Evidence for Protein Leverage in Children and Adolescents with Obesity
Abstract
Objective
The aim of this study was to test the protein leverage hypothesis in a cohort of youth with obesity.
Methods
A retrospective study was conducted in a cohort of youth with obesity attending a tertiary weight management service. Validated food questionnaires revealed total energy intake (TEI) and percentage of energy intake from carbohydrates (%EC), fats (%EF), and proteins (%EP). Individuals with a Goldberg cutoff ≥ 1.2 of the ratio of reported TEI to basal metabolic rate from fat-free mass were included. A subgroup had accelerometer data. Statistics included modeling of percentage of energy from macronutrients and TEI, compositional data analysis to predict TEI from macronutrient ratios, and mixture models for sensitivity testing.
Results
A total of 137 of 203 participants were included (mean [SD] age 11.3 [2.7] years, 68 females, BMI z score 2.47 [0.27]). Mean TEI was 10,330 (2,728) kJ, mean %EC was 50.6% (6.1%), mean %EF was 31.6% (4.9%), and mean %EP was 18.4% (3.1%). The relationship between %EP and TEI followed a power function (L coefficient −0.48; P < 0.001). TEI was inversely associated with increasing %EP. In the subgroup with < 60 min/d of moderate to vigorous physical activity (n = 48), lower BMI z scores were associated with higher %EP and moderate %EC.
Conclusions
In youth with obesity, protein dilution by either carbohydrates or fats increases TEI. Assessment of dietary protein may be useful to assist in reducing TEI and BMI in youth with obesity.
Study Importance
What is already known?
- ► The protein leverage hypothesis (PLH) posits that a diet low in energy from protein sources causes a compensatory increase in food intake to achieve a target protein intake (protein leverage), and that this mechanism consequently contributes to excess energy intake and obesity in humans living in modern Westernized food environments. Evidence for protein leverage is based on studies in animals and adult humans; however, studies investigating protein leverage or the PLH in children and adolescents are lacking.
What does this study add?
- ► This study in a cohort of youth with obesity illustrates an increased total energy intake with decreasing energy intake from protein sources, irrespective of whether carbohydrates or fats were the diluents, consistent with the mechanism of protein leverage. In a physically inactive subgroup, a diet high in proteins and moderate in carbohydrates was associated with decreased BMI z scores.
How might these results change the direction of research?
- ► Modulating dietary protein may assist in reducing total energy intake in youth with obesity.
Introduction
Obesity in childhood and adolescence is caused by a mismatch between energy intake and energy expenditure, whether or not an underlying genetic syndrome predominates (1). The amount and composition of macronutrients in daily nutrition (i.e., carbohydrate, fat, and protein) determine total energy intake (TEI). Energy expenditure varies with age, sex, body composition, and duration and intensity of physical activity. Proteins contain less energy than the equivalent mass amount of fats, and their percentage contribution to the daily TEI is approximately 20% compared with 50% from carbohydrates and 30% from fats in a standard diet (2). Nonetheless, daily protein intake in childhood is essential for normal development and growth, and protein requirements vary with age, sex, and physical activity (3). Subtle changes in the percentage of intake from proteins may have a disproportionately high impact on TEI, a mechanism termed protein leverage (PL). Based on PL, Simpson and Raubenheimer (4) were the first to formulate the PL hypothesis (PLH), which posits that in humans, PL contributes to weight gain and obesity. In a recent issue of Obesity, the founders have extended the theoretical foundations and updated and clarified evidence for the PLH (5).
Where diets are low in the proportion of energy from proteins, PL stimulates a compensatory increase in food intake and hence TEI to attempt to achieve a certain absolute protein intake, as shown in studies in mice (6). In animals such as insects, birds, fish, and mammals, separate appetite systems control intake for different macronutrients, which guide animals toward an optimal intake of multiple nutrients. However, under circumstances in which the nutritional environment limits attaining an optimal diet, an adequate intake of proteins takes precedence over carbohydrates and fats (7, 8). Data supporting PL in adult humans come from experimental studies, systematic compilations of data from numerous trials, and analyses of cohort and population data (5). It was also shown in humans that dilution of proteins by either carbohydrates or fats had the same effect on PL (9). Hall (10) most recently confirmed, as originally shown by Simpson and Raubenheimer (4), that PL was a potential major contributor to the US obesity epidemic, where an increase in TEI of 950 kcal per capita was accompanied by a decline of 1.5% of energy from protein sources between 1961 and 2013.
Studies in infants, however, have shown positive associations between a higher percentage of energy intake from protein sources with later development of obesity (11). There are no studies that have assessed PL or the PLH in children. In this retrospective cohort study, we aimed to assess PL and the PLH in children and adolescents with obesity aged 6 to 18 years old who were enrolled in a cohort study of children and youth with obesity by using a validated food questionnaire, the Australian Child and Adolescent Eating Survey Food Frequency Questionnaire (ACAES-FFQ). Our hypothesis was that in children and adolescents with obesity, TEI increases with decreasing proportion of dietary energy from proteins. Our secondary aim was to investigate the PLH, that is, whether PL is associated with increased BMI z score in youth with obesity.
Methods
Study cohort
Data were collected from participants of the Childhood Overweight Biorepository of Australia cohort, Australia’s largest cohort of children and adolescents with obesity. Details of recruitment, sample collection protocols, and methodology are described elsewhere (12). For the purpose of this retrospective cohort study, participant data on anthropometry and results from food questionnaires and accelerometry were used for analysis. Written consent by the legal guardian was obtained. The study protocol was approved by the Royal Children’s Hospital Human Research Ethics Committee, Melbourne, Australia (reference number 28082Q, October 9, 2017) and is in accordance with Helsinki principles.
Participant characteristics
Height was measured without shoes to the nearest 0.5 cm, using a fixed Harpenden stadiometer (Holtain Ltd., Crymych, Dyfed, UK). Weight was assessed in light clothes to the nearest 100 g. BMI was calculated according to the formula weight in kilograms divided by height in meters squared and then converted into BMI z scores adjusted for age and sex by using the US Centers for Disease Control and Prevention growth reference charts (13). Total body fat percentage was assessed by a four-point bioimpedance device (Tanita), which was previously validated for use in children > 6 years (14); therefore, children > 6 years of age were included in the study. The fat-free mass (FFM) was calculated according to the formula FFM = weight (kilograms) × (100 − body fat percentage) and then used to calculate basal metabolic rate (BMR) according to the Schofield equation (15). A specialist pediatric endocrinologist or a consultant general pediatrician assessed the Tanner stage for pubertal development, in which Tanner 1 was considered prepubertal, Tanner 2 to 3 was peripubertal, and Tanner 4 to 5 was postpubertal (16). For the analysis, we used data only from individuals aged 6 to 18 years with obesity (i.e., BMI > 95th percentile on sex- and age-matched Centers for Disease Control and Prevention growth charts).
Food questionnaires
Data on dietary intake were collected using the ACAES-FFQ (17) between the years 2010 and 2018. As part of the Australian Eating Survey suite of food questionnaires, these questionnaires have undergone comprehensive evaluation for validity and reproducibility, and they can be self-administered or completed by parents for young children. Reproducibility and comparative validity for this survey have previously been established (17). The ACAES-FFQ is a 120-item semiquantitative FFQ with 15 supplementary questions regarding age, use of vitamin supplements, food behaviors, and sedentary behaviors. The ACAES-FFQ was sent to the University of Newcastle for scanning, and nutrient analysis was assessed using FoodWorks software (version 8.0.3553; Xyris Software, Xyris, Australia) to elicit TEI (kilojoules) and macronutrient composition as a percentage of energy from TEI (%TEI). Energy from dietary carbohydrate, fat, and protein was calculated by multiplying grams of intake by 16.7 kJ/g for carbohydrate and protein and 37.7 kJ/g for fat (2).
Reporting of TEI in dietary assessments
Misreporting, specifically underreporting of TEI with food questionnaires, is a recognized phenomenon in adolescence, particularly in individuals with obesity (18). In this study, we used a Goldberg cutoff ≥ 1.2 of the ratio of reported TEI to BMR in order to exclude underreporters of TEI with high specificity (19). BMR was calculated from FFM, which previously explained 79.8% of the BMR variance in a cohort of children and adolescents aged 9.5 to 16.5, including individuals with obesity (20).
Accelerometry data
A subgroup had physical activity measured by Actical accelerometers (Philips Respironics, Philips, USA; REF 198-0200-01, 109-0302-00, and 1063544) worn on the left hip on an elasticized belt continuously for 7 days, including water-based activities and sleep. Accelerometer results were considered valid for participants with data on three or more weekdays and one or more weekend days, with a wearing time of at least 600 minutes and a maximum nonwear time of 360 minutes in each 24-hour period, as per published guidelines regarding acceptability (21). Actical accelerometry physical activity intensity was categorized according to published intensity cutoff points (22).
Descriptive statistics
Categorical participant variables (sex, pubertal stage) are shown in absolute numbers and percentages. Continuous participant characteristics and food questionnaire data were analyzed for their distribution and log-transformed if skewed. Descriptive statistics are shown as mean, SD, and range. Univariate linear correlation analysis between the explanatory variable and the response variable was performed with Pearson test, including 95% CI and P values, where the condition of bivariate normality for the variable was checked. Student’s t test was used to compare associations between pubertal stages, sex, and TEI. Linear regression models were used to analyze differences in TEI based on pubertal stage, sex, FFM, and age.
Modeling of macronutrients and TEI using power functions
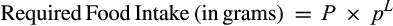
where P is the targeted intake of proteins in grams, p is the proportion of proteins from a given diet, and L is the strength of leverage. P and p are derived from the analysis, and the exponent L reveals the strength of leverage. Assuming full PL, that is, L = −1, small changes in p will cause substantial changes in TEI. Assuming no PL, that is, L = 0, changes in p will not cause changes in TEI (5).
Compositional data analysis to explain TEI from macronutrient composition
Multiple regression with compositional predictors (e.g., percentages of energy from relevant macronutrients) may lead to inferential errors because of the covariance that exists among the percentages of different components; for example, an increase in the percentage of energy from proteins (%EP) must necessarily lead to a decrease in the percentage of energy from fats (%EF) and/or carbohydrates (%EC). Mixture models, also known as Scheffé polynomials, are an analytical framework based on multiple regression that is robust to the analysis of outcomes with compositional predictors (23). To test the sensitivity of our results to the choice of analysis, we analyzed TEI and BMI z score data as a function of dietary macronutrient content (proportion EC, proportion EF, and proportion EP) in a mixture model framework, which allows us to test for effects of all three macronutrients simultaneously. We fitted five different models for each outcome. Model 1 was a null model, which assumes no effect of dietary composition on the outcomes. Models 2 through 5 were mixture models, corresponding to equations 1 through 4 in Lawson and Willden (23) (see online Supporting Information), to test for linear additive through increasingly complex nonlinear interactive effects of macronutrients on TEI and BMI z score. We note here that model 2, which tests for linear effects, is identical to the partition substation model commonly used in nutritional epidemiology (Supporting Information) (24, 25). To select among models, we used an information theoretic approach based on Akaike information criterion (AIC) (26). Of the five models fitted, that with the minimal AIC was favored, and in the event that two models were within two AIC points of one another, the simplest model was selected. Where the null model is favored, no effect of macronutrient composition on the outcome is inferred, with subsequent models suggesting more complex effects of diet composition on the outcome of interest. Mixture models were implemented using the “MixModel” function in the R package mixexp (The R Foundation for Statistical Computing, Vienna, Austria). For interpretation, we plotted the predictions from the AIC-favored model as surfaces on a right-angle mixture triangle (27). All analyses were performed using R statistics. P < 0.05 was considered significant.
Results
FFQ data were available for 203 individuals. Of those, 137 fit with the age criteria, were classified as having obesity, had plausible energy intake according to a Goldberg cutoff ≥ 1.2, and were considered for further analysis. The mean age was 11.3 (SD 2.7) years, 68 (50%) were female, and mean BMI z score was 2.47 (SD 0.27). Mean TEI was 10,330 (SD 2,728) kJ, mean %EC was 50.6% (SD 6.1%), mean %EF was 31.6% (SD 4.9%), and mean %EP was 18.4% (SD 3.1%). Tables 1 and 2 provide descriptive characteristics of participants and food questionnaire–related data.
| Variable | n | Mean (SD) | Range | % |
|---|---|---|---|---|
| Age (y) | 137 | 11.3 (2.7) | 6-18 | |
| Sex (female) | 68 | 50 | ||
| Pubertal stage | ||||
| Prepubertal | 66 | 48.2 | ||
| Peripubertal | 40 | 29.2 | ||
| Postpubertal | 31 | 22.6 | ||
| Weight (kg) | 137 | 77.6 (26.3) | 30.7-175.9 | |
| Height (m) | 137 | 1.52 (0.15) | 1.10-1.90 | |
| BMI (kg/m2) | 137 | 32.5 (6.0) | 21.9-58.8 | |
| BMI z score | 137 | 2.47 (0.27) | 1.86-3.09 | |
| Accelerometry | 57 | 42 | ||
| Avg. counts/d | 249,734 (97,904) | 96,841-493,728 | ||
| MVPA (min/d) | 43.4 (24.1) | 3.5-110-8 | ||
| < 60 min/d MVPA | 48 | 84 |
- BMI z score according to Centers for Disease Control and Prevention growth charts.
- Avg. counts/d, number of signals reflecting deceleration and acceleration forces counted by accelerometer, averaged per day; MVPA, time in minutes spent with moderate to vigorous physical activity, derived from avg. counts/d according to published intensity cutoff points (22).
| Variable | Mean (SD) | Range |
|---|---|---|
| TEI (kJ) | 10,330 (2,728) | 5,467-17,886 |
| Total daily protein intake (g) | 110 (30) | 56-213 |
| Energy intake from proteins (kJ) | 1,831 (497) | 933-3,565 |
| % TEI protein | 18.4 (3.1) | 11.0-30.0 |
| Total daily carbohydrate intake (g) | 307 (95) | 154-611 |
| Energy intake from carbohydrates (kJ) | 5,119 (1293) | 2,563-10,202 |
| % TEI carbohydrate | 50.6 (6.1) | 32.0-68.0 |
| Total daily fat intake (g) | 84 (27) | 37-195 |
| Energy intake from fats (kJ) | 3,173 (1,013) | 1,408-7,354 |
| % TEI fat | 31.6 (4.9) | 21.0-51.0 |
- Total energy intake (TEI) as calculated from Australian Child and Adolescent Eating Survey Food Frequency Questionnaire given as TEI and macronutrients (protein, carbohydrate, and fat) in grams, kilojoules, and percentages from TEI.
Energy intake per age, pubertal stage, BMI z score, and sex
TEI increased with age for both sexes (Figure 1A) (Pearson correlation coefficient 0.20; 95% CI: 0.03-0.36; P = 0.02). TEI was associated with increasing BMI z score (correlation coefficient 0.20; 95% CI: 0.04-0.36; P = 0.02; Figure 1B). The t tests to compare TEI between pubertal stages (pre- to peri-, peri- to post-, and pre- to postpuberty) were nonsignificant when looking at both sexes (Figure 1C). Linear regression models to assess whether TEI varies independently of FFM, age, and pubertal stage were not significant for females. In postpubertal males, however, TEI was significantly reduced independently of these cofactors (estimated −2,577 kJ in TEI postpubertal vs. prepubertal males; P = 0.017).

Modeling of macronutrients and TEI
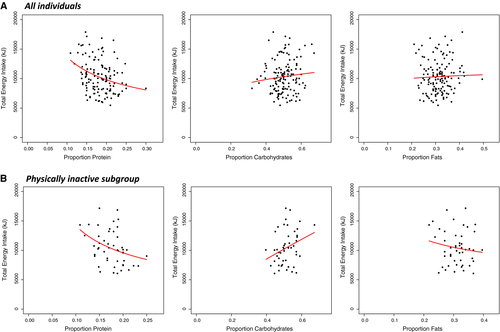
Figure 2 illustrates associations between the proportion of energy derived from each macronutrient (x-axis) and the TEI (y-axis) in all individuals and in the physically inactive subgroup. In all individuals (Figure 2A), the distribution of percentage of intake from macronutrients and TEI significantly followed a power function for proteins (%EP) but not for carbohydrates or fats (%EC, %EF). The strength of leverage for proteins was −0.48 (P < 0.001), whereas the exponent (L) for carbohydrates and fats was 0.22 (P = 0.23) and 0.06 (P = 0.67), respectively (Figure 2A and Table 3).
| Macronutrient | Strength of leverage (L) | P value |
|---|---|---|
| All individuals, n = 137 | ||
| Proteins | −0.48 | < 0.001 |
| Carbohydrates | 0.22 | 0.23 |
| Fats | 0.06 | 0.67 |
| Physically inactive subgroup, n = 47 | ||
| Proteins | −0.57 | < 0.05 |
| Carbohydrates | 0.82 | < 0.05 |
| Fats | −0.31 | 0.30 |
- Model coefficients for fitted power functions for whole cohort and for physically inactive subgroup. L indicates strength of leverage for each macronutrient (−1 signifies complete leverage, 0 means no leverage). In whole cohort, leverage on total energy intake (TEI) was significant only from proportion of proteins. In physically inactive group, leverage on TEI was significant from proportions of proteins and carbohydrates.
Compositional data analysis
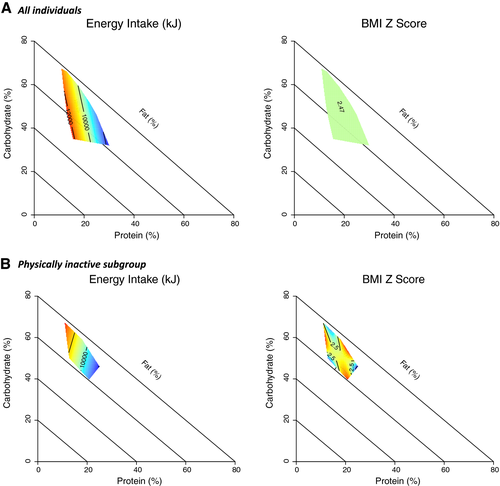
To assess whether there was a relationship between the macronutrient composition of diet, TEI, and BMI z score, mixture model analysis was performed. For TEI, model 2 had the most favorable AIC (Supporting Information Tables S1 and S2 provide all model coefficients), which suggests a linear effect of dietary macronutrients on TEI. TEI was highest in those diets with the lowest %EP (Figure 3A). In this population of youths with obesity, dietary macronutrient composition was not a significant driver of BMI z score, as the null model was favored (Figure 3A and Supporting Information Table S1).
Subgroup analysis for physically inactive youth with obesity
The Australian guidelines recommend 60 min/d of moderate to vigorous activity for children and adolescents aged 5 to 17 years. Of the 57 individuals with valid accelerometry data, 48 (84%) did not meet these recommendations and, for the purpose of our study, were considered to be physically inactive individuals (Table 1, accelerometry data) (28). Figure 2B illustrates associations between percentages of energy intake from each macronutrient (x-axis) and the TEI (y-axis) in the physically inactive subgroup. The %EP and %EC followed a power function with respect to TEI. Relevant exponents (L) were −0.57 (P < 0.05) for proteins, 0.82 (P < 0.05) for carbohydrates, and 0.82 (P = 0.30) for fats (Figure 2B and Table 3).
For compositional data analysis in the physically inactive group, model 2 was favored by AIC, suggesting linear effects of dietary macronutrients on TEI (Supporting Information Tables S3 and S4). For BMI z score in physically inactive youths, compositional model 4 was favored, suggesting that effects of macronutrient composition on BMI z score were complex and nonlinear. Physically inactive individuals with a diet high in %EP with moderate %EC had lower BMI z scores, whereas individuals with a diet moderate in %EP and low %EC had higher BMI z scores (Supporting Information Tables S3 and S5).
Discussion
In this retrospective cohort study, we analyzed macronutrient food composition data derived from the ACAES-FFQ in 137 children and adolescents with obesity, aged 6 to 18 years. This is the first study to provide evidence for PL in youth with obesity. Specifically, we found that with decreasing percentages of TEI from protein sources, total daily energy intake increased, consistent with the mechanism of PL. The strength of leverage was L = −0.48 (P < 0.001), indicating partial PL that is comparable to available studies from adults (5). In a physically inactive subgroup that did not meet daily recommended physical activity levels, lower %EP remained the only macronutrient to increase TEI. Also, in the physically inactive subgroup, a diet high in protein and moderate in carbohydrate contents was associated with lower BMI z score, whereas a diet moderate in proteins and low in carbohydrates was associated with higher BMI z score. This is the first time that PL and the PLH have been tested in a cohort of youth with obesity, and further studies are now required to see whether similar effects are seen in children or adolescents of all weight categories.
PL versus PLH in this study
As recently explained by Raubenheimer and Simpson (5), PL is necessary for the PLH but not sufficient. We found evidence for PL, as seen in the negative association between dietary energy from proteins and TEI. We also found a positive association between TEI and BMI z score. However, in our analyses, we did not detect statistical support for an association between macronutrient intake and BMI z score in this cohort of children and adolescents with obesity. There are several possible explanations for this. Detecting relationships between diet and body composition is challenging because of the fact that obesity results from long-term accumulation of small daily differences in intake (29). Additionally, all participants in our study had high BMI z scores, and consequently, our data provided a limited range over which to detect significance in the relationship between diet and body composition. Missing information about dietary nonmacronutrient composition in our cohort might also be relevant. In particular, the content of dietary fiber has been shown to attenuate PL effects on TEI and obesity in mice (5). Also, our results have to be interpreted in the context of specific growth characteristics for children with obesity and within the context of varying protein targets in childhood. Throughout childhood, normative BMI percentiles are steadily, in a near-linear manner, trending upward from about 6 years of life, and so is the difference between the 50th and the 95th percentile increase (the latter representing the threshold to determine obesity in childhood) (13). Hence, for PL in this age group to be associated with increasing BMI z score, the effect size must exceed the one from adulthood, for which overweight and obesity are determined by static thresholds (i.e., BMI of 25 and 30). Also, protein targets vary throughout life, influenced by, among other factors, age, early nutritional experience, and physical activity (5). Limited data for physical activity analysis only allowed us to test PL and the PLH in a physically inactive subgroup, in which we found an effect of PL on TEI and of dietary macronutrients (protein and carbohydrate) on BMI z score.
Comparison with recommendations for dietary protein intake
Based on nitrogen studies in adults, the recommended dietary allowance (RDA) is 0.8 g of protein per kilogram of body weight. Because of rapid growth and development, children need a positive nitrogen balance. Therefore, the RDA of protein in children aged 4 to 13 and 14 to 18 years is slightly higher, at 0.95 g and 0.85 g per kilogram of body weight, respectively, as recommended by the Institute of Medicine (30). Yet, importantly, these RDA measures aim to provide the minimum need of most of an investigated group to avoid adverse effects from undersupply. The true requirement for protein intake is affected by protein quality (plant-based vs. meat protein), individual physiological characteristics (age, fitness), individual health (e.g., chronic disease, obesity), and environmental factors (temperature) (3). Comparing with the nutrient reference values for Australia and New Zealand, the protein intake in this cohort is considered moderate (between 1 and 1.5 g/kg) and well below reported long-term intake of up to 2.8 g/kg for male athletes without adverse health consequences (2). Similar to the RDA from the United States, the Australian National Health and Medical Research Council published acceptable macronutrient distribution ranges (AMDR) associated with reduced risk for chronic disease while guaranteeing adequate intake; for children aged 4 to 18 years, the relevant AMDR per TEI for carbohydrates, fats, and proteins is 45% to 65%, 20% to 35%, and 15% to 25% (2). The average intake of proteins in this study (1.42 g of protein per kilogram of body weight) was above the RDA and the percentage range of energy intake from protein sources (11%-30%) spanned the AMDR by roughly 5% in either direction. The percentage of intake from fats exceeded the AMDR (ranging up to 51% in this study), whereas the percentage of intake from carbohydrates, only 32% at the lower end of the range, was considerably lower than recommended. This may partly explain the complex interactions between macronutrient intake and BMI z score in the physically inactive subgroup, in wich BMI z scores were higher for those on a diet moderate in proteins and low in carbohydrates.
Dietary protein related to body weight in different age groups
In infants and toddlers, several longitudinal studies have revealed positive associations between formula with higher protein content and later elevated adiposity markers (31). In the European Childhood Obesity Project (CHOP) study, a European multicenter, double-blind, randomized clinical trial, healthy infants were randomly assigned to receive higher versus lower protein content in formula during the first year of life. At age 6 years, the high-protein formula–fed children had higher BMI (0.51; 95% CI: 0.13-0.90; P = 0.009) and an odds ratio of 2.43 (95% CI: 1.12-5.27; P = 0.024) to develop obesity compared with the low-protein formula–fed children (11). This may be explained by the following two mechanisms: (1) if the protein intake target during infancy was set higher than normal because of higher protein intake, PL may drive an increased TEI later in life to achieve this higher protein set point, therefore contributing to obesity (5) and (2) a mechanism termed “the early protein hypothesis” assumes that higher protein intake in infancy triggers elevated levels of insulinlike growth factor 1 and insulin (32) and therefore stimulates early weight gain. However, a 2016 systematic review (including the CHOP trial) revealed insufficient evidence to urge a reduction in the protein content in infant formula to avoid later weight gain (33).
Systematic reviews of studies in late childhood and early adolescence that investigate the effects of higher intake of proteins on later BMI and cardiometabolic risk factors (including hypertension, dyslipidemia, and insulin resistance) have found limited and inconclusive results for adverse effects (34, 35).
In adults, diets with low carbohydrate and high protein contents have been shown to be effective in weight management (36). However, there has been growing evidence from longitudinal studies that high-protein, low-carbohydrate diets are associated with increased mortality and cancer. A detailed discussion about macronutrient composition and health-related outcomes in animals and humans is available elsewhere (37).
Implications of protein content in foods
Population-based studies investigating macronutrient composition trends over the past 4 decades in the United States (10) have shown a decrease in the percentage of energy intake from protein sources accompanied by an increase in the overall intake of total energy, correlating with increasing trends in BMI. A recent analysis of National Health and Nutrition Examination Survey (NHANES) data from 2009 to 2010 identified the most likely causative food product for this trend as being ultraprocessed foods. Ultraprocessed food is a group of food products, including soft drinks, industrialized desserts, and reconstituted meat products, “ready to consume,” representing almost 60% of all energy intake in the US diet but containing just around 9.5% energy from protein sources (38). On a population level, the mechanism of the PLH may well contribute to an overall obesity epidemic as recently commented by Hall (10), investigating data from the US food supply since 1973.
Strengths of this study are the availability of validated food questionnaire data in a reasonably large cohort of children and adolescents with obesity, including data on all three relevant macronutrients. We addressed the problem of underreporting by excluding those reporting a TEI below a Goldberg cutoff of 1.2. This sanctioned the total number of participants but also increased the validity of the data. Accelerometry data was available from 57 individuals, with 42 of those not meeting physical activity recommendations, allowing for analysis in what was considered a physically inactive subgroup.
This study does have important limiting factors. Though we addressed the effects from underreporting of TEI, we could not do so for misreporting of relative macronutrient content. However, when we looked at the whole cohort (n = 203), including underreporters of TEI, we found consistent effects from PL on TEI (data not shown). This allows the assumption that misreporting of overall energy intake should apply equally to protein, carbohydrate, and fat intakes in this cohort. Further limiting factors are the cross-sectional study design, which does not allow extrapolation to causality. In addition, the available food questionnaire data unfortunately do not allow investigation into the quality of proteins, the specific amino acids, or the fiber content.
This study was undertaken in a cohort of children and adolescents with obesity because the majority of them are estimated to maintain obesity at the age of 35 years (39). For a child aged 6 years with obesity, there is a 21.8% chance of reducing his or her weight classification to nonobese (BMI < 30) at the age of 35 years, and at 18 years, this chance is further reduced to 14.2%. Identifying modifiable mechanisms, such as changing the nutrient composition of diets, provides an opportunity to decrease TEI and positively affect this risk trajectory. Further studies are now warranted in much larger cohorts of population-based groups of children and adolescents, ideally assessing dietary composition with subsequent weight changes. However, these studies will require extensive data collection relating to potential confounders.
Conclusion
This is the first study investigating PL and the PLH in a cohort of children and adolescents with obesity aged 6 to 18 years. We identified decreased proportion of dietary protein to be the only macronutrient associated with increased overall energy intake. Importantly, dietary macronutrient composition may assist to reduce TEI in children and adolescents with obesity seeking weight management counseling.
Acknowledgments
The authors would like to thank the Childhood Overweight Biorepository of Australia participants and their families.
Funding agencies
Authors from the Murdoch Children’s Research Institute are supported in part by the Victorian Government Operational Infrastructure Support Program. CS was supported by a 1-year clinical research fellowship position at the Endocrinology Department at the Royal Children’s Hospital. CS was also funded by Batzebaer Foundation, Inselspital Bern; Fondazione Ettore e Valeria Rossi; Freie akademische Gesellschaft Basel; and Novo Nordisk, all Switzerland. BEH is a National Health and Medical Research Council (NHMRC) Peter Doherty Early Career Fellow (APP:1072086). DPB is supported by a NHMRC Senior Research Fellowship (APP:1064629). AVR is supported by the NIHR Leicester Biomedical Research Centre and the Collaboration for Leadership in Applied Health Research and Care (CLAHRC) East Midlands. MJ was supported by Juho Vainio Foundation and federal research grants to Turku University Hospital. AMS was supported by a Discovery Early Career Researcher Award from the Australian Research Council (DE180101520). The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), National Institute for Health Research (NIHR), NHMRC, or Department of Health. The funding bodies did not play any role in this study or the decision to publish.
Disclosure
The authors declared no conflicts of interest.
Author contributions
CS and DT conceptualized the study, did the statistical analysis and interpreted results, and wrote and revised the manuscript; BEH conceptualized the study, collected data, and revised the manuscript; KTK provided the initial study design and data collection; ZM and EJA collected data; TO and AVR analyzed accelerometer data and revised the manuscript; DPB revised the manuscript; SJS and DR assisted with results interpretation and revised the manuscript; AMS provided statistical background for power analysis and compositional data analysis; MJ revised the manuscript; MAS conceptualized the study, interpreted results, and revised the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.





