Enterprises, Portage, Michigan), and all participants were weighed at each visit on a calibrated scale (Scale-Tronix Model 6002; White Plains, New York). BMI was calculated as body weight in kilograms divided by height in meters squared and entered into the EHR during any visit to the health system. Thus, we had data available for individuals who did not remain in the program. We calculated %ΔBMI at three points in time: 1, 2, and 3 years following enrollment to compare our results with frequently reported results from observational trials and to provide estimates for discrete-time simulation models. The closest visit within 90 days of the time point was used.
Predictors
We examined several factors as candidate variables to predict %ΔBMI using a multivariable model. Demographic variables included age, sex, and race. Physiologic variables included BMI and blood pressure at enrollment. We also considered the change in BMI at 4 weeks of treatment as a predictor of the longer-term change in BMI. Baseline lab values included fasting glucose, hemoglobin A1c (HbA1c), total cholesterol (TC), high-density lipoprotein cholesterol, triglycerides (TG), low-density lipoprotein cholesterol, and lipid ratio (TC/high-density lipoprotein cholesterol). Baseline HbA1c was considered as both a continuous and a categorical variable representing levels associated with diabetes risk (HbA1c < 5.7% [normal], 5.7%-6.4% [prediabetes], and ≥6.5 [diabetes]). Finally, we included interaction terms between the attrition category and baseline BMI, change in BMI at 4 weeks, and HbA1c category as candidate predictors.
The primary predictor of interest was program attrition. Attrition was classified by the stage of intervention completed prior to program withdrawal or loss to follow-up. Individuals who left the program within 6 months did not complete the full intensive treatment phase (i.e., VLED plus transition) and were considered to have “early attrition.” People who completed the intensive treatment phase but left the program before 22 months were considered to have “late attrition.” Individuals who left the program between 22 and 28 months were deemed to have completed the program and were termed “program completers.” Some individuals continued the program beyond the 2-year period and were considered “program extenders.” Our a priori hypothesis was that individuals with attrition (either early or late) would lose less weight and have smaller %ΔBMI than those who completed the program (both completers and extenders).
Statistical methods
First, we examined bivariate associations between the predictors and %ΔBMI at 1, 2, and 3 years. We used p-splines ((16)) to examine the functional relationship between continuous predictors and the %ΔBMI. We used forward stepwise regression in combination with the Schwarz Bayesian information criterion ((17)) to select variables for inclusion in the final model. Program completers comprised the reference category. A Tukey test was used to test post hoc differences between attrition categories controlling for multiple tests. Standard methods for model diagnostics ((18)) were used, including inspection of residual plots, outliers, and leverage points.
In our analysis of the first year of intervention, we included all attrition categories because our intent was to enable policy makers to synthesize published results. In this scenario, attrition is known at the time of data synthesis.
Individuals were censored beyond the time of their last measurement. To explore whether %ΔBMI could be explained by covariates other than attrition, we repeated the analysis but forced all of the variables into the model and examined the significance of attrition.
For the nine individuals who had bariatric surgery following their last visit to the program (eight individuals with early attrition and one individual with late attrition), we ran the analysis with and without censoring those individuals at the time of surgery. The conclusions did not change, and our results report individuals censored at the time of surgery.
Results
We identified 881 individuals who had been enrolled for at least 3 years before January 1, 2019 and had at least one follow-up visit (Figure 1). Of these, 687 (78%) had supplemental data from the EHR beyond their last program visit. Baseline demographic descriptors are displayed in Table 1. The mean (SD) age of participants was 49 (10) years, 63% were women, and 84% were white. At baseline, the average BMI was 41 (6) kg/m2, systolic blood pressure was 131 (15) mmHg, diastolic blood pressure was 68 (9) mmHg, and HbA1c was 6.1% (1.4%) (Table 1). Among individuals who left the program at the various stages, there were significant differences in age, race, systolic blood pressure, and TC. People who did not complete the program (both early and late attrition) were younger and more likely to be Black than those who completed the program or extended their participation.
| Retention in the program | All participants | ||||
|---|---|---|---|---|---|
| Early attrition | Late attrition | Completers | Extenders | ||
| N | 216 | 286 | 240 | 139 | 881 |
| Age (y), mean (SD) | 45.7 (10.6) | 47.7 (9.6) | 51.1 (9.3) | 52.1 (9.7) | 48.7 (10.1) |
| Female (%) | 66% | 64% | 60% | 63% | 63% |
| Race (%) | |||||
| White | 80% | 81% | 89% | 88% | 84% |
| Black | 16% | 14% | 9% | 12% | 13% |
| Other | 4% | 5% | 2% | 0% | 3% |
| BMI (kg/m2), mean (SD) | 40.9 (6.4) | 41.1 (6.7) | 39.7 (6.1) | 39.9 (5.6) | 40.5 (6.3) |
| Systolic blood pressure (mmHg), mean (SD) | 131 (16) | 132 (14) | 133 (16) | 128 (14) | 131 (15) |
| Diastolic blood pressure (mmHg), mean (SD) | 69 (10) | 69 (10) | 68 (8) | 68 (9) | 68 (9) |
| Glucose (mg/dL), mean (SD) | 110 (53) | 110 (42) | 107 (38) | 113 (43) | 110 (46) |
| Hemoglobin A1c (%), mean (SD) | 6.2 (1.7) | 6.1 (1.3) | 6.0 (1.1) | 6.2 (1.1) | 6.1 (1.4) |
| Total cholesterol (mg/dL), mean (SD) | 191 (70) | 181 (37) | 180 (37) | 177 (38) | 182 (47) |
| High-density lipoprotein (mg/dL), mean (SD) | 50 (12) | 47 (12) | 49 (15) | 48 (14) | 48 (13) |
| Triglycerides (mg/dL), mean (SD) | 161 (129) | 150 (85) | 144 (85) | 151 (110) | 151 (100) |
| Low-density lipoprotein (mg/dL), mean (SD) | 107 (33) | 104 (33) | 103 (29) | 99 (30) | 104 (32) |
| Lipid ratio (%), mean (SD) | 4.2 (4.6) | 4.1 (2.4) | 3.9 (1.0) | 3.9 (1.2) | 4.0 (2.7) |
When program data were supplemented with EHR data, the mean (SD) decrease in BMI was 11.8 (9.2), 8.6 (9.3), and 5.2 (10.0) kg/m2 at 1, 2, and 3 years after enrollment (720, 604, and 367 individuals, respectively). In contrast, estimates based on complete case analysis of program data alone (not using supplemental EHR data) suggested that BMI decreased by a mean (SD) of 13.5 (8.5), 11.2 (9.0), and 10.5 (9.6) kg/m2 at 1, 2, and 3 years after enrollment (650, 430, 80 individuals, respectively). Supplemental EHR data increased the available sample size compared with program data alone because, for example, individuals with early attrition, by definition, had no program data beyond 6 months. Thus, by supplementing the data, we had at least 69 participants each year, rather than none. Similarly, using supplemental EHR data, we increased our 3-year sample size from 80 participants (who extended their treatment an additional year) to 367 individuals who received care from the health system 3 years following program enrollment.
When we examined predictors of %ΔBMI at 1 year in bivariate analyses (Supporting Information Table S1), attrition category, race, change in BMI in the first 4 weeks, baseline TC, baseline TG, and baseline glucose were significantly associated with %ΔBMI. On average, individuals who stayed in the program had a greater percentage of decrease in BMI than those who left prior to completion. Blacks, on average, had a smaller decrease in BMI than whites. For continuous variables such as baseline BMI, the coefficients displayed in Supporting Information Table S1 are interpreted as the association relative to the average decrease in BMI. Baseline BMI had a negative association with %ΔBMI. That is, individuals with higher BMI at enrollment had a larger decrease in %ΔBMI than average. In contrast, the initial 4-week change in BMI was positively associated with %ΔBMI. That is to say, people with a larger-than-average decrease in BMI in the first 4 weeks had a larger-than-average percentage of decrease in BMI at 1 year. The significant lab values were all positively associated with %ΔBMI. That is to say, higher lab values were associated with lower-than-average decrease in %ΔBMI.
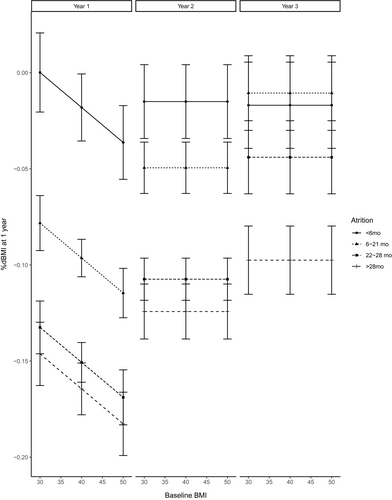
Although these variables were associated with %ΔBMI in the bivariate analyses, only baseline BMI and attrition status were independently associated with %ΔBMI in the multivariable model at 1 year (Table 2). Figure 2 plots the average %ΔBMI as a function of baseline BMI. Every 10-kg/m2 increase in baseline BMI was associated with an additional 2% (95% CI: 1%-3%) decrease in BMI at 1 year. Individuals with early attrition, on average, had a percentage of decrease in BMI that was 13% (11%-15%) less than the percentage of decrease in BMI for individuals who completed the program. Individuals with late attrition had a percentage of decrease in BMI that was 5% (4%-7%) less than the percentage of decrease in BMI for individuals who completed the program. Percentage of decrease in BMI was not statistically significant for program extenders compared with program completers.
| Year 1 | Year 2 | Year 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Early attrition vs. completers | 0.133 | 0.010 | <.0001 | 0.092 | 0.011 | <.0001 | 0.027 | 0.015 | 0.06 |
| Late attrition vs. completers | 0.054 | 0.007 | <.0001 | 0.058 | 0.009 | <.0001 | 0.033 | 0.013 | 0.01 |
| Postgraduate vs. completers | −0.014 | 0.009 | 0.11 | −0.017 | 0.009 | 0.06 | −0.054 | 0.012 | <.0001 |
| Baseline BMI | −0.002 | 0.0005 | 0.0002 | ||||||
At 2 years, bivariate analyses found that attrition category, age at enrollment, race, and baseline TC and TG were significantly associated with %ΔBMI. Individuals who stayed in the program longer had a greater decrease in percentage of BMI than participants who left the program earlier. Participants who were older at enrollment had a higher decrease in %ΔBMI. There were 63 participants who self-reported as neither Black nor white, and they exhibited less decrease in BMI at 2 years than white participants. Higher baseline lipids were associated with smaller decreases in %ΔBMI.
In the multivariable model at 2 years, only program retention was a significant independent predictor with an average 9% (7%-11%) less decrease in BMI for individuals with early attrition and 6% (4%-8%) less decrease in BMI for those with late attrition compared with program completers (Table 2). Again, program extenders were not statistically different from program completers.
At 3 years, only attrition, age at enrollment, other race, and initial 4-week change in BMI were associated with %ΔBMI. Individuals who continued beyond the end of the 2-year program had a greater percentage of decrease in BMI than those who completed the program. Participants who self-reported as neither Black nor white, had less %ΔBMI than white participants, and participants whose age at enrollment was higher than average had a greater percentage of decrease in BMI.
In the multivariable model at 3 years, attrition category was the only significant independent predictor of %ΔBMI. Program extenders had the highest average percentage of decrease in BMI from baseline: 5% (3%-8%) more than program completers. Tukey test for post hoc pairwise comparisons indicated that individuals who remained in the program beyond 2 years were significantly (α = 0.05) different from the other retention groups, but the other groups were not significantly different from each other.
For all three follow-up times, attrition category was a significant predictor (P < 0.001) of %ΔBMI even when we simultaneously conditioned on other baseline physiologic biomarkers.
Discussion
We found that by supplementing weight management program data with EHR data, we could estimate the bias in estimates of %ΔBMI for individuals who left the program before its end. Attrition was associated with lower percentage of decrease in BMI 1, 2, and 3 years following enrollment. One year following enrollment, attrition before 6 months and before 12 months was associated with 5% to 15% less decrease in BMI than for people who competed the program, depending on an individual’s BMI at enrollment. At 2 years after enrollment, attrition before 6 or 12 months was associated with a 5% to 10% less decrease in BMI. At 3 years, program completers who continued treatment had a 7% greater decrease in BMI than program completers who did not extend their participation in the program. These estimates are useful for simulation models based on published results that do not account for loss to follow-up.
Although a number of biomarkers, including baseline TC and baseline TG, were associated with %ΔBMI over time in bivariate analyses, when we accounted for attrition, the biomarkers did not provide independent information and were not as highly associated with %ΔBMI as attrition status. In addition, although we initially hypothesized that the 4-week %ΔBMI would be associated with future weight change, the initial trajectory did not provide additional information in our analysis when attrition was also considered.
More importantly, our multivariable models suggest that, over time, the influence of baseline characteristics diminishes and retention in the program becomes the dominate predictor of the percentage of decrease in BMI. Efforts to improve population-level outcomes will need to focus on retention. Our results suggest that reducing attrition by 50% is associated with an additional 2% to 3% decrease in a program’s reported mean percentage of decrease in BMI (Supporting Information). Attrition is associated with numerous risk factors, and interventions to improve retention will have to address the underlying causal factors. Psychosocial assessments that measure changes in mood, health-related quality of life, and eating behaviors may add value to the more objective data to help determine who is more susceptible to withdrawal from the program or where to maximize (or perhaps reduce) the frequency of visits and intensity of treatment.
Notably, at 3 years (1 year after program completion), individuals with attrition before 6 months or before 12 months had no difference in decrease in %ΔBMI than program completers. However, the program extenders’ percentage of decrease in BMI was greater than program completers. This suggests that ongoing lifestyle support may improve outcomes ((13)). In another study of 50 individuals with overweight and obesity who incorporated a VLED for 8 weeks to promote a 10% weight loss from baseline weight, Sumithran and colleagues showed that at 1 year following weight loss, circulating levels of the mediators of appetite regulation that favor weight regain persisted ((19)). In another study evaluating long-term weight-loss maintenance after an intensive lifestyle behavioral program focused on dietary change, exercise, and provision of psychological counseling administered over 21 weeks and delivered at weight-loss camp in Denmark, 249 participants who had severe obesity had reduced their weight from baseline weight by 15%. However, the average weight-loss maintenance was 5.3% at a follow-up after 2 to 4 years, and only 28.3% had maintained a weight loss above 10% after 4 years of follow-up. Per these authors, “this emphasizes that obesity is a chronic condition that needs additional strategies after a weight loss intervention in the efforts to maintain a sufficient weight loss” ((20)). Our data suggest that ongoing behavioral support is needed beyond 2 years to maintain weight loss.
Obesity is the second leading cause of preventable death in the United States and is considered a chronic disease. Therefore, approaches for long-term weight control need to be implemented and delivered similarly to other chronic disease management paradigms that ensure long-term follow-up for patients. In our study, ongoing lifestyle support beyond 2 years was confounded by self-selection, and the benefits of prolonged program accessibility need to be examined in a randomized controlled trial. However, ongoing support appears to benefit individuals who extend participation. Policies should provide ongoing access to behavioral weight-management interventions for individuals for whom interventions were successful. Doing so may provide benefits to both individuals and payers.
Our results also emphasize the importance of retention. At every follow-up time, active participants in our program had a greater decrease in %BMI than those who had left the program. As such, reducing barriers to retention and increasing adherence is critical. For example, in the 3 years of the Diabetes Prevention Program, 75% of participants in the lifestyle intervention adhered to the exercise program, and the average weight loss was 5% ((7)). Weight loss among those who adhered was not reported. In contrast, in our study, adherence was synonymous with retention. Among the 43% of individuals who completed the program, the average decrease in BMI was 10%. We need to better understand the trade-off between a program with high adherence that yields less weight loss compared with a program with high attrition but greater weight loss.
The reasons for attrition are also poorly understood. In our study, nearly 60% of individuals who left did so during the maintenance phase rather than during the restrictive diet phase. This suggests that it is not just the intensive calorie restriction that leads to attrition. Also, the reasons for attrition will likely differ between modalities. Efforts to improve retention are needed during all phases of a program and are critical to the success of all weight-loss programs. More importantly, health care providers need the ability to match individuals with weight-management programs in which they will be successful.
When making policy decisions about treatments, the role of attrition is important to consider, particularly when patients can withdraw prior to the end of a treatment program. In particular, if attrition is highly associated with the outcome of interest, the appropriateness of underlying statistical assumptions may be difficult to verify. In the case of treatment using VLED, attrition category was significantly associated with the percentage of change in BMI, even when other variables were included in the model. This suggests that applying a statistical technique like multiple imputation may be inappropriate because the percentage of decrease in BMI depends on attrition status, even after controlling for other risk factors. Therefore, explicit measurement of the bias due to attrition is critical.
We found that attrition was associated with many other baseline variables, especially biomarkers that are also associated with the percentage of change in BMI. As such, we cannot say that attrition causes differences in %ΔBMI. There are other unmeasured variables that are likely to confound the relationship between attrition and %ΔBMI, such as depression and low self-efficacy. Moreover, because our model for year 1 included information about attrition that occurred at 2 years, our model cannot be interpreted as a causal model in which attrition at an earlier time leads to outcomes at another. However, when developing policy recommendations, program retention can be directly tied to incentives and reimbursement. Thus, when modeling the effectiveness of a proposed policy, attrition as known at the end of the study can be used to correct the reported outcomes for potential bias.
Although our analysis is limited to a single program at a major medical center, we are not aware of any comparable estimates of bias due to program attrition. However, our results may not be generalizable to small programs that do not have access to the comprehensive medical records that are available at our institution. Assessing the bias in smaller programs will be more difficult. Similarly, our results are not likely to generalize to other lifestyle regimens, such as the Diabetes Prevention Program. Another limitation is that we had supplemental data for only 78% of participants. However, our lack of supplemental data is largely due to patients who receive their routine care from other health systems. Because these data were unavailable for administrative reasons, the missing data are less likely to induce bias among weight-loss outcomes than data that are missing because of participant self-selection.
Our study is also limited in application to studies of VLED. Although our analytic technique could be applied to other treatment modalities, different interventions have different mean long-term weight loss ((8)). Thus, our results may or may not generalize to other lifestyle regimens, such as the National Diabetes Prevention Program. More research is needed to ascertain whether or not the attrition effect is consistent between treatment modalities.
Finally, we do not suggest that study authors routinely report adjusted averages rather than reporting attrition rates. Ideally, authors would supplement their data with health system data and transparently report outcomes with respect to attrition. However, this is not always possible. For this reason, we provide adjustments for use by policy makers and illustrate the importance of considering the role of attrition bias.
The gold standard for addressing missing data is to collect it. In our case, we collected the data using the EHR. Using these supplemental data, we present models that quantify the effect of attrition. Our estimates could be used to guide imputation or to adjust published results for attrition bias.
Funding agencies
Funded by the National Institutes of Health (NIDDK P30 DK92926 and DK089503).
Disclosure
AER: Personal fees from RHYTHM, outside the submitted work. The other authors declared no conflict of interest.
Clinical trial registration
ClinicalTrials.gov identifier NCT02043457
Data sharing statement
Deidentified data from this study will be available to researchers who submit a methodologically sound proposal to the corresponding author within 36 months of publication.





