Genetically Engineered Escherichia coli Nissle 1917 Secreting GLP-1 Analog Exhibits Potential Antiobesity Effect in High-Fat Diet-Induced Obesity Mice
Abstract
Objective
This study aimed to investigate the potential antiobesity effect of genetically modified Escherichia coli Nissle 1917 (EcN-GM) in mice fed a high-fat diet (HFD).
Methods
The mice were randomly divided into six groups: a normal diet group (ND), a HFD group, a HFD + EcN group, and three HFD + EcN-GM groups. The effects of EcN-GM on body weight, food intake, fat pad and organ weight, and an oral glucose tolerance test were measured, in addition to hepatic biochemistry and histological analysis. The mRNA expression of neuropeptides related to food intake regulation in the hypothalamus was also detected.
Results
The results showed that EcN-GM decreased body weight, body weight gain, food intake, fat pad weight, and hepatic weight of HFD mice. There were beneficial effects of EcN-GM on blood glucose, hepatic biochemistry, and hepatic histological alterations. A dramatic switch of food intake-regulating gene expression in the hypothalamus was also observed in mice.
Conclusions
This work has revealed that a modified live bacterial therapeutic, EcN-GM, has potential beneficial effects on obesity. This effect may be related to the regulating of the neuropeptide expression of energy intake and expenditure in the hypothalamus. This study demonstrates a successful example of engineered EcN-GM as a novel approach for weight management.
Study Importance
- Various medications have been used to treat obesity over the years. However, most of them have been withdrawn from the market because of serious side effects. Thus, novel and alternative strategies are urgently needed for long-term prevention and treatment of obesity.
- The gut hormone glucagon-like peptide 1 (GLP-1), a 30-amino acid peptide hormone, has been increasingly understood for its effect on and its control over glucose metabolism and body weight.
- The genetic manipulation of Escherichia coli Nissle 1917 (EcN) as a “live biotherapeutic product” could deliver therapeutic factors to treat obesity, diabetes, intestinal disease, and other chronic diseases.
- Circulating GLP-1 has a short half-life of 2 to 5 minutes because of rapid degradation by dipeptidyl peptidase 4. Some modifications were made on the sequence of GLP-1 (7-37) to form a new GLP-1 GM (genetically modified) sequence (Patent Application Number 201811519742.X and PCT/CN2019/071736), and EcN was engineered to secrete GLP-1 GM.
- In the present study, the potential effect of the genetically modified EcN (EcN-GM) against obesity was investigated in mice fed a high-fat diet.
- This work has, for the first time, revealed that a modified live bacterial therapeutic, EcN-GM, has beneficial effects on obesity, blood glucose, and hepatic steatosis.
Introduction
Obesity is defined as abnormal or excessive fat accumulation, and it has been one of the most critical public health concerns worldwide in recent years (1). It is a major contributor to a variety of chronic diseases, including type 2 diabetes, hypertension, cardiovascular disease, nonalcoholic fatty liver disease, stroke, and osteoarthritis (2-5), with enormous economic burden on both families and society (6, 7).
Previous studies have indicated that lifestyle and behavioral modification such as diet intervention and exercise is the cornerstone for prevention and management of obesity, but these have mostly failed to achieve long-term and sustainable success for most patients (8). If lifestyle modification is ineffective, alternative therapy such as pharmacological approaches should be taken into consideration. Various medications have been used to treat obesity over the years. However, most of them have been withdrawn from the market as a result of serious side effects (9). Thus, novel strategies are urgently needed for the long-term treatment of obesity.
Researchers have paid close attention to gut-derived peptide hormone, which plays a significant role in energy homeostasis, in recent decades (10). Glucagon-like peptide 1 (GLP-1), a 30-amino acid peptide hormone, has been increasingly investigated for its effect on glucose metabolism and body weight (11). GLP-1 is secreted by enteroendocrine L cells of the intestinal mucosa in response to glucose and other nutrients during food intake. It exerts various effects, such as reducing glucagon secretion under conditions of hyperglycemia and stimulating pancreatic β-cell proliferation, which could reduce body weight with a low risk of hypoglycemia (12). However, circulating GLP-1 has a short half-life of 2 to 5 minutes because of rapid degradation by dipeptidyl peptidase 4 (11, 13). Some modifications were made by our group on the sequence of GLP-1 (7-37) to form a new GLP-1 GM (genetically modified) sequence.
Moreover, the challenge with the application of GLP-1 and the analog of GLP-1 is that peptide medications can be administered only by injection, which is not suitable for long-term application, driving the need for more universally applicable delivery approaches (14, 15). Escherichia coli Nissle 1917 (EcN) does not carry any virulence genes and therefore it is not pathogenic to humans; it is specifically used as a probiotic to treat intestinal infections and disorders (16).
In recent years, ongoing research has focused on genetic manipulation of EcN as a carrier of therapeutics for the treatment of obesity, diabetes, intestinal disease, and other diseases (17-19). In addition, this approach could also be used in a novel oral administration form to deliver bioactive molecules to the intestine without degradation; oral administration remains the most preferred route of drug delivery for the management of chronic diseases, owing to patient compliance. Therefore, EcN was genetically engineered to secrete GLP-1 GM. The goal of the current study was to investigate the potential antiobesity effect of this genetically modified EcN (EcN-GM) in mice fed a high-fat diet (HFD).
Methods
Bacterial strains and preparation
Several modifications were made in the primary sequence of GLP-1 (7-37), and the new sequence was called GLP-1 GM (Patent Application Number 201811519742.X and PCT/CN2019/071736). The plasmid was constructed to express GLP-1 GM. The sequence was synthesized at Chinese Peptide Co. (Hangzhou, China). and was inserted into pGEX carrier to make pGEX-GLP-1 GM, and plasmid pGEX-GLP-1 GM was transformed into EcN to construct the EcN-pGEX-GLP-1 GM strains. The EcN-GM was grown in Luria-Bertani (LB) medium.
Animals and protocols
Specific pathogen free (SPF) Kunming (KM) mice were obtained from Chengdu Dasuo Biotechnology Co. (Chengdu, China). The mice were housed under humidity- and temperature-controlled conditions and a 12-hour light/dark cycle with free access to food and water. All procedures strictly conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
After a 1-week adaptation, mice were randomly divided into six groups (n = 10 in each group): a normal diet (ND) group, a HFD group (ND 67%, sucrose 20%, lard oil 10%, cholesterol 2%, and sodium cholate 1%), a HFD + EcN group, and three HFD + EcN-GM groups (HFD + EcN-GM8 group, HFD + EcN-GM9 group, and HFD + EcN-GM10 group). The mice in the HFD, EcN, and EcN-GM groups were gavaged with 0.3 mL of control medium, EcN (1010 CFU/mL), and EcN-GM (108, 109, and 1010 CFU/mL), respectively. All treatments were given once per day for 8 weeks.
Body weight, body length, and food intake
The body weight, body length, and food intake of mice were measured every week for 8 weeks.
Tissue sample collection
The fat pad (epididymal and perirenal), liver, heart, spleen, lung, and kidney of mice were removed and weighed.
Oral glucose tolerance test
For the glucose tolerance test, animals fasted overnight were injected intraperitoneally with glucose solution (2 mg/kg body weight). Blood glucose was measured at 0, 15, 30, 60, and 120 minutes with a blood glucose meter (Sinocare GA-3; Sinocare Inc., Changsha, China), and areas under the curve (AUC) were calculated.
Measurement of hepatic triglyceride and total cholesterol content
Hepatic triglyceride and total cholesterol content were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to manufacturer instructions.
Histological analysis
Liver tissues were fixed in paraformaldehyde, processed in paraffin blocks, embedded and sectioned at 5 μm, and stained with hematoxylin and eosin using a standard procedure. The cellular structure and lipid accumulation in tissue samples were observed and photographed (× 200, original magnification) under a microscope (Olympus, BX43; Olympus Co., Tokyo, Japan).
Quantitative real-time polymerase chain reaction analysis for expression of neuropeptides in hypothalamus
β-actin: GAAGATCAAGATCAT TGCTCCT (forward), TACTCCTGCTTGCTGATCCA (reverse);
AgRP: TCTCAC AGAGGCACCCAGAG CAGAG (forward), CGAGCAGAGATAGAGCGAGGGTCAGT (reverse);
NpY: TTAGGCTTGGAGCAGGTCCTGGAGTC (forward), GCGTC TGGCTCTTCTCGGAGGTCAT (reverse);
and POMC: TGGCGGAGGTGCTAGATCC ACAGAA (forward), CCATTGGCTAGGTGCGACTACAGAGG (reverse).
Statistical analysis
All data are presented as mean (SEM). One-way ANOVA was determined by SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, New York). Differences were considered statistically significant at P < 0.05.
Results
EcN-GM reduced body weight, body weight gain, and food intake in HFD-fed mice
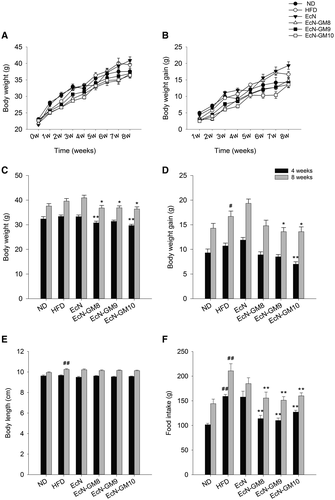
As expected, the body weight and body weight gain of the HFD group increased compared with the ND group. EcN-GM treatment decreased body weight and suppressed body weight gain significantly. There was a significant elevation of food intake in the HFD group compared with the ND group, and EcN-GM treatment markedly reduced food intake to a similar level as the ND group. The body length increased significantly in the HFD group compared with the ND group at 8 weeks. There was no significant difference in body length between the EcN-GM-treated groups and the HFD group (Figure 1).
EcN-GM decreased adipose tissue weight and hepatic weight in HFD-fed mice
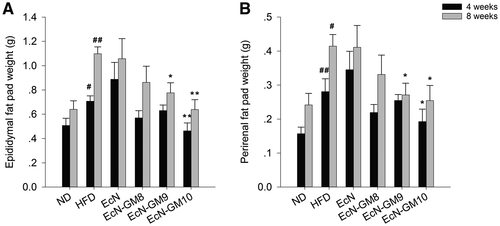
As shown in Figure 2, the epididymal and perirenal fat pad weight of the HFD group was significantly greater than that in the ND group. Fat pad weight was reduced remarkably in the EcN-GM-treated groups compared with the HFD group, especially in the EcN-GM10 group at 4 and 8 weeks.
A significant increase of hepatic weight was observed in the HFD group compared with the ND group. In the EcN-GM-treated groups, there was a significant reduction of hepatic weight compared with the HFD group, and the most marked change was detected in the EcN-GM10 group (Figure 3A).
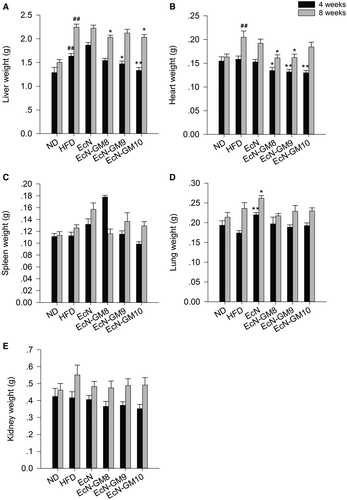
The weight of other vital organs, including heart, spleen, lung, and kidney, was also measured (Figure 3B-3E). An increase in heart weight was detected in HFD mice, and administration of EcN-GM significantly reduced the heart weight. The weight of spleen, lung, and kidney was not significantly different between EcN-GM-treated groups and the HFD group.
EcN-GM reduced glucose level and AUC of blood glucose in HFD-fed mice
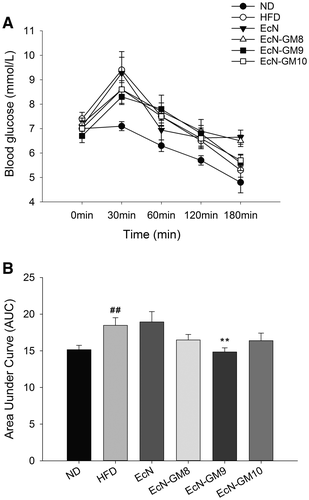
An oral glucose tolerance test was performed at week 8. During the glucose tolerance test, the AUC of glucose of the HFD group was increased compared with the ND group, and EcN-GM treatment reduced the AUC compared with the HFD group. The most significant reduction was observed in the EcN-GM9 group (Figure 4).
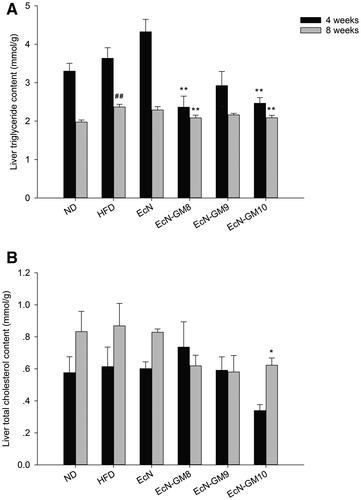
Impact of EcN-GM on hepatic triglyceride and total cholesterol content in HFD-fed mice
The content of hepatic triglyceride increased remarkably in the HFD group compared with the ND group. In contrast, the administration of EcN-GM diminished its levels, especially in the EcN-GM8 and EcN-GM10 groups at 4 and 8 weeks. There was an increase in total cholesterol levels in the HFD group; this was reduced by EcN-GM treatment, but a significant reduction was observed only in the EcN-GM10 group at 8 weeks (Figure 5).
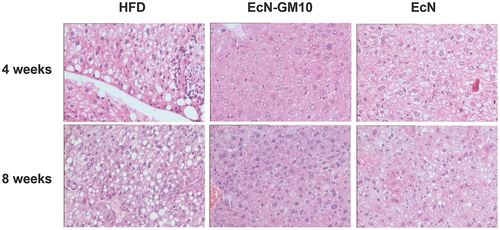
EcN-GM attenuated histological alterations in liver of HFD-fed mice
Pathological tests were performed on liver tissues at week 8. As shown in Figure 6, macrovesicular steatosis was observed in hepatic cells of HFD mice. Meanwhile, fibrosis was also observed in the area around central veins and hepatic cells in HFD mice. However, EcN-GM10 treatment ameliorated hepatic steatosis and fibrosis of HFD-fed mice.
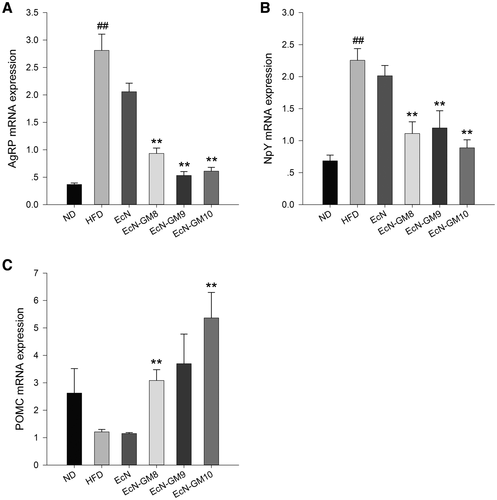
Impact of EcN-GM on mRNA expression of neuropeptides in hypothalamus of HFD-fed mice
The mRNA expression of AgRP and NpY was remarkably upregulated, and POMC was downregulated in the hypothalamus of the HFD group. EcN-GM treatment suppressed the mRNA expression of AgRP and NpY and induced the expression of POMC (Figure 7).
Discussion
Multiple factors that lead to an imbalance between energy intake and expenditure are the main cause of obesity, and most obesity treatment is to modify energy balance (20). Because of the complexity of obesity, it is difficult to achieve and sustain the goal of weight loss with lifestyle intervention only. It is unfortunate that the effects of most of the antiobesity agents have been modest and the adverse effects significant. Nowadays, only five agents have been approved for use in weight loss in the United States and three in Europe, among which analogs of GLP-1 are promising medications for their outstanding effects and safety (20).
The use of engineered bacteria to deliver therapeutic molecules has been reported in various studies (15, 18, 19). Notably, probiotics, including EcN, bifidobacteria, and lactobacilli, have already been engineered to produce GLP-1 or GLP-1 analogs to treat diabetes or inflammation-induced memory impairment (15, 21-23).
Therefore, in previous work, an alteration of the original sequence of GLP-1 was made, and our team selected the most effective sequence (GLP-1 GM) to engineer a strain of EcN to express GLP-1 GM. The main goal of this research was to investigate the hypothesis that engineered EcN-GM could serve as a novel treatment method for obesity. The data from this work demonstrated that 8 weeks of treatment with EcN-GM remarkably reduced body weight and body weight gain in HFD-induced obesity mice. Liver, epididymal, and perirenal fat pad weight was also diminished in EcN-GM-treated mice. These results are paralleled by significantly reduced food intake, which is consistent with other studies showing that GLP-1 and an analog of GLP-1 reduced weight by appetite suppression in rodents and minipigs (24-26).
Moreover, glucose level and AUC of blood glucose were decreased by 8 weeks of EcN-GM treatment. EcN-GM also diminished the content of hepatic triglyceride and total cholesterol and attenuated the histological alterations in liver tissue, which confers a protective effect of liver in obese mice.
The pathways that modulate food intake and energy homeostasis are the main target for obesity research (27, 28). This homeostatic control is maintained by neurons in various brain regions, among which the hypothalamus is believed to be the key brain area in regulation (29). The arcuate melanocortin neurons consist of two distinct neuronal populations, which have totally opposite effects on energy intake and expenditure (29, 30). AgRP and NpY increase food intake and decrease energy expenditure; POMC acts as a break to reduce food intake and induce energy expenditure (31-34).
Previous research has indicated that GLP-1 diminishes food intake at least in part via hypothalamic pathways (35). Therefore, to determine whether the antiobesity effect of EcN-GM is correlated with the neuropeptide of appetite in the central nervous system, the mRNA expression of POMC, AgRP, and NpY in the hypothalamus was measured. The results showed that EcN-GM treatment remarkably induced the mRNA expression of POMC and reduced the expression of AgRP and NpY in the hypothalamus of HFD mice. Therefore, the previously mentioned findings hint at a possible fact that the effect of EcN-GM against obesity might be attributed to the reduced food intake and increased energy expenditure via modulating neuropeptide level related to homeostatic control in the hypothalamus. Further research is required to demonstrate the effect of EcN-GM on appetite regulation using a GLP-1 receptor antagonist or GLP-1 receptor knockout mice and to present more detailed molecular mechanisms underlying the antiobesity effect of EcN-GM.
Conclusion
Our work has, for the first time, revealed that a genetically modified probiotic, EcN-GM, has beneficial effects on obesity, blood glucose, and hepatic steatosis. This effect might be related to regulation of the neuropeptide expression of energy intake and expenditure in the hypothalamus. The present study demonstrates a successful example of engineered EcN-GM as a novel approach for weight management. More detailed studies are necessary to elucidate the molecular mechanism underlying the regulation.





