Effect of High-Intensity Interval Training on Visceral and Liver Fat in Cardiac Rehabilitation: A Randomized Controlled Trial
Abstract
Objective
This study aimed to investigate the effect of exercise intensity on visceral adipose tissue (VAT) and liver fat reduction in patients with coronary artery disease (CAD) over 3 months and the maintenance of improvements over 12 months.
Methods
Forty-two participants with CAD were randomized to three sessions/week of either 4 × 4-minute high-intensity interval training (HIIT) or 40 minutes of usual care moderate-intensity continuous training (MICT) for a 4-week supervised cardiac rehabilitation program, followed by three home-based sessions/week for 11 months. Liver fat (as intrahepatic lipid) and VAT were measured via magnetic resonance techniques. Data are mean change (95% CI).
Results
HIIT and MICT significantly reduced VAT over 3 months (−350 [−548 to −153] cm3 vs. −456 [−634 to −278] cm3; time × group effect: P = 0.421), with further improvement over 12 months (−545 [−818 to −271] cm3 vs. −521 [−784 to −258] cm3; time × group effect: P = 0.577) and no differences between groups. Both groups improved liver fat over 3 months, with HIIT tending to show greater reduction than MICT (−2.8% [−4.0% to −1.6%] vs. −1.4% [−2.4% to −0.4%]; time × group effect: P = 0.077). After 12 months, improvements were maintained to a similar degree. Higher exercise intensity predicted liver fat reduction (β = −0.3 [−0.7 to 0.0]; P = 0.042).
Conclusions
HIIT and MICT reduced VAT over 3 and 12 months. For liver fat, HIIT tended to provide a slightly greater reduction compared with MICT. These findings support HIIT as a beneficial adjunct or alternative to MICT for reducing visceral and liver fat in patients with CAD.
Study Importance
What is already known?
- ► Visceral adiposity and ectopic fat (including liver fat) are significant contributors to lifetime risk of atherosclerosis and cardiometabolic disease.
- ► Previous studies have shown exercise training can reduce visceral and liver fat even in the absence of weight loss.
- ► High-intensity interval training (HIIT) is a potent stimulus for improving cardiorespiratory fitness in patients with cardiometabolic disease. However, the effect of HIIT on reducing visceral and liver fat compared with moderate-intensity exercise is unclear, particularly over the long term.
What does this study add?
- ► We found that HIIT elicits similar reductions in visceral adiposity to moderate-intensity exercise, and long-term training delivers further improvement. For liver fat, HIIT tends to result in a slightly greater reduction than moderate-intensity exercise over 3 months, with a twofold improvement maintained over 12 months.
How might these results change the direction of research and the focus of clinical practice?
- ► These findings support HIIT as a beneficial adjunct or alternative to moderate-intensity exercise for reducing visceral and liver fat in patients with coronary heart disease.
- ► Further research is warranted to investigate the effect of exercise intensity on liver fat reduction.
Introduction
Visceral adipose tissue (VAT) is a significant contributor to lifetime risk of atherosclerosis and cardiometabolic disease and an independent predictor of morbidity and mortality ((1)). Liver fat is also an emerging risk factor for atherosclerosis and cardiovascular disease ((1)). Exercise training has been shown to reduce liver fat ((2-4)) and VAT ((5, 6)), even in the absence of weight loss. However, whether exercise intensity influences the degree of reduction is less clear ((1)). Results have been conflicting on whether high-intensity exercise produces similar or superior reductions in VAT and/or liver fat ((3, 4, 7)) compared with moderate-intensity exercise. Currently, no studies have investigated the effect of exercise intensity on VAT or liver fat in patients with coronary artery disease (CAD), using gold standard measurement techniques of magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (1H-MRS), respectively.
The aim of this study was to (1) compare the effect of exercise intensity on reducing VAT and liver fat, quantified using gold standard magnetic resonance techniques, in patients with CAD over 3 months; and (2) determine whether improvements can be maintained in the long term over 12 months. Furthermore, we conducted exploratory analyses to investigate relationships between liver fat, VAT, biomarkers, and other body composition measures.
Methods
This randomized controlled trial was part of a larger clinical trial, The FITR Heart (Feasibility, Safety, Adherence, and Efficacy of High Intensity Interval Training in Rehabilitation for Coronary Heart Disease) Study ((8)), comparing high-intensity interval training (HIIT) with usual care moderate-intensity continuous training (MICT) in patients undertaking a 4-week cardiac rehabilitation program and home-based training over 12 months. A detailed description of the design, eligibility, and outcome measures of The FITR Heart Study has been previously published ((8)). It was prospectively registered with Australian New Zealand Clinical Trial Registry (anzctr.org.au; identifier: ACTRN12615001292561; November 26, 2015). This trial was approved by The University of Queensland and UnitingCare Health Ethics Committees and it adhered to the Helsinki Declaration. This manuscript will focus on predefined secondary outcomes of VAT and liver fat (measured as intrahepatic lipid).
Participants and study design
Participants were considered for inclusion in the study following a recent cardiac-related hospital admission and/or procedure at a single tertiary hospital (The Wesley Hospital, Brisbane, Australia). Patients were eligible if they had angiographically proven CAD, were aged 18 to 80 years, and were able to participate in the private hospital cardiac rehabilitation program. Exclusion criteria included any absolute or relative contraindications to exercise testing ((9)) and other criteria previously described ((8)). Written informed consent was obtained at enrollment, and following baseline testing, participants were randomized 1:1 to (1) HIIT or (2) usual care MICT.
The study involved three stages. Stage 1 was a 4-week cardiac rehabilitation program involving two supervised sessions and one home-based session of participants’ randomized training per week. Following the supervised stage, participants were instructed to continue three home-based sessions of their randomized training for a further 11 months. For the first 2 months of home-based training (stage 2), participants received routine support, which involved weekly submission of exercise logs by the participant and weekly phone/email follow-up by the study team. The remaining 9 months of home-based training (stage 3) involved informal support only. Supervised training occurred within the hospital cardiac rehabilitation program (The Wesley Hospital). All testing was performed at The University of Queensland (Brisbane, Australia), and follow-up testing for this substudy was conducted at 3 months (following stage 2) and 12 months (following stage 3).
Exercise training protocols
Exercise training protocols were designed to be matched for energy expenditure, as previously described ((8)). Following a 4-minute warm-up, HIIT involved 4 × 4-minute high-intensity intervals at a rating of perceived exertion (RPE) 15 to 18 (hard to very hard) on a 6 to 20 Borg scale, interspersed with 3 minutes of active recovery. MICT (usual care) involved 34 minutes of moderate-intensity exercise at an RPE 11 to 13 (fairly light to somewhat hard) following a 3-minute warm-up (minimum of 20 minutes per exercise machine). A 3-minute cooldown was used in both groups. A heart rate (HR) range was also given to participants to assist in managing their exercise intensity; this was 85% to 95% HRpeak for HIIT and 65% to 75% HRpeak for MICT. The %HRpeak was derived from the HRpeak achieved during the baseline maximal exercise test. As a high proportion of participants with CAD are prescribed beta-blocker therapy, this relative method of exercise intensity takes into account the likely lower HRpeak achieved by these participants during the exercise test. To ensure training exercise intensity was reflective of medication effects, all participants were instructed to take their usual medications prior to the maximal exercise test. Participants in HIIT and MICT exercised together in the same class, and a variety of machines were utilized according to participant preference and limitations. During the supervised training, HR was measured by three-lead electrocardiography or pulse oximetry. For MICT, HR was measured at least 10 minutes into the exercise session and RPE at the end of each exercise modality. For HIIT, the highest HR and RPE were recorded for each high-intensity interval. Exercise intensity was then assessed as average training RPE, peak training RPE, average training %HRpeak, and peak training %HRpeak as previously suggested ((10)). Average RPE and HR were calculated by averaging the measurements taken during each exercise modality (e.g., treadmill, bike) for MICT or each high-intensity interval for HIIT. Training HR was not measured continuously throughout the entire exercise session.
Outcome measures
Liver fat was assessed by the quantification of intrahepatic lipid, measured by 1H-MRS, using a 3.0 × 2.0 × 2.0-cm voxel. A 3-T MRI system (Magnetom; Siemens, Muenchen, Germany) was used for the measurement of intrahepatic lipid, VAT, and subcutaneous adipose tissue (SAT). Participants were scanned in a supine position obtaining 5-mm transverse sections (without intersection gaps) from the pelvis to the diaphragm. As previously outlined ((8, 11)), a combination of axial T1- and T2-weighted images was acquired during suspended end expiration with breath hold for abdominal VAT and SAT determination.
Analysis of intrahepatic lipid concentration was performed using the MRI user-interface software and expressed as a percentage of the water signal. Processing and analysis of liver spectra were conducted as previously described ((3)). For VAT and SAT, cross-sectional areas were measured using Slice-O-matic software (version 5.0; TomoVision, Magog, Canada). Volumes were calculated through summation of sections (from the L5/S1 junction to the first appearance of the diaphragm) and adjusted for section thickness. A localizing scan enabled accurate detection of sections at the diaphragm and L5/S1 junction. The same number of sections was analyzed at baseline and follow-up for each participant. Repeatability of the analysis for VAT and SAT quantification was assessed with a coefficient of variation.
Body composition (by dual-energy x-ray absorptiometry) and blood biomarkers were measured as previously described ((8)). Lipid profile, liver enzymes, fasting glucose, and high-sensitivity C-reactive protein were measured on a Randox analyzer (Randox Laboratories, Crumlin, UK), and plasma insulin was detected and quantified using radioimmunoassay on a Cobas 3411 (Hitachi High-Technologies Corp., Tokyo, Japan). Insulin resistance was estimated by the homeostatic model assessment ((12)). Interleukin- 6 and -10, leptin, and vascular adhesion molecule-1 were analyzed using a Milliplex magnetic bead panel (Merck KGaA, Darmstadt, Germany) on a Magpix analyzer (Luminex Corp., Austin, Texas), and high-molecular-weight adiponectin was analyzed using a specific enzyme-linked immunosorbent assay kit (Merck KGaA, Darmstadt, Germany). All assessments were performed in duplicate, with the average value used for data analysis.
Exercise adherence was measured by a combination of supervised exercise records, self-report exercise logs, and exercise training questionnaires. Adherence to the exercise protocol is presented as the percentage of participants with ≥ 70% attendance at the recommended number of exercise sessions and who trained at the prescribed exercise intensity prescription during the exercise sessions. Physical activity (including exercise training), assessed as daily minutes in moderate to vigorous physical activity (MVPA) for ≥ 1-minute bouts, was measured by triaxial accelerometers with specific thresholds used for the wrist (100 mg) and hip (70 mg) ((13, 14)). Accelerometers were worn on the nondominant wrist (GENEActiv; ActivInsights, Cambridgeshire, UK) or waist (ActiGraph, Pensacola, Florida) as previously described ((8)), with open source code used for analysis ((15)). Habitual diet was measured over 2 days at each time point by 24-hour diet recall using a multiple pass method ((8)). Average daily intake of energy and macronutrients was calculated from the Australian Food, Supplement, and Nutrient Database, using dietary analysis software (Foodworks, Xyris, Brisbane, Australia) or Automated Self-administered 24-hour dietary assessment tool (Australian version; National Cancer Institute, Bethesda, Maryland) ((16)). The same analysis method was used for each participant at baseline and follow-up.
Randomization and blinding
The random allocated sequence was computer generated and sealed in sequentially numbered opaque envelopes. To ensure allocation concealment, this was performed by an individual external from the investigation team. The principal investigator of the study enrolled participants and, after baseline testing was completed, assigned them to an exercise group using the next envelope in the sequence. The randomization process was stratified by sex and initial fitness level as previously described ((8)). MRI technicians were blinded to group allocation. Images were also coded by independent personnel to ensure blinding during analysis of VAT and liver fat.
Statistical analysis
The sample size for predefined secondary outcomes (including VAT and liver fat) was based on the predefined primary outcome of The FITR Heart Study, cardiorespiratory fitness (VO2peak) ((8)). In brief, we determined 80 participants (40 per group) would be sufficient to detect a one-metabolic-equivalent (3.5 mL/kg/min) difference between groups, with an SD of 4.75 mL/kg/min, power of 0.9, and 0.05 significance level.
For secondary outcomes in this study, intention-to-treat analyses were performed using a linear mixed model to investigate time × group interactions over 3 and 12 months. Time and group were included as fixed effects and subject was included as the random effect. Linear mixed modeling accounts for missing data with the assumption that the data is missing at random. Data are presented as the mean change (95% CI). Baseline characteristics and exercise training data were analyzed using Fisher exact for categorical data and t test for continuous data. These data are presented as mean (SD). Where data were not normally distributed, log transformation was used for statistical analysis; however, actual data are presented in the results tables. If log transformation was unable to normally distribute the data, sensitivity analyses by a nonparametric Kruskal-Wallis test were conducted to compare (1) the group change scores and (2) the absolute value of each group at follow-up. To determine the robustness of the primary analysis (intention to treat), sensitivity analyses were also conducted for complete case analysis and a prespecified per protocol analysis. Complete case analysis was performed because of the large percentage of missing data (32% HIIT and 35% MICT) for liver fat, VAT, and SAT at 12 months. Per protocol analysis included only participants meeting the criteria for exercise adherence. For liver fat and VAT, exploratory analyses for predictors of change were further investigated. Baseline or change outcomes with the strongest correlation were identified by bivariate correlations and then analyzed using multivariate linear regression to explore independent predictors of change.
Results
Participant characteristics
Recruitment for the clinical trial commenced in May 2016 and concluded in November 2017. Recruitment ended when the sample size for the primary outcome was attained. A summary of participant inclusion, withdrawal, and loss to follow-up is presented in Figure 1. A total of 42 participants underwent baseline MRI and 1H-MRS assessment for visceral and liver fat in this subgroup analysis study, with 19 and 23 participants randomized to HIIT and MICT, respectively. The intended sample of 40 participants per group was unable to be attained primarily because of a lack of funding (n = 47). There were no differences in baseline characteristics between groups (Table 1), and both groups had a similar percentage of participants with elevated liver fat (> 5.5%). At the conclusion of the study, a total of 29 participants (13 HIIT, 16 MICT) were analyzed for liver fat, and 29 participants (14 HIIT, 15 MICT) were analyzed for adipose tissue. Follow-up testing for the final participant concluded in December 2018.
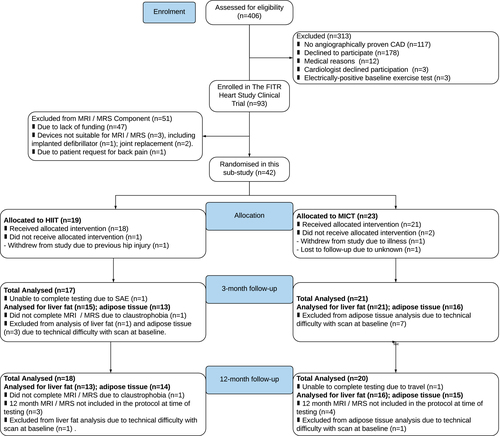
| HIIT (n = 19) | MICT (n = 23) | P value | |
|---|---|---|---|
| Sex (male, female), n | 15, 4 | 19, 4 | 1.000 |
| Age (y) | 65 ± 7 | 63 ± 7 | 0.433 |
| VO2peak (mL/kg/min) | 27.6 ± 6.1 | 29.0 ± 7.9 | 0.532 |
| BMI (kg/m2) | 27.7 ± 4.2 | 28.3 ± 4.0 | 0.617 |
| Liver fat (%) | 7.3 ± 8.9 | 4.8 ± 4.1 | 0.267 |
| Coronary event/intervention, n (%) | |||
| Acute coronary syndrome | 2 (11) | 5 (22) | 0.428 |
| Coronary artery bypass graft surgery | 4 (21) | 4 (17) | 1.000 |
| Percutaneous coronary intervention | 11 (58) | 12 (52) | 0.763 |
| Pharmacotherapy only | 4 (21) | 7 (30) | 0.726 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 1 (5) | 3 (13) | 0.613 |
| Current smoking | 0 (0) | 2 (9) | 0.492 |
| Hepatic steatosis (liver fat ≥ 5.5%) | 7 (39) | 7 (30) | 0.742 |
| Medications, n (%) | |||
| Aspirin | 18 (95) | 21 (91) | 1.000 |
| Antiplatelet | 12 (63) | 13 (57) | 0.757 |
| Statin | 18 (95) | 21 (91) | 1.000 |
| Nonstatin cholesterol lowering | 3 (16) | 5 (22) | 0.709 |
| Oral hypoglycemic agent | 1 (5) | 2 (9) | 1.000 |
| Insulin | 0 (2) | 1 (4) | 1.000 |
| β-blocker | 6 (32) | 7 (30) | 1.000 |
| Angiotensin-converting enzyme inhibitor | 1 (5) | 7 (30) | 0.054 |
| Angiotensin II receptor blocker | 6 (32) | 9 (39) | 0.750 |
| Calcium channel blocker | 1 (5) | 2 (9) | 1.000 |
| Diuretic | 4 (21) | 2 (9) | 0.384 |
- Values are mean ± SD or n (%). Significance is < 0.05.
- HIIT, high-intensity interval training; MICT, moderate-intensity continuous training; VO2peak, peak oxygen uptake.
Liver fat
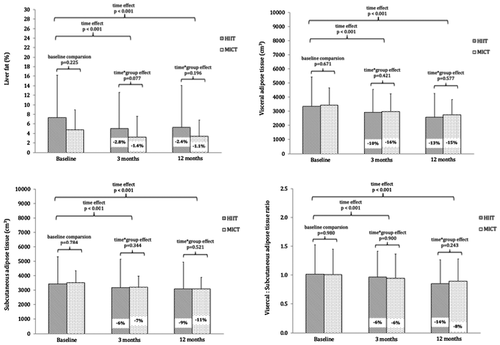
Following 3 months of training, both groups reduced liver fat; however, HIIT tended to show a greater reduction, which was twofold compared with MICT (−2.8% [−4.0% to −1.6%] vs. −1.4% [−2.4% to −0.4%]; time × group effect: P = 0.077) (Table 2; Figure 2). At 12 months, HIIT and MICT maintained their reductions in liver fat to a similar degree (−2.4% [−4.0% to −0.7%] vs. −1.1% [−2.6% to −0.3%]; time × group effect: P = 0.196). To determine whether the higher baseline liver fat in the HIIT group influenced the greater reduction, sensitivity analysis was performed with removal of two extreme outliers (liver fat > 25%) in the HIIT group (Supporting Information Table S1). Removing these outliers reduced the HIIT baseline liver fat to 4.8% ± 5.0% for the intention-to-treat analysis (similar to baseline for MICT) but had little effect on the absolute reduction for HIIT over 3 months (−2.6% [−3.8% to −1.3%]) and 12 months (−2.8% [−4.5% to −1.2%]). This resulted in larger relative changes for HIIT with reductions of 54% (compared with 38%) at 3 months and 58% (compared with 38%) at 12 months. Given the reduced sample size for liver fat at 12 months, complete case analysis was also performed (Supporting Information Table S1). This showed a similar twofold reduction in liver fat at 3 months and 12 months with HIIT compared with MICT when the entire sample size was included. When excluding outliers in the complete case analysis, a threefold reduction was shown by HIIT compared with MICT at 12 months (−3.1% vs. −1.0%). Finally, per protocol analysis (Supporting Information Table S2) showed similar results for 3 months with a twofold improvement favoring HIIT (−2.9% [−4.4% to −1.4%] vs. −1.4% [−1.6% to −0.2%]; time × group effect: P = 0.069) but a smaller mean difference between groups at 12 months (−2.2% [−4.5% to −0.1%] vs. −1.4% [−3.9% to 1.1%]; time × group effect: P = 0.628).
| Outcome measure | n | Stages 1-2 | P value | Stages 1-3 | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change in 3 months | Time | Time × group | Change in 12 months | Time | Time × group | |||||
| HIIT | MICT | HIIT | MICT | HIIT | MICT | ||||||
| Liver fat and adipose tissue | |||||||||||
| Liver fat (%) | 41 | 7.3 ± 8.9 | 4.8 ± 4.1 | −2.8*, * (−4.0 to −1.6) | −1.4*, * (−2.4 to −0.4) | < 0.001 | 0.077 | −2.4*, * (−4.0 to −0.7) | −1.1 (−2.6 to 0.3) | < 0.001 | 0.196 |
| Visceral adipose tissue volume (cm3) | 32 | 3,357 ± 2,060 | 3,449 ± 1,212 | −350*, * (−548 to −153) | −456*, * (−634 to −278) | < 0.001 | 0.421 | −545*, * (−818 to −271) | −521*, * (−784 to −258) | < 0.001 | 0.577 |
| Subcutaneous adipose tissue volume (cm3) | 32 | 3,445 ± 1,865 | 3,515 ± 846 | −213*, * (−382 to −43) | −320*, * (−473 to −167) | < 0.001 | 0.344 | −244*, * (−481 to −7) | −384*, * (−612 to −157) | < 0.001 | 0.521 |
| Visceral:subcutaneous adipose tissue ratio | 32 | 1.0 ± 0.5 | 1.0 ± 0.4 | −0.06*, * (−0.11 to −0.01) | −0.06*, * (−0.11 to −0.02) | 0.001 | 0.900 | −0.14*, * (−0.21 to −0.07) | −0.08*, * (−0.15 to 0.02) | < 0.001 | 0.243 |
| Body composition | |||||||||||
| BMI (kg/m2) | 42 | 27.7 ± 4.2 | 28.3 ± 4.0 | −0.3*, * (−0.6 to −0.1) | −0.6*, * (−0.8 to −0.3) | < 0.001 | 0.253 | −0.4 (−0.8 to 0.1) | −0.7*, * (−1.1 to −0.2) | < 0.001 | 0.490 |
| Body mass (kg) | 42 | 81.3 ± 14.4 | 85.7 ± 14.3 | −1.0*, * (−1.9 to −0.2) | −1.8*, * (−2.6 to −1.1) | < 0.001 | 0.165 | −1.1 (−2.5 to 0.4) | −2.1*, * (−3.4 to −0.8) | < 0.001 | 0.372 |
| Waist circumference (cm) | 42 | 97.8 ± 13.5 | 99.2 ± 9.6 | −2.5*, * (−4.1 to −0.9) | −3.3*, * (−4.7 to −1.9) | < 0.001 | 0.447 | −2.7*, * (−4.9 to −0.4) | −5.1*, * (−7.3 to −3.0) | < 0.001 | 0.147 |
| Fat mass (kg) | 42 | 26.0 ± 7.7 | 28.1 ± 7.7 | −0.9 (−2.0 to 0.2) | −1.8*, * (−2.8 to −0.9) | 0.001 | 0.222 | −0.4 (−1.7 to 1.0) | −1.4*, * (−2.7 to −0.1) | 0.003 | 0.341 |
| Fat-free mass (kg) | 42 | 54.1 ± 9.4 | 56.2 ± 10.4 | 0.1 (−0.7 to 0.9) | 0.4 (−0.4 to 1.1) | 0.357 | 0.546 | −0.9 (−2.2 to 0.4) | −0.4 (−1.6 to 0.8) | 0.028 | 0.801 |
- Baseline results data presented as mean ± SD. Follow-up data presented as mean change (95% CI). P values provided for time effects and time × group interaction effects after 4 weeks and 12 months.
- * Significant difference from baseline.
- HIIT, high-intensity interval training; MICT, moderate-intensity continuous training.
Abdominal adipose tissue
There were no differences between groups for VAT reduction, with both groups significantly reducing VAT at 3 months (Table 2; Figure 2). After 3 months of training, HIIT reduced VAT by 10% compared with 13% for MICT (time × group effect: P = 0.421). After 12 months, HIIT reduced VAT by 16% compared with 15% for MICT (time × group effect: P = 0.577). Results for SAT showed a similar trend (Figure 2). After 3 months, HIIT reduced SAT by 6% compared with 9% for MICT (time × group effect: P = 0.344). After 12 months, HIIT maintained a reduction in SAT by 7% compared with 11% for MICT (time × group effect: P = 0.521). Nonparametric sensitivity analysis showed similar results (Supporting Information Table S1). The VAT:SAT ratio was also reduced in HIIT and MICT over 3 and 12 months with no differences between groups (time × group effect: P = 0.900 and P = 0.243, respectively). After 3 months, the VAT:SAT ratio was reduced by 6% for both HIIT (−0.06 [−0.11 to −0.01] cm3) and MICT (−0.06 [−0.011 to −0.02] cm3) (Table 2; Figure 2). After 12 months, the reduction was 14% (0.14 [−0.21 to −0.07] cm3) for HIIT and 8% (−0.08 [−0.15 to −0.02] cm3) for MICT. The coefficient of variation for VAT and SAT measurements was 5% and 0%, respectively. The coefficient of variation for change in VAT was 2%.
Body composition, cardiovascular risk factors, liver aminotransferases, and adipokines
Both HIIT and MICT had small reductions in body weight, BMI, waist circumference, fat mass, and fat-free mass over 3 and 12 months, with no differences between groups (Table 2). However, there was a trend for greater reductions with MICT over 12 months. In particular, waist circumference reduced by 5% in MICT, which was almost a twofold improvement compared with HIIT (time × group effect: P = 0.147). There was an increase in high-density lipoprotein cholesterol over 12 months for both groups (time effect: P < 0.001) but no group difference (Supporting Information Table S3). There was no change over time or differences between groups for any other biomarkers or cardiovascular disease risk factors (Supporting Information Table S3).
Exercise adherence
Supervised exercise data (from weeks 1-4) indicated that both HIIT and MICT were exercising at intensities within intended targets for both RPE and %HRpeak (Supporting Information Table S4). For HIIT, both average training RPE (16.5 [1.2]) and average training %HRpeak (88% [6%]) were different from average training RPE (12.6 [0.4]) and average training %HRpeak (71% [7%]) for MICT (P < 0.001). At 3 months (following home-based training), intensities were similar to the supervised training with an average training RPE of 16.3 (1.3) for HIIT and 12.5 (0.4) for MICT. Exercise adherence was highest at 3 months (HIIT: 77%; MICT: 86%; P = 0.678) and declined over 12 months (HIIT: 56%; MICT: 39%; P = 0.505), with no differences between groups (Table 3). At 3 months, the MICT group was performing a greater number of self-reported weekly exercise minutes compared with HIIT (240 [151] vs. 144 [73]; P = 0.015); however, groups were not different at 12 months (Table 3).
| Measure | 3 months | 12 months | ||||
|---|---|---|---|---|---|---|
| HIIT (n = 17) | MICT (n = 21) | P value | HIIT (n = 18) | MICT (n = 20) | P value | |
| Adherence to exercise training protocols, n (%) | 13 (77) | 18 (86) | 0.678 | 10 (56) | 7 (39) | 0.505 |
| Adherence to attendance at exercise sessions, n (%) | 13 (77) | 18 (86) | 0.678 | 11 (61) | 12 (67) | 1.000 |
| Adherence to prescribed exercise intensity, n (%) | 16 (94) | 19 (91) | 1.000 | 11 (61) | 7 (39) | 0.318 |
| Total exercise sessions per week, n | 3.2 ± 1.5 | 3.8 ± 1.9 | 0.270 | 3.2 ± 2.0 | 3.5 ± 2.2 | 0.720 |
| Total exercise minutes per week, n | 144 ± 73 | 240 + 151 | 0.015*, * | 130 ± 108 | 191 ± 164 | 0.196 |
- * Significant difference between groups.
- Values presented as mean ± SD unless otherwise stated.
- Adherence to exercise training protocol involves ≥ 70% attendance at recommended number of exercise sessions and participant training at recommended exercise intensity prescription during exercise sessions.
- HIIT, high-intensity interval training; MICT, moderate-intensity continuous training.
Habitual physical activity and diet
There was also a group difference for objectively measured physical activity minutes at 3 months, with an increase in MVPA minutes from baseline for MICT but not HIIT (10 [3 to 18] vs. 1 [−9 to 6]; P = 0.038). However, no group difference was found at 12 months (Supporting Information Table S3). For habitual dietary intake, there was no effect of time or differences between groups at 3 months or 12 months (Supporting Information Table S3).
Safety
There were three serious adverse events requiring hospitalization reported to the ethics committees throughout the study period (HIIT: n = 2; MICT: n = 1). None of these serious adverse events was deemed to be related to the exercise interventions.
Relationships between change in liver fat, VAT, and other outcome measures
Bivariate correlations for liver fat, VAT, SAT, exercise training, and other clinical measures are displayed in Supporting Information Tables S5-S7 for baseline, 3-month, and 12-month correlations, respectively. Independent predictors for liver fat and VAT by linear regression are shown in Supporting Information Tables S8-S9, respectively. Exercise intensity (as peak training RPE) was an independent predictor for change in liver fat over 3 months (β = −0.5 [-0.9 to −0.1]; P = 0.031), as was baseline liver fat (β = −0.2 [−0.3 to −0.1]; P < 0.001) and age (β = 0.1 [0.0 to 0.2]; P = 0.019). For VAT, weekly exercise minutes was an independent predictor of change over 12 months (β = −1 [−2 to 0]; P = 0.022) in addition to changes in body composition measures.
Discussion
This study investigated the changes in visceral adiposity and liver fat with HIIT and usual care MICT during and following a cardiac rehabilitation program over 12 months. This is the first study to investigate the effect of exercise intensity on liver fat and VAT in a cardiac rehabilitation population by using gold standard magnetic resonance techniques. We observed similar reductions in VAT and SAT for both groups at 3 months and 12 months. For liver fat, HIIT tended to result in a greater reduction compared with MICT at 3 months with a twofold improvement, although this was not statistically significant. Both groups maintained their liver fat reductions over 12 months to a similar degree. Previous studies comparing HIIT with MICT have been conflicting, showing similar reductions for liver fat and VAT in healthy adults with obesity ((3)) or a superior reduction in liver fat with HIIT (without changes in VAT) in patients with nonalcoholic fatty liver disease ((4)). An additional unique feature of the current study was that the design employed exercise training in a hospital-based cardiac rehabilitation setting, with 4 weeks of supervised training at the beginning of the 12-month study period. Given the established relationships between visceral adiposity, liver fat, and cardiometabolic risk, these findings support HIIT as a beneficial adjunct or alternative to MICT for improvement of visceral and liver fat in cardiac rehabilitation programs.
The absolute 2.8% reduction in liver fat with HIIT over 3 months is consistent with previous studies in adults with overweight ((3)) and patients with nonalcoholic fatty liver disease ((2)). Though we observed that baseline liver fat was an independent predictor of liver fat reduction, this did not appear to be a factor relating to the greater reduction in liver fat seen with HIIT compared with MICT. Our sensitivity analysis, which excluded outliers in the HIIT group and brought the baseline values closer together, had little influence on the absolute reduction for HIIT. Therefore, our finding that HIIT tended to result in slightly greater liver fat reduction was unlikely influenced by regression to the mean.
For VAT and SAT, previous studies comparing HIIT and MICT have found conflicting results, with similar reductions between groups ((3)) or a superior effect of HIIT ((7)). Our reduction in VAT of 15% over 3 months with MICT was similar to Keating et al. ((3)) but less than Zhang et al. ((7)). Within these studies, participants exercised four times per week at a moderate intensity (50% VO2peak and 60%-70% HRpeak, respectively). Our MICT group exercised at a higher intensity but with fewer weekly exercise sessions. The reduction in VAT of 10% over 3 months with HIIT was similar to previous studies ((17, 18)) but less than Keating et al. ((7)). All studies prescribed similar exercise intensity to our HIIT group. As the latter study prescribed an additional exercise session per week ((7)), it is logical to infer the degree of VAT reduction is related to total energy expenditure. Indeed, a dose-response relationship between aerobic exercise and VAT reduction has been reported ((19)). Furthermore, we found weekly exercise minutes was an independent predictor for VAT reduction over 12 months. This relationship was even stronger for SAT but not evident for liver fat. Rather, the reduction in liver fat was independently predicted by exercise intensity. Given that the total volume of VAT reduction in the present study was greater than the total volume of SAT, the VAT:SAT ratio was also improved over the study period. Though the link between VAT and liver fat with cardiometabolic risk is well established, there is less consensus regarding the health consequences of SAT. Indeed, SAT may be protective when capacity to expand is adequate to accommodate additional fat storage, thereby preventing visceral and ectopic fat deposition ((1)). The VAT:SAT ratio reflects this protective effect with an increased ratio linked to increased severity of obstructive CAD ((20)).
Although MICT resulted in a greater magnitude of improvement (almost twofold) in body mass and waist circumference compared with HIIT, the group difference was not clinically meaningful (< 5% body weight loss) ((21)). This is consistent with a previous meta-analysis concluding that improvements in body composition tended to favor MICT, compared with HIIT, when energy expenditure was greater ((22)). Despite the exercise protocols being designed for similar energy expenditure, some participants chose to complete additional exercise to the recommended protocol. At 3 months, weekly exercise minutes and minutes in MVPA were greater in the MICT group. At 12 months, weekly exercise minutes remained 30% higher in the MICT group. Changes in body mass and waist circumference were significantly associated with reductions in VAT and SAT but not liver fat.
This study is strengthened by the use of gold standard magnetic resonance methods for quantifying abdominal adipose tissue and liver fat. Multiple MRI sections were obtained from the diaphragm to the pelvis without intersection gaps, improving accuracy by negating the need to estimate total volumes from a single section or use algorithms to account for intersection gaps. Additional sensitivity analyses were performed to provide robustness to our results. Finally, this study is strengthened by the rigorous effort to capture differences in diet, physical activity, and exercise that may influence our findings.
The major limitation of this study is the reduced sample size and power to detect statistical differences between groups for liver fat and VAT. As these were secondary outcomes in a larger clinical trial, funding was available only for a subgroup of participants. Post hoc analyses revealed that for the effect sizes we found with our sample size over 3 months in our intention-to-treat analyses, the probability of a false negative (or type II error) was 60% for liver fat results (power 0.4) and 90% for VAT (power 0.1). However, the moderate effect size found in our analyses for liver fat (0.6), the low power, and the marginally significant P value (0.077) suggest that HIIT might perform slightly better than MICT. While the current study is underpowered and therefore cannot provide firm conclusions, these results reinforce the need for further research investigating the relative importance of exercise intensity on liver fat reduction. Given these effect sizes, the sample size required to achieve sufficient power (> 0.8) is 44 participants per group (total n = 88) for liver fat and 165 per group (total n = 330) for VAT. This will aid in the research design of future studies. Furthermore, given there is no existing data regarding a clinically meaningful change for liver fat or VAT for reducing cardiovascular risk, we have reported on the magnitude of change and confidence intervals, with HIIT showing a twofold improvement in liver fat compared with MICT over 3 months. Another limitation of this study is the lack of a nonexercise control group. In a cardiac rehabilitation trial, there are ethical concerns in randomizing patients to no exercise or delaying a rehabilitation program. Instead, our control group was the usual care exercise (MICT) prescribed during cardiac rehabilitation. Therefore, it is possible the reductions in VAT and liver fat in this study may have been influenced by attending cardiac rehabilitation rather than directly related to the exercise training. Finally, as our study involved a clinical population with cardiovascular disease and high percentage of males (80%), these results may not allow generalization to females or healthy populations. The low rate of female participation also weakens this study because of the inability to explore potential sex differences on the benefits of HIIT and MICT for reducing VAT and liver fat. Previous work has highlighted sex-based differences in hepatic metabolism and lipid storage ((23)).
Conclusion
This study in patients undertaking cardiac rehabilitation found that both HIIT and MICT significantly reduced VAT and abdominal SAT over 3 months and 12 months. Liver fat was also reduced in both groups over 3 months. While HIIT tended to result in a greater reduction, which was twofold compared with MICT, this was not statistically significant. Over 12 months, both groups maintained their reductions in liver fat to a similar degree. Improvements in VAT, SAT, and body composition were found to be associated with increased weekly exercise minutes, whereas improvements in liver fat were independently predicted by higher exercise intensity. These findings support HIIT as a beneficial adjunct or alternative to MICT in cardiac rehabilitation programs, allowing for prescription based on patient goals, preferences, and capabilities. Further adequately powered studies are warranted to confirm any effect of exercise intensity on liver fat reduction.
Acknowledgments
We thank our participants for their involvement in the study. We acknowledge Aiman Najjar and Nicole Atcheson for their magnetic imaging technical support as well as Ainsley Bolton for her assistance with dietary recall data collection and Kelly Bang for her assistance in Foodworks software analysis. A study protocol has been published in an open access journal that may contain additional information to that contained in the manuscript ((8)). Deidentified individual participant data that underlie the results reported in this article (text, tables, figures, and appendices) will be available for sharing immediately following publication with no end date. Any researchers who provide a methodologically sound proposal can access this data to achieve the aims in this proposal or for individual participant data meta-analysis. Proposals should be directed to [email protected] or [email protected]. Data requestors will need to sign a data access agreement to gain access.
Funding agencies
This study was supported by grant funding from Wesley Medical Research (grant 2015-17) and the Centre of Advanced Imaging at The University of Queensland (grant 16035), as well as philanthropic funding by Neatstone Pty Ltd. The primary author, JLT, was supported by Postgraduate Research Scholarships from The National Health and Medical Research Council (1133622) and The University of Queensland (44018586). SEK was supported by the National Health and Medical Research Council of Australia via an Early Career Research Fellowship (1122190). GIM is supported by a Development Fellowship from The University of Queensland (UQFEL1830501). AVR is with the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre and the NIHR Collaboration for Leadership in Applied Health Research and Care, East Midlands. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure
The authors declared no conflicts of interest.
Authors contributions
JLT was responsible for conception and design of the study, data collection and analysis, and manuscript writing. SEK contributed to conception and design of the study, data analysis and interpretation, and critical revision of the manuscript. DJH, NAJ, MDL, SRG, and JSC contributed to design of the study and critical revision of the manuscript. GIM and TGB contributed to data interpretation and critical revision of the manuscript. AVR contributed to data analysis and interpretation and critical revision of the manuscript.
Clinical trial registration
Australian New Zealand Clinical Trial Registry (anzctr.org.au) identifier ACTRN12615001292561 (November 26, 2015).





