Circadian Rhythm of Substrate Oxidation and Hormonal Regulators of Energy Balance
Abstract
Objective
The circadian system provides an organism with the ability to anticipate daily food availability and appropriately coordinate metabolic responses. Few studies have simultaneously assessed factors involved in both the anticipation of energy availability (i.e., hormones involved in appetite regulation) and subsequent metabolic responses (such as energy expenditure and substrate oxidation) under conditions designed to reveal circadian rhythmicity.
Methods
Eight healthy adults (four females; age: 28.0 ± 2.3 years; BMI: 24.3 ± 2.9 kg/m2) participated in a 26-hour constant routine protocol involving continuous wakefulness with constant posture, temperature, dim light, and hourly isocaloric snacks. Indirect calorimetry was performed every 3 hours for measurement of energy expenditure and substrate oxidation. Subjective hunger was obtained hourly using questionnaires. Saliva and plasma were obtained hourly to assess melatonin (circadian phase marker) and hormones (leptin, ghrelin, and peptide YY).
Results
Fat and carbohydrate oxidation was highest in the biological evening and morning, respectively. Subjective hunger ratings peaked during the middle of the biological day. Significant circadian rhythms were identified for ghrelin and peptide YY with peaks in the biological evening and morning, respectively.
Conclusions
These findings support a role for the circadian system in the modulation of nutrient oxidation, subjective measures of appetite, and appetitive hormones.
Study Importance
What is already known?
- ► Previous research has identified circadian rhythms in energy expenditure, substrate oxidation, subjective ratings of hunger, and concentrations of appetite-related hormones under fasting and nonfasting conditions.
- ► However, rarely are all of these variables measured simultaneously in the same participants to evaluate interrelationships.
What does this study add?
- ► Using a highly controlled constant routine protocol (26 hours of wakefulness with constant posture, temperature, dim light, and hourly isocaloric snacks), we demonstrate endogenous circadian rhythms in substrate oxidation, subjective hunger ratings, ghrelin, and peptide YY in healthy adults.
- ► These findings contribute to our understanding of how factors involved in energy balance are modulated by the circadian system.
How might these results change the direction of research?
- ► Future studies are needed to determine how rhythms in energy metabolism differ between lean adults and adults with conditions such as obesity and diabetes.
- ► It will also be important to know how rhythms in energy metabolism respond to therapeutic interventions such as weight loss and exercise as well as the potential implications of these responses for metabolic health.
Introduction
Energy balance refers to a physiologic state in which total daily energy expenditure (TDEE) (composed of resting energy expenditure [EE] and diet induced- and exercise-induced thermogenesis) is equal to energy intake (EI). Daily EE and EI are tightly regulated by homeostatic mechanisms to maintain energy balance ((1)). During the fasted state, the orexigenic hormone ghrelin is secreted from the gut, enters the plasma, and stimulates energy-sensing regions of the hypothalamus triggering EI. During the postprandial period, hormonal responses favor satiety and increased EE, marked by the release of anorexigenic humoral signals from the pancreas (insulin), adipose tissue (leptin), and the gastrointestinal tract (glucagon-like peptide 1, cholecystokinin, and peptide YY [PYY]) ((1)). In healthy individuals, the interplay among orexigenic and anorexigenic signals results in a remarkably stable body mass over time despite large day-to-day fluctuations in EI and EE ((1)).
Many hormones (e.g., leptin, ghrelin, and PYY) ((3, 5)) and metabolic processes involved in the homeostatic regulation of energy balance exhibit distinct diurnal rhythmicity that is cued to the feeding-fasting cycle. However, some are also secreted in an anticipatory manner in part regulated by the circadian system ((6-8)). The circadian timekeeping system is designed to anticipate environmental changes (e.g., light, food availability) so that metabolic physiology and behavior are timed appropriately. Clock-driven rhythms have been identified in EE ((8-10)), substrate oxidation ((8)), subjective hunger ((6, 11-14)), leptin, and ghrelin ((2, 6, 15)), though results have been inconsistent. For example, carbohydrate oxidation has been shown to peak during the biological day with fat oxidation peaking during the biological night in one study ((8)), but another failed to demonstrate a rhythm in substrate oxidation as measured by the respiratory quotient (RQ) ((10)).
Furthermore, few previous studies have simultaneously measured variables relevant to energy balance in the same participants. To understand how the timing of factors involved in energy intake are temporally related to the timing of factors involved in nutrient oxidation, it is important to examine these variables in the same individuals. Moreover, there appears to be vast interindividual variability in the circadian profiles of variables associated with energy balance regulation.
Therefore, the present study utilized a highly controlled constant routine protocol to simultaneously evaluate group- and individual-level variability and rhythms in EE, substrate oxidation (i.e., RQ and calculated fat and carbohydrate oxidation), subjective hunger, and appetite-related hormones (leptin, ghrelin, and PYY) over one circadian cycle. The constant routine protocol evenly distributes or holds constant environmental and behavioral factors that impact outcomes of interest (e.g., dim light, continuous wakefulness, ambient thermoneutral temperature, hourly snacks, posture, and activity levels [bedrest]) ((16)). Under these conditions, it is possible to determine whether a given metabolic or physiologic variable possesses a circadian rhythm.
Given the discrepant reports between rhythms in EE and substrate oxidation, we aimed to verify circadian rhythmicity in these variables. We hypothesized that circadian rhythms in both ghrelin and leptin would oscillate in parallel with subjective ratings of hunger and fullness, respectively. We further hypothesized that the satiety signal PYY would display a circadian rhythm, which has not been assessed previously.
Methods
Participants
Eight healthy participants (four females/four males) aged 28.0 (2.3) years (mean [SD]), with a normal to overweight BMI of 24.3 (2.9) kg/m2 and percent body fat of 30.0% (5.9%), completed the study protocol. Men were 27.5 (2.6) years old with a BMI of 25.4 (1.6) and percent body fat of 27.1% (5.0%), and women were 28.5 (1.9) years old with a BMI of 23.2 (3.2) and percent body fat of 33.0% (5.3%). Specific inclusion and exclusion criteria are provided in the Supporting Information. One female participant had to discontinue the inpatient protocol early because of nausea and was excluded from the present analyses. Therefore, data for seven participants are presented in this manuscript. All study procedures were approved by the scientific and advisory review committee of the Colorado Clinical and Translational Sciences Institute and the Colorado Multiple Institutional Review Board.
Run-in period
Prior to admission to the Clinical Translational Research Center (CTRC), participants completed a 7-day outpatient run-in protocol with an 8-hour sleep schedule based on habitual sleep/wake times and verified by wrist actigraphy (Actiwatch-2; Philips Respironics, Murrysville, Pennsylvania), sleep logs, and text messages of bed and wake times to an online form. Drugs, medications, and nicotine were proscribed during the 7-day home assessment prior to CTRC admission for the inpatient protocol. For 3 days prior to the inpatient protocol, vigorous exercise, caffeine, and alcohol were proscribed, and participants consumed an energy-balanced diet provided by the CTRC Metabolic Kitchen (TDEE = resting metabolic rate [RMR] × an activity factor of 1.4). Macronutrient composition consisted of 30% fat, 55% carbohydrate, and 15% protein. To control for potential effects of prior meal timing on circadian phase of variables of interest, the 3-day outpatient diet meals were consumed at 1.5 hours, 5.5 hours, 10.5 hours, and 14.5 hours after awakening for breakfast (30% of TDEE), lunch (30% of TDEE), dinner (30% of TDEE), and an evening snack (10% of TDEE). All three female participants were studied in the early follicular phase of the menstrual cycle (estradiol, 26.7 [2.9] pg/mL; progesterone, 0.6 [0.2] ng/mL).
Inpatient constant routine protocol
All protocol events were scheduled relative to habitual wake time as determined during the 7-day run-in phase for each individual. Participants were admitted to the inpatient CTRC approximately 4 hours prior to their habitual bedtime. Urine pregnancy tests confirmed female participants were not pregnant during the study. Light exposure was maintained at less than 5 lux during scheduled wake and 0 lux during scheduled sleep. Lighting in the room consisted of three main overhead fixtures each containing 3 m × 1.2 m-long, 28-watt fluorescent lamps in the 3,500 k spectrum. Each overhead light fixture was covered with diffusion filter paper and dimmed to its lowest setting to achieve < 5 lux measured from the participants angle of gaze. On night one, participants were provided a baseline 8-hour sleep opportunity. Immediately upon waking the following morning, an indwelling venous catheter was inserted into the antecubital space of the arm. During the constant routine, wakefulness was maintained for 26 hours in a semirecumbent posture (head of the bed raised to 35 degrees) in dim light as described above, and participants were provided hourly isocaloric snacks (matched to the macronutrient content of the run-in diet). The hourly meals consisted of either a custom liquid nutritional supplement or a solid food option (small sandwiches) prepared by the metabolic kitchen. Both options had the same macronutrient composition as the run-in diet. Participants picked one of these options and consumed it exclusively for the duration of the study period. Each snack represented 1/24th of the participants’ TDEE. Participants were given ~100 mL water with each hourly snack. Individual energy requirements during the constant routine were calculated as RMR × an activity factor of 1.07 to account for the measured energy cost of 24 hours of waking bedrest ((17)). Wakefulness was confirmed by continuous monitoring by staff. After the completion of the constant routine, participants were given a recovery sleep opportunity (8-10 hours) prior to discharge.
Indirect calorimetry
Minute-by-minute EE and the RQ were measured every 3 hours starting 3 hours after waking using hood calorimetry (ParvoMedics TrueOne 2400 Metabolic Measurement System; Sandy, Utah). Recordings lasted between 10 and 20 minutes, with the first 5 minutes excluded from the analysis (see Supporting Information for additional details). EE was calculated using the Weir equation ((18)), and RQ was calculated as the ratio of volumes of CO2 to O2 (VCO2/VCO2). Rates of carbohydrate oxidation and fat oxidation were calculated as milligrams per minute according to the formula of Frayn ((19)). Percent contribution of fat and carbohydrate to total oxidation was calculated as follows: percent carbohydrate oxidation = ([RQ − 0.71] / 0.29) × 100; percent fat oxidation = 100 − percent carbohydrate oxidation ((20)).
Subjective hunger and satiety
Visual analog scale questionnaires obtained hourly assessed ratings of global hunger and fullness, desire to eat, subjective quantity of food that can be consumed, preoccupation with thoughts of food, desire for meat, desire for fruit, desire for dairy, desire for vegetables, desire for salty foods, and desire for sweet foods.
Blood and saliva sampling
Blood was sampled hourly from an indwelling catheter (~5 mL per draw) starting 1 hour after waking. Plasma was separated from whole blood after centrifugation and stored at −80°C until analysis. Plasma samples were assayed for ghrelin, leptin, and PYY. Saliva was collected every hour beginning immediately upon waking for melatonin. Assay details (kit manufacturer, coefficient of variation, and sensitivity values) are provided in Supporting Information Table S1.
Dim light melatonin onset
Salivary melatonin concentrations were assessed hourly beginning immediately after waking. Dim light melatonin onset (DLMO25%) was defined as the linearly interpolated point in time at which melatonin levels reached 25% of the fitted peak-to-trough amplitude of individual data, as determined by a three-harmonics least-squares regression analysis ((21, 22)).
Analysis of circadian rhythmicity
Because of increasing homeostatic sleep pressure over the course of the constant routine protocol, many variables exhibit a positive linear trend over time ((2)). Therefore, the linear trend was subtracted from each variable using least-squares regression to produce a steady baseline without distorting the period or phase of the oscillations prior to any analyses. Detrended data were then aligned to individual DLMO25% clock times and averaged into 3-hour bins for EE and substrate oxidation and hourly for hunger ratings and appetitive hormones. Aligning variables to DLMO25% produced an expected amount of missing data given individual variability in DLMO25%. For example, a participant with an earlier DLMO25% will have more available data points after DMLO25% than a participant with a later DLMO25% time. As such, four participants were missing data from indirect calorimetry occurring + 9 hours and/or + 12 hours after DLMO25%. To avoid excluding these participants, detrended data from −12 and −9 hours prior to DLMO25% were used to replace the missing time points. This assumption was based on visual inspection of the individual raw data, which suggested rhythmicity in each measured variable (see Supporting Information). To test whether replacing the missing data created bias for detecting rhythms, statistical analyses were run on the data set with and without missing time points. Results between data sets were similar.
Data are presented as mean (SEM) unless otherwise indicated. All variables are plotted in circadian degrees (1 hour = 15°) with DLMO25% set to 0° and a relative clock hour equal to 2000 hours. Variables are double plotted, with the average melatonin curve provided in grey to improve visualization of rhythms and biological timing of peaks and troughs. However, analyses were only performed on one circadian cycle during the constant routine. Linear mixed models were used to evaluate changes in variables over time (SAS Institute Inc., Cary, North Carolina). To evaluate rhythmicity, each variable was converted to normalized units (i.e., percent difference from the mean), and statistical analyses were performed using linear harmonic regression (CircWave by R. Hut; The University of Groningen, Groningen, Netherlands). Using ANOVA, CircWave fits one or more sine waves (harmonics) to the data and compares this with a horizontal line through the data. An R2 value is produced to describe goodness of fit (see Supporting Information for detail).
Results
Wake, sleep, and DLMO25%
Average self-selected habitual wake and bedtime during the run-in period occurred at 0606 hours (56 minutes) and 2152 hours (47 minutes), respectively. These sleep periods were used to calculate the habitual bedtime and wake time for the first inpatient night prior to the start of the constant routine (2201 hours [50 minutes] and 0559 hours [49 minutes], respectively). All study procedures were relative to the individual participant’s habitual bed and wake times. During the constant routine, DLMO25% occurred at a clock time of 2003 hours (93 minutes). Average melatonin profiles are presented in Figures 1-4 as shaded gray areas.
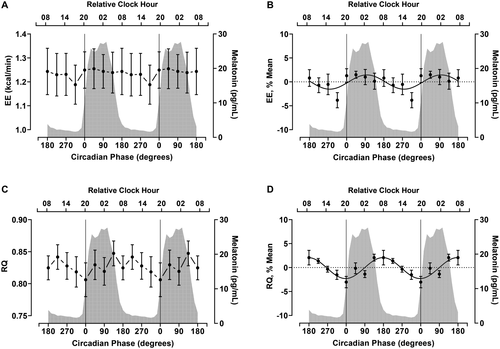
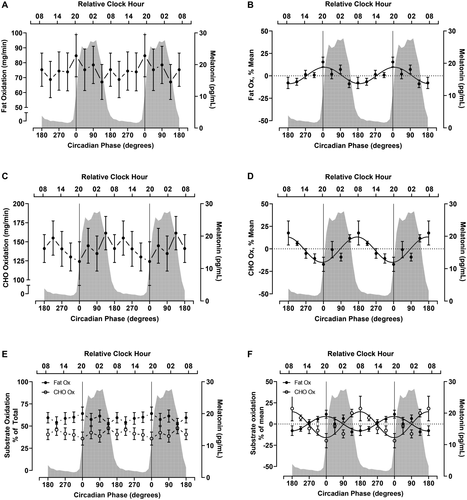
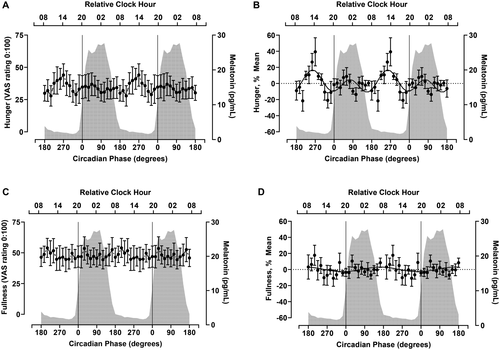
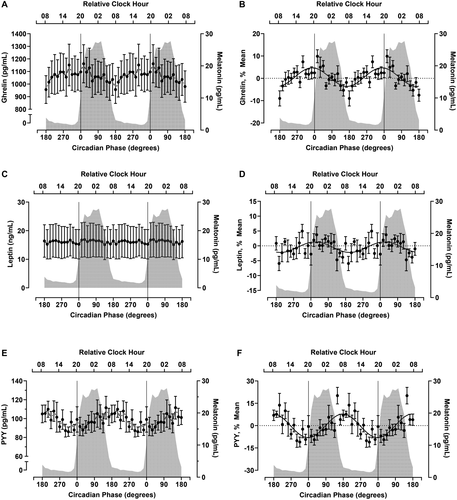
Circadian variation in EE, RQ, and substrate oxidation
EE showed a significant effect of time (P = 0.03) (Table 1, Figure 1A), but the CircWave curve fit at the group level was not significant (P = 0.17) (Table 1, Figure 1B), with only one out of seven participants showing rhythmicity in EE (Supporting Information Table S2, Supporting Information Figure S1A). In contrast, RQ showed both a significant effect of time (P = 0.007) (Table 1, Figure 1C) and a significant CircWave curve fit at the group level, with a sine wave explaining 29% of the variance in RQ during the constant routine (P < 0.001) (Table 1, Figure 1D). The fitted peak of the group-level rhythm in the RQ occurred at 177° (~0750 hours), with a peak to nadir amplitude of 4.4%. At the individual level, RQ was rhythmic in three of seven participants (Supporting Information Table S2, Supporting Information Figure S1B).
| Variable | Data mean (SEM) | LMM (P) | Peak phase degrees (rel. clock hour) | Peak-to nadir-amplitude | ANOVA: F statistic (P) | CircWave: R2 (P) |
|---|---|---|---|---|---|---|
| Indirect calorimetry | ||||||
| EE, kcal/min | 1.24 (0.03) | 0.53 | -- | -- | 1.08 (0.40) | 0.06 (0.17) |
| RQ | 0.83 (0.007) | 0.005 | 177° (~0750 h) | 4.4% | 3.54 (< 0.001) | 0.29 (< 0.001) |
| Fat ox, mg/min | 74.8 (4.0) | 0.01 | 7° (~2030 h) | 18.1% | 2.92 (0.009) | 0.23 (< 0.001) |
| CHO ox, mg/min | 141.4 (7.1) | 0.006 | 174° (~0730 h) | 28.8% | 2.89 (0.01) | 0.25 (< 0.001) |
| Fat ox, % of total | 58.5 (2.1) | 0.02 | 357° (~1950 h) | 17.0% | 3.12 (0.006) | 0.25 (< 0.001) |
| CHO ox, % of total | 41.5 (2.1) | 0.02 | 178° (~0750 h) | 30.3% | 2.76 (0.01) | 0.24 (< 0.001) |
| VAS | ||||||
| Hunger, 0-100 scale a | 34.0 (1.5) | 0.04 | 257° (~1300 h) | 27.6% | 2.03 (0.006) | 0.12 (0.001) |
| Fullness, 0-100 scale | 48.1 (1.5) | 0.82 | -- | -- | 0.57 (0.10) | 0.01 (0.49) |
| Hormones | ||||||
| Ghrelin, pg/mL | 1,063.4 (25.6) | < 0.001 | 349° (~1910 h) | 8.6% | 2.74 (< 0.001) | 0.16 (< 0.001) |
| Leptin, ng/mL | 16.1 (1.0) | 0.41 | -- | -- | 0.99 (0.48) | 0.03 (0.06) |
| PYY, pg/mL | 97.9 (1.9) | 0.01 | 178° (~0750 h) | 15.6% | 2.20 (0.002) | 0.15 (< 0.001) |
- a Linear harmonic regression performed by CircWave determined that the profile for hunger was best fit by two sinusoidal waves. All other variables were described by a single sine wave.
- “--” indicates that the sine wave fit was not significantly different from a horizontal line.
- EE, energy expenditure; RQ, respiratory quotient; fat ox, fat oxidation; CHO ox, carbohydrate oxidation; VAS, visual analog scale; LMM, linear mixed model effect of time; Rel. clock, relative clock time.
Fat oxidation displayed a significant effect of time (P < 0.001) (Table 1, Figure 2A) and a significant CircWave curve fit at the group level, with a sine wave explaining 23% of the total variance in fat oxidation during the protocol (P < 0.001) (Table 1, Figure 2B). The fitted peak in the fat oxidation rhythm occurred at 7° (at a similar time as the DLMO) with an 18.1% peak to nadir amplitude. At the individual level, fat oxidation was rhythmic in three of seven participants (Supporting Information Table S2, Supporting Information Figure S1C).
Carbohydrate oxidation also demonstrated a significant effect of time (P = 0.006) (Table 1, Figure 2C) and a significant CircWave group-level fit, explaining 25% of the variance in the data (P < 0.001) (Table 1, Figure 2D). The fitted peak in the carbohydrate oxidation rhythm occurred at 174° (~0730 hours) with a 28.8% peak to nadir amplitude. Like fat oxidation, three of seven participants showed a significant rhythm in carbohydrate oxidation at the individual level (Supporting Information Table S2, Supporting Information Figure S1D).
For reference, we also present the percent contribution of fat and carbohydrate oxidation to total substrate oxidation in Figures 2D and 2E, respectively. On average, fat contributed 58.5% (2.1%) to total oxidation, whereas carbohydrate contributed 41.5% (2.1%) to total oxidation during the constant routine (Table 1).
Circadian variation in subjective hunger and fullness ratings
Subjective hunger showed a significant effect of time (P = 0.04) (Table 1, Figure 3A) and a significant CircWave fit consisting of two harmonics (i.e., two separate peaks; P = 0.001) (Table 1, Figure 3B). This contrasts with the variables reported above that were best described by a single harmonic. Twelve percent of the variance in the hunger rhythm was explained by the CircWave analysis, with the larger fitted peak in hunger occurring at 257° (~1300 hours) and a smaller peak occurring during the biological night (76°, ~0100 hours). At the individual level, four of the participants demonstrated a rhythm in hunger (Supporting Information Table S2, Supporting Information Figure S2A). Ratings of fullness did not vary by time (P = 0.082) (Table 1, Figure 3C) or rhythmic pattern (P = 0.49) (Table 1, Figure 3D).
Additional ratings of subjective hunger and desire for certain food groups are presented in Supporting Information Table S3 and Supporting Information Figure S3. Desire to eat, preoccupation with thoughts of food, desire for meat, desire for fruit, desire for dairy, desire for vegetables, desire for salty foods, and desire for sweet foods all showed a significant CircWave fit (P < 0.006) (Supporting Information Table S3). Fitted peaks for ratings of desire to eat and preoccupation with thoughts of food aligned with the peak of overall hunger, whereas the other ratings peaked later in the biological evening (Supporting Information Table S3 for statistics; Supporting Information Figures S3A-S3I for the plots).
Circadian variation in appetite hormones
Ghrelin, the only known orexigenic hormone, demonstrated a significant effect of time (P < 0.001) (Table 1, Figure 4A) and a significant CircWave fit at the group level explaining 16% of the variance in the data (P < 0.001) (Table 1, Figure 4B). The fitted peak of the ghrelin rhythm was at 349° (~1910 hours) with an 8.6% peak to nadir amplitude. At the individual level, ghrelin was rhythmic in three of seven participants (Supporting Information Table S2, Supporting Information Figure S4A). Leptin, an anorexigenic hormone, did not vary by time (P = 0.41) (Table 1) or fit a rhythmic profile, although the CirWave fit was a nonsignificant trend (P = 0.06) (Table 1, Figure 4D). PYY, also an anorexigenic hormone, varied by time (P = 0.01) (Table 1, Figure 4E) and had a significant rhythm as determined by CircWave (P < 0.002) (Table 1, Figure 4F). The fitted peak of the PYY rhythms was at 178° (~0750 hours) with a 15.6% peak to nadir amplitude. Five of seven participants demonstrated a rhythm in PYY (Supporting Information Table S2, Supporting Information Figure S4C).
Discussion
In the present study, we observed circadian variation in RQ, fat and carbohydrate oxidation, hunger ratings, ghrelin, and PYY in a sample of healthy adults under constant routine conditions. Despite significant rhythms in these variables at the group level, we observed considerable individual variability. Inconsistent with previous reports ((2, 8)), we did not observe rhythms in EE or leptin. Our data suggest the possibly that drivers of energy balance operate in anticipation of feed-fasting cycles to optimize metabolic efficiency over 24 hours. Eating or sleeping out of phase with endogenous rhythms in regulators of energy balance may play a role in the link between circadian misalignment and obesity ((23)).
Findings from two previous constant routine studies have identified a rhythm in EE ((9, 10)) Spengler et al. ((10)) studied 10 adult males under constant behavioral and environmental conditions over 41 hours of wakefulness and measured VO2, VCO2, and ventilation via indirect calorimetry every 2 hours. Kräuchi et al. ((9)) performed a 30.5-hour constant routine protocol in seven men with indirect calorimetry measurements occurring hourly. Spengler et al. ((10)) demonstrated a significant rhythm in EE peaking in the biological morning, whereas Kräuchi et al. ((9)) showed a more complex circadian pattern in EE with two peaks, a small peak slightly preceding the core body temperature minimum and a larger peak in the biological morning. Kräuchi et al. ((9)) also noted significant interindividual variability in EE rhythms but did not present individual-level data.
Findings from our study are also inconsistent with findings from a forced desynchrony study ((8)) that reported a significant circadian rhythm in fasting EE with a peak in the biological afternoon/evening and a trough near the core body temperature minimum, with a peak-to-trough amplitude of ± 110 kcal. A clear distinction between the forced desynchrony protocol and the constant routine protocol used in the present study (and those of Spengler et al. and Kräuchi et al.) ((9, 10)) is the food intake/fasting state. In the current protocol, participants were not fasting; thus, the measure of EE was composed of both resting EE plus the EE produced by diet-induced thermogenesis induced by the hourly snacks. Diet-induced thermogenesis is the most challenging component of EE to measure independently, and to assess its potential circadian rhythm would require near continuous measurement of EE during a constant routine (i.e., using a whole room calorimeter). More frequent as well as longer duration measures of EE (as was done in the Kräuchi et al. study) ((9)) might be required to capture a rhythm in EE under fed conditions.
In agreement with Zitting et al. ((8)), but in disagreement with two prior constant routine studies ((9, 10)), we observed a rhythm in the RQ, with higher RQ during the biological morning/day compared with the evening. Our results (and those of Zitting et al.) ((8)) suggest that carbohydrate oxidation is highest during the biological morning (~12 hours prior to DLMO25%) and lowest during the evening (near the DLMO25%). In contrast, fat oxidation is antiphase (highest during the biological evening and lowest during the biological morning). Our finding of a circadian rhythm in fat oxidation with a peak near the DLMO25% is consistent with the anticipatory nature of the circadian system, which would be primed to oxidize fat during a nocturnal fast under normal sleep/wake and fasting/food intake conditions ((21, 22, 24)). These data are also consistent with previous reports of higher circulating free fatty acid concentrations in plasma and higher fat oxidation during the biological night compared with the day ((25-27)). It should be noted that melatonin itself has been implicated in body weight regulation and lipid metabolism in animal models, and it remains possible that rhythms in substrate oxidation are driven by the circadian melatonin rhythm ((28-32)).
We observed a rhythm in subjective hunger with a larger peak during the early afternoon and a smaller peak during the biological night (~3 hours after the DLMO). These data are in contrast to a forced desynchrony study by Scheer et al. ((14)), who reported a peak in hunger at 230° (~2200 hours relative time) and a trough at 50° (~0800 hours relative time). Hunger oscillated with a 17% peak-to-trough amplitude in the study by Scheer et al. ((14)) compared with ~28% in the current study. Results from another forced desynchrony study by McHill et al. ((6)) produced similar results as Scheer et al. ((14)) and showed a peak in subjective hunger at 240° and a nadir at 60°. One possible explanation for the differences in the phase of subjective hunger between our study and the results of Sheer et al. ((14)) and McHill et al. ((6)) may be related to differences in food intake and sleep between constant routine and forced desynchrony protocols.
We observed a circadian rhythm in ghrelin during the constant routine with peak levels prior to DLMO25% and a trough ~12 hours later. Results from a prior study in which 33 young adults were fasted for 12 to 48 hours with blood sampling every 3 hours showed a marked diurnal rhythm in serum ghrelin with a nadir in the morning, peak levels in the afternoon, and a gradual decline during the night ((4, 7, 33)). This pattern in ghrelin secretion was observed despite lack of meal stimuli, suggesting ghrelin plays a role in the anticipation of EI. A circadian rhythm in fasting ghrelin has also been reported under the conditions of forced desynchrony ((6)). Because ghrelin is an orexigenic hormone, we were surprised to find such a large difference between the peak timing of ghrelin and hunger. This is in contrast to findings from forced desynchrony studies in which peaks in hunger ratings and fasting ghrelin colocalized to the biological evening ((6, 14)). Again, differences may be due to protocol selection (constant routine vs. forced desynchrony).
Leptin follows a diurnal rhythm under food intake-fasting conditions with a nadir in the mid-afternoon and peak during the evening/sleep ((3, 23, 34-38)). However, findings are conflicting as to whether leptin varies with circadian phase ((2, 6, 36)). In the present study, we did not observe a rhythm in leptin, which contrasts with previously published findings using a constant routine protocol ((2)). During a 37-hour constant routine, Shea et al. ((2)) reported a significant rhythm in leptin, with a peak during the biological night (close to the core body temperature minimum) and a peak-to-trough amplitude of 16%. Analysis of data from individual participants from that study revealed the presence of circadian rhythms in leptin in four of the six participants as assessed using cosinor analysis. In the present study, we observed significant circadian rhythms in leptin in three of seven participants (Supporting Information Tables S1-S2), but the group level fit was not significant. Our results are also at odds with previous forced desynchrony studies that found significant rhythms in fasting concentrations of leptin ((6, 36)). While available evidence has suggested a small circadian component to leptin, the hormone appears to be largely driven by sleep/wake and fasting/food intake ((23, 35, 36)).
PYY is considered a satiety hormone secreted primarily from L cells in the distal gastrointestinal tract and is involved in macronutrient absorption. Circulating PYY concentrations are lower in adults with obesity compared with those who are lean ((39)) and are increased in “obesity-resistant” individuals in response to overfeeding ((40)). Like daily patterns of ghrelin and leptin, PYY predominantly follows the sleep-wakefulness fasting-feeding cycle ((23)); however, our findings also reveal a circadian component. The PYY rhythm peaked in the biological morning (~12 hours prior to DLMO25%). This is in opposition to our hypothesis in which we predicted PYY to peak during the biological night (parallel with the nadir of the hunger rhythm). Given the role of the circadian system in sleep consolidation, we thought that a circadian drive in PYY (as well as leptin) during the biological night would support fasting across the sleep period. While this may be happening to some extent (i.e., PYY levels are increasing across the biological night in the current study), we observed the peak in PYY in the biological morning. Although a morning peak in PYY is consistent with Sheer et al. ((14)), who reported a nadir in hunger at ~0800 hours in forced desynchrony conditions, the apparent dissociation between the phase of PYY and hunger in a constant routine requires further study.
One limitation of the present study is the small sample size. However, findings from previous studies have reported significant circadian rhythms in outcome variables using similar sample sizes. Given the highly controlled nature of the constant routine protocol, circadian rhythms can be detected using these smaller sample sizes. Another limitation is the relatively short duration of our constant routine protocol, which resulted in missing time points when data were aligned to individual DLMO25%. In addition, the current paper did not compare the amplitude of the circadian rhythms to the magnitude of diurnal patterns. This comparison would have allowed us to comment on the strength of the circadian drive versus the influence of behavior and environment. Finally, as we did not measure protein metabolism (e.g., by collecting 24-hour urinary nitrogen), we were not able to adjust our respirometry measures for protein metabolism; thus, observed oscillations in carbohydrate and fat oxidation may be influenced to some extent by oscillations in protein oxidation.
Conclusion
Using a constant routine protocol, we provide evidence of circadian rhythms in fat and carbohydrate oxidation under nonfasted conditions. We also provide evidence of circadian rhythms in the hunger and satiety hormones ghrelin and PYY. Fluctuations in nutrient oxidation, ghrelin, PYY, and appetite are thought to respond primarily to nutrient ingestion. However, the present results suggest the endogenous circadian system also plays an important role, which may have important implications for regulation of body weight and energy metabolism.
Acknowledgments
We thank the participants for volunteering for this research, the CTRC Core Laboratory for running the assays, the CTRC Nursing Core for assistance with data collection, and the CTRC Metabolic Kitchen for providing the controlled diets.
Funding agencies
This research was supported by the NIH/NIDDK Colorado Nutrition Obesity Research Center (P30 DK048520); NIH/National Center for Research Resources Colorado Clinical and Translational Sciences Institute grants (UL1 RR025780), K01 DK113063 (CAR), K24 DK02935 (DHB), RO1 DK62874 (DHB), and K01 DK110138 (JLB); and a University of Colorado Innovative Seed Grant Award (JLB). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Disclosure
The authors declared no conflict of interest.
Author contributions
CAR, SJM, and JLB analyzed and interpreted the data and wrote the first draft of the manuscript. DHB and KPW assisted in the interpretation of the data and writing. CAR, SJM, and JLB carried out the experiments. CAR and JLB conceived of the experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Clinical trial registration
ClinicalTrials.gov identifier NCT02809482.





