Differential Effects of Diet and Weight on Taste Responses in Diet-Induced Obese Mice
Abstract
Objective
Previous studies have reported that individuals with obesity have reduced taste perception, but the relationship between obesity and taste is poorly understood. Earlier work has demonstrated that diet-induced obesity directly impairs taste. Currently, it is not clear whether these changes to taste are due to obesity or to the high-fat diet exposure. The goal of the current study was to determine whether diet or excess weight is responsible for the taste deficits induced by diet-induced obesity.
Methods
C57BL/6 mice were placed on either high-fat or standard chow in the presence or absence of captopril. Mice on captopril did not gain weight when exposed to a high-fat diet. Changes in the responses to different taste stimuli were evaluated using live cell imaging, brief-access licking, immunohistochemistry, and real-time polymerase chain reaction.
Results
Diet and weight gain each affected taste responses, but their effects varied by stimulus. Two key signaling proteins, α-gustducin and phospholipase Cβ2, were significantly reduced in the mice on the high-fat diet with and without weight gain, identifying a potential mechanism for the reduced taste responsiveness to some stimuli.
Conclusions
Our data indicate that, for some stimuli, diet alone can cause taste deficits, even without the onset of obesity.
Study Importance
What is already known?
- Obesity is often associated with impaired taste.
- The mechanisms associating obesity and taste are poorly understood.
What does this study add?
- Our study on diet-induced obese mice demonstrates that high-fat diet and excess weight can each contribute to impaired taste.
- There is selectivity in the effects of diet and weight that varies by stimulus.
Introduction
Studies have suggested that perception of taste stimuli is reduced in populations with obesity (1-8), which leads to increases in intake (9-11). Despite this link between taste and obesity, little work has focused on identifying the mechanisms that are responsible for this relationship. We previously reported that diet-induced obesity (DIO) inhibited the responsiveness of peripheral taste receptor cells (TRCs) to taste stimuli, particularly sweet stimuli, and that these changes translated into behavioral effects (12). There is strong support for the idea that a reduction in taste signaling drives increases in food intake (9, 10, 13), and our earlier study (12) demonstrated that DIO affects multiple aspects of taste starting with the initial signaling event.
There are two aspects of DIO that can potentially affect taste responses: excess weight or diet. We explored the respective roles of these factors for the identified DIO-dependent taste deficits. We focused on sweet taste because it was the most affected by DIO (12) and because it is reported to be affected by obesity (9-11, 14-16). The goal of this study was to identify whether there are independent effects of diet or excess weight on taste function.
To separate weight gain from high-fat (HF) diet exposure, we added a low dose of captopril (CAP) to the water of mice on an HF diet. Previous work showed that CAP, an angiotensin-converting enzyme inhibitor, protected against the development of DIO (17) because animals on CAP voluntarily reduced their caloric consumption. Exposing DIO animals to CAP causes weight loss and reverses associated metabolic issues (17-19). This approach avoids the stress that using food restriction and single housing might impose on the mouse. Using this pharmacological method, we compared DIO mice with mice on the same HF diet that did not become obese.
Materials
Mice
Husbandry practices were in compliance with the University at Buffalo Institutional Animal Care and Use Committee. All experiments used C57BL/6 mice (1-6 months). At weaning, mice were placed on either standard (Harlan Labs, Inc., Madison, Wisconsin; calories were 18% from fat, 58% from carbohydrates, and 24% from protein) or HF chow (Harlan Labs; calories were 60% from fat, 22% from carbohydrates, and 18% from protein). Mice were placed on the HF diet or standard chow in the presence and absence of CAP (0.05 mg/mL water). Initially, CAP was replaced weekly, but at week 2, CAP water was replaced every other day. Experiments were performed approximately 6 to 8 weeks after presentation of the HF diet. Omentum (OM) and retroperitoneal (RP) fat weights were collected and were calculated as either [(OM g ÷ total weight g) × 100] or [(RP g ÷ total weight g) × 100].
Taste cell isolation
TRCs were harvested from taste papillae of adult mice as previously described (12, 20-25). Briefly, tongues were removed and injected beneath the lingual epithelium with 0.2 mL of enzyme solution (3 mg of Dispase II, 0.7 mg of collagenase B [Roche Diagnostics, Indianapolis, Indiana], and 1 mg of trypsin inhibitor in 1 mL of Tyrode’s). The epithelial layer of the tongue was placed in Ca2+-Mg2+-free solution (140mM NaCl, 5mM KCl, 10mM HEPES, 2 mMEGTA, and 2 mMBAPTA [pH 7.4]) after being incubated at room temperature in oxygenated Tyrode’s. Cells were removed from circumvallate (CV) taste papillae via gentle suction and placed on a slide coated in Cell-Tak (Discovery Labware, Bedford, Massachusetts).
Calcium imaging
Isolated TRCs were incubated for 20 minutes in 2µM Fura-2 AM and nonionic dispersing agent, Pluronic F-127 (Invitrogen, Eugene, Oregon), and then washed for 20 minutes. Isolated cells were visualized using an Olympus IX71 scope and 40x oil immersion objective (Olympus, Center Valley, Pennsylvania). TRCs were stimulated and Ca2+ changes were recorded every 4 seconds using a Sensicam QE camera (Cooke Corp., Romulus, Michigan) at 340/380-nm excitation and 510-nm emission. Data were collected using Imaging Workbench 6.0 (Indec Biosystems, Santa Clara, California) and analyzed using Origin 8.6 (Origin Lab Corp., Northampton, Massachusetts). Response amplitude was calculated as [(peak value − baseline value) ÷ baseline value] × 100. Only isolated TRCs with a resting Ca2+ baseline between 40 and 150 nm were analyzed, and an evoked response was defined as measurable if the increase in fluorescence was greater than 2 standard deviations above baseline.
Analysis of licking behavior
Unconditioned licking responses were recorded to varying concentrations of taste stimuli in a Davis Rig chamber (Davis MS80 Rig; Dilog Instruments and Systems, Tallahassee, Florida), which has been described elsewhere (26). Each individual lick was detected and recorded by a contact lickometer via DavisPro collection software (Dilog Instruments and Systems).
Mice were adapted to the Rig and trained to drink from the sipper tubes for eight consecutive days (26). During testing, animals were allowed to take as many trials as possible in 30 minutes. Mice were tested on varying concentrations of sucrose (0, 3, 10, 30, 60, 100, 300, and 1,000mM), saccharin (0, 0.1, 0.5, 1, 3, 5, 10, and 50mM), acesulfame potassium (AceK; 0, 0.5, 2, 6, 8, 16, 20, and 32mM), KCl (0, 12.5, 25, 50, 100, 200, 300, and 400mM), and denatonium benzoate (0, 0.1, 0.3, 1, 3, 5, 10, and 20mM), in that order. Each stimulus was presented in randomized blocks on Monday, Wednesday, and Friday in a single week. Animals were tested on sucrose and AceK while water replete; however, trial initiation was lower than expected, so animals were 22-hour water deprived for all of the remaining stimuli. Once the mouse began licking the tube, it was allowed 10 seconds of access before the shutter closed.
For stimuli tested in the water-deprived condition, lick ratios were calculated by dividing the average number of licks at each concentration by the average number of licks to water. For sucrose and AceK, lick scores were calculated by subtracting the average number of licks at each concentration by the average number of licks to water. Lick scores were compared by ANOVA with treatment and solution concentration as factors. Significant interactions between treatment and concentration were followed with sub-ANOVA. Comparisons between control (CTL) ± CAP ruled out any potential effects of CAP. CTL-treated animals were collapsed and then compared with either the HF +CAP group (to determine an effect of diet alone) or with HF −CAP (to determine the effects of weight). Bonferroni-corrected t tests were conducted to identify concentrations that differed between groups.
Immunohistochemistry
Tongues were removed and placed in 4% paraformaldehyde/0.1M phosphate buffer (pH 7.2) overnight at 4°C. After cryoprotection, 40-μm sections of the CV papillae were cut, washed in phosphate-buffered saline (PBS), and incubated in blocking solution (0.3% Triton X-100, 1% normal goat serum, and 1% bovine serum albumin [BSA] in 0.1M PBS). Samples were incubated in primary antibody overnight at 4°C. All primary antibodies were diluted in blocking solution, and CTL mice with no primary antibody were included in each experiment. After washing, sections were incubated in secondary antibody for 2 hours, washed, mounted in Fluoromount media with DAPI staining (Southern Biotechnology Associates, Birmingham, Alabama), and visualized using confocal microscopy (Axioimager Z1 and Axiovert 200M, Zeiss, White Plains, New York). Primary antibodies tested include α-gustducin (1:50, Santa Cruz Biotechnology, Santa Cruz, California) and phospholipase Cβ2 (PLCβ2; 1:1000, Santa Cruz Biotechnology). Primary antibody labeling was visualized using goat-anti-rabbit Cy5 secondary antibody (1:500, Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania).
Cell count analysis
Cells in individual CV taste buds were analyzed using Zen 2012 Blue Edition (Zeiss, San Diego, California) with the analysis done blind to experimental condition. Images were taken at every micron, and the nuclei in every fifth slice were counted as an estimate of the number of taste cells within each bud. Cells expressing the target protein were then counted at every fifth slice. Data are reported as the ratio of protein-expressing cells to total cells in the buds. Taste buds from at least three mice were analyzed for each experimental condition.
Complementary DNA synthesis
Isolated TRCs were pelleted for RNA extraction. RNA was isolated using the NucleoSpin RNA XS kit (Macherey-Nagel, Dϋren, Germany), treated with DNAase (Fermentas Life Sciences, Waltham, Massachusetts), and then used as a template to produce complementary DNA (cDNA) using SuperScript III Reverse Transcriptase (Invitrogen). Polymerase chain reaction (PCR) analysis was performed for GAPDH to ensure sample quality and check for genomic contamination. Contaminated samples were discarded. Primers are listed in Supporting Information Table S1.
Real-time PCR
Real-time PCR was performed using a MiniOpticon system (Bio-Rad Laboratories, Hercules, California), with BioRad SYBR Green reagents. cDNA from three mice for each experimental condition was run in triplicate. If there was more than a 5% difference between the replicates, the data were discarded. Data were normalized to GAPDH expression for each sample to correct for any loading differences and reported as fold differences.
Solutions
All chemicals were purchased from Sigma Aldrich (St. Louis, Missouri) unless otherwise noted. Tyrode’s solution contained the following: 140mM NaCl, 5mM KCl, 1mM MgCl2, 3mM CaCl2, 10mM HEPES, 10mM glucose, and 1mM pyruvic acid (pH 7.4). The following chemicals were diluted into Tyrode’s and used for cellular stimulation during experiments: sweet, 2mM saccharin, 50mM sucrose (50mM NaCl was replaced with 50mM sucrose), and 20mM AceK; bitter, 5mM denatonium benzoate; and salt, 50mM NaCl replaced with 50mM KCl.
Results
HF diet causes significant weight gain in absence of CAP
Mice fed an HF diet rapidly gained weight. After 1 week, mice on HF food were significantly heavier than mice on standard chow (CTL). To determine whether exposure to CAP reduces weight gain in mice on HF food, we measured the weights of a subset of mice that were on HF or standard chow, with or without CAP exposure. Mice on HF food with CAP in their water (HF +CAP) and mice on standard chow with CAP (CTL +CAP) were not significantly different from CTL (Figure 1A). During the initial 2 weeks, when water was replaced weekly, HF +CAP mice gained weight at a slightly higher rate than mice on CTL chow (± CAP). Providing fresh CAP water every other day resulted in the weights of HF +CAP mice being comparable to CTL mice.
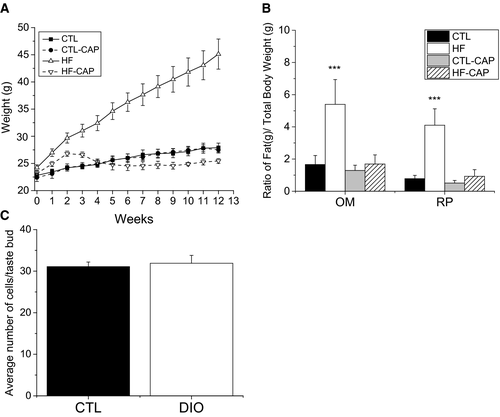
Greater OM and RP adipose tissues (Figure 1B) were larger for both the ratio of OM to total body weight (one-way ANOVA, P < 0.001) and RP to total body weight in mice on the HF diet compared with mice in the other experimental conditions (one-way ANOVA, P < 0.001). No other significant differences were found. Based on these criteria, mice on the HF (−CAP) diet were identified as obese.
DIO does not cause loss of TRCs
To determine whether DIO causes a significant loss of TRCs, we analyzed taste buds from mice (n = 3) that were on the HF diet for 10 weeks and had OM > 3 and RP > 3. The number of TRCs per bud in these mice was compared with age-matched littermates on the CTL diet (n = 3). Taste buds from the CV papillae were fixed and stained with DAPI to identify the nuclei (Supporting Information Figure S1) for cell counting. A total of 13 taste buds were analyzed for each condition, and no significant differences were found between the CTL or DIO mice (Figure 1C).
Diet and weight differentially affect sweet taste
We evaluated the effects of excess weight and exposure to the HF diet for three sweet stimuli (AceK [20mM], saccharin [2mM], and sucrose [50mM]), a bitter stimulus (denatonium benzoate [5mM]), and a salt stimulus (50mM KCl). Three different measures were taken: (1) the percentage of TRCs that respond to a given stimulus, (2) the amplitude of the taste-evoked calcium signal in the responding cells, and (3) the unconditioned licking response in the behaving animal for each stimuli (Figures 2, 3). To ensure that CAP does not independently affect taste, we included a group of mice kept on standard chow with CAP exposure for all experiments. No significant differences for any of the experimental conditions were found. The percentage of responsive TRCs was compared using χ2 analyses (27) with Yates correction. Other analyses were compared using ANOVA followed by student t tests or sub-ANOVA, as specified.

TRC responsiveness was defined as the number of TRCs that responded to a specific stimulus divided by the total number of TRCs that were exposed to that stimulus. These data are presented as a percentage on a bar graph and are shown in the first column of Figure 2 for AceK (Figure 2A), saccharin (Figure 2B), and sucrose (Figure 2C). Individual values are shown in Supporting Information Table S2. No significant differences were found for the number of TRCs that responded to AceK (Figure 2A). While the average amplitude of the TRC responses to AceK was significantly reduced in the mice on the HF diet (−CAP) compared with the other groups (Figure 2A, second panel, P = 0.03), no other significant differences were found. Unconditioned licking was significantly reduced only in the HF −CAP mice compared with other treatments (specific values shown in Table 1), and there was no significant difference in the number of trials initiated (P = 0.56) (Figure 2A, right two panels) or the number of licks to water (P = 0.8). These data indicate that weight is likely the primary factor resulting in the reduced taste response to the artificial sweetener AceK and that the significantly reduced licking by the HF mice was not due to low motivation in the task that would be reflected in trial initiation.
| Hypothesis group | All groups comparison | Effect of CAP | Effect of HF diet | Effect of weight | Effect of CAP in HF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All groups | P | CTL ± CAP | P | CTL vs. HF +CAP | P | CTL vs. HF −CAP | P | CTL ± CAP | P | |||||||
| Effect | F | df | F | df | F | df | F | df | F | df | ||||||
| AceK | Treatment | 15.613 | (3,28) | < 0.001 | 0.004 | (1,14) | 0.947 | 2.777 | (1,22) | 0.110 | 19.140 | (1,22) | < 0.001 | |||
| Concentration | 23.848 | (6,168) | < 0.001 | 25.786 | (6,84) | < 0.001 | 33.830 | (6,132) | < 0.001 | 32.449 | (6,132) | < 0.001 | ||||
| Interaction | 6.928 | (18,168) | < 0.001 | 1.587 | (6,84) | 0.161 | 0.360 | (6,132) | 0.903 | 5.873 | (6,132) | < 0.001 | ||||
| Saccharin | Treatment | 5.663 | (3,28) | 0.004 | 0.214 | (1,14) | 0.651 | 1.498 | (1,22) | 0.234 | 15.968 | (1,22) | 0.001 | |||
| Concentration | 81.404 | (6,168) | < 0.001 | 55.981 | (6,84) | < 0.001 | 62.198 | (6,132) | < 0.001 | 38.013 | (6,132) | < 0.001 | ||||
| Interaction | 5.244 | (18,168) | < 0.001 | 0.368 | (6,84) | 0.898 | 2.142 | (6,132) | 0.053 | 14.854 | (6,132) | < 0.001 | ||||
| Sucrose | Treatment | 20.710 | (3,28) | < 0.001 | 1.587 | (1,14) | 0.228 | 22.587 | (1,22) | < 0.001 | 47.005 | (1,22) | < 0.001 | 4.622 | (1,14) | 0.500 |
| Concentration | 73.212 | (6,168) | < 0.001 | 86.372 | (6,84) | < 0.001 | 49.475 | (6,132) | < 0.001 | 40.186 | (6,132) | < 0.001 | 5.240 | (6,84) | <0.001 | |
| Interaction | 12.452 | (18,168) | < 0.001 | 1.287 | (6,84) | 0.272 | 18.742 | (6,132) | < 0.001 | 24.715 | (6,132) | < 0.001 | 0.500 | (6,84) | 0.806 | |
| Den | Treatment | 9.922 | (3,28) | < 0.001 | 0.170 | (1,14) | 0.686 | 12.574 | (1,22) | 0.002 | 27.208 | (1,22) | < 0.001 | 3.525 | (1,14) | 0.081 |
| Concentration | 39.811 | (6,168) | < 0.001 | 28.400 | (6,84) | < 0.001 | 30.064 | (6,132) | < 0.001 | 20.973 | (6,132) | < 0.001 | 13.649 | (6,84) | <0.001 | |
| Interaction | 2.516 | (18,168) | 0.001 | 0.055 | (6,84) | 0.999 | 3.918 | (6,132) | 0.001 | 5.068 | (6,132) | < 0.001 | 0.860 | (6,84) | 0.528 | |
| KCl | Treatment | 0.144 | (3,28) | 0.933 | 0.048 | (1,14) | 0.830 | |||||||||
| Concentration | 20.648 | (6,168) | < 0.001 | 13.587 | (6,84) | < 0.001 | ||||||||||
| Interaction | 1.529 | (18,168) | 0.108 | 0.609 | (6,84) | 0.723 | ||||||||||
Analyses of the saccharin responses revealed that TRC responsiveness was significantly reduced in the obese mice compared with CTL (Figure 2B, left panel, P = 0.04); however, it was not significantly different from number of responsive cells in the HF +CAP mice. While the number of TRCs from the HF +CAP mice that were responsive to saccharin was lower than the CTL ± CAP mice, there were no significant differences identified. Evaluation of the response amplitudes identified significant differences in the mice on the HF diet (Figure 2B, second panel, P = 0.001). Student t tests determined that the saccharin responses in the obese mice were significantly smaller than CTL (P = 0.003), with comparable reductions in HF +CAP compared with CTL +CAP (P = 0.017). There were no significant differences between the mice on the HF diet (± CAP) or the CTL diet (± CAP). Unconditioned licking was significantly reduced in the obese mice (HF −CAP) compared with CTL, and there was a strong trend (P = 0.053) for a reduced response in HF +CAP compared with CTL +CAP (Figure 2B, third panel), with no differences in the number of trials taken (Figure 2B, right panel, P = 0.35) or number of licks to water (P = 0.2). Unlike the AceK results, these data suggest that diet is driving the reduction in saccharin responses, which is magnified when the animal is also obese.
We found no differences in the percentage of sucrose-responsive cells (P = 0.79), but the amplitudes of the sucrose responses were reduced in the obese mice (P = 0.02). Interestingly, in the behavioral analysis, both groups of HF mice (± CAP) showed reduced unconditioned licking compared with CTL (± CAP) as sucrose concentration increased (Table 1). There was no difference between groups in the number of trials initiated (P = 0.97) or number of water licks (P = 0.5). This suggests that the decrease in licking is due to the exposure to the HF diet, not changes in body mass. Representative live cell imaging traces are shown in Supporting Information Figure S2.
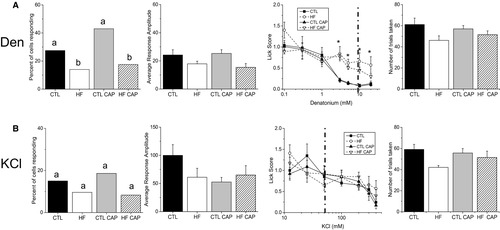
Diet affects bitter but not KCl taste
We also tested bitter (denatonium, 5mM) and salt (50mM KCl) stimuli. Exposure to the HF diet, regardless of weight, significantly reduced the number of denatonium-responsive TRCs (Figure 3A, left panel, P = 0.029 for HF +CAP and P = 0.007 for HF −CAP) but did not have a significant effect on the amplitude of the denatonium-evoked signals (Figure 3A, second panel, P = 0.05). The behavior data demonstrate that exposure to the HF diet reduced their sensitivity (increased unconditioned licking) to denatonium (Figure 3A, third panel, Table 1) with no change in the number of trials taken (Figure 3A, right panel, P = 0.27) or water licks (P = 0.9). In agreement with our earlier study (12), 50mM KCl responses were not affected by weight gain or diet exposure (Figure 3B, P = 0.13). Representative cell imaging traces are shown in Supporting Information Figure S3.
Taste signaling proteins change in obese mice
To investigate why taste-evoked calcium responses were inhibited, we measured the expression of α-gustducin and PLCβ2, two proteins in the signaling pathway that transmits sweet and bitter taste signals in TRCs (28). Immunohistochemical analysis suggested that there was a reduction in the expression of both of these signaling proteins, so we quantitated the number of TRCs expressing α-gustducin and PLCβ2 in the CV taste buds for mice in each experimental condition (n = at least 3 mice for each). Gustducin and PLCβ2 were analyzed separately.
The relative expression of α-gustducin for each experimental condition is shown in Supporting Information Figure S4A. χ2 analysis with Yates correction identified a significant reduction in the number of cells expressing α-gustducin in taste buds of obese mice (HF) compared with CTL (Figure 4A, left panel, P = 0.02). No other significant differences were found. Real-time PCR was used to quantitate the mRNA levels for α-gustducin from isolated taste buds (n = 3/group). There was a significant reduction in α-gustducin transcript levels in both groups of HF mice (± CAP) (Figure 4A, right panel, P < 0.01) compared with CTL.
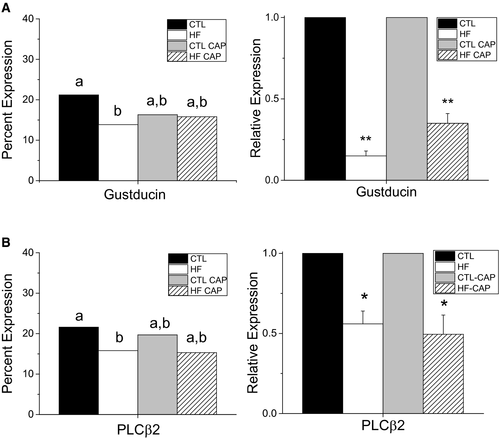
PLCβ2 expression appeared to be reduced in the CV taste buds of obese mice (Supporting Information Figure S4B). Analysis of PLCβ2 expression identified a significant reduction in the number of CV TRCs expressing PLCβ2 in the obese mice compared with CTL (Figure 4B, left panel, P = 0.04). The number of PLCβ2-expressing cells in the HF +CAP mice was not significantly different from CTL; however, PLCβ2 transcript levels were significantly reduced in HF ± CAP (Figure 4B, right panel, P < 0.05) compared with CTL.
Discussion
Obesity is a complex disease, and its relationship with the taste system is still not well understood. While multiple studies have suggested that perception of taste stimuli is reduced in populations with obesity (1-8) and that this leads to increased consumption (9-11), the mechanisms underlying these effects have not been identified. We have now demonstrated that an HF diet and excess weight can each inhibit taste responses and that these effects vary depending on the stimulus.
We chose to treat a subset of mice on the HF diet with a low dose of CAP as a way to expose them to an HF diet while preventing weight gain. Because these mice were exposed only to the HF diet, we were able to isolate the effect of diet exposure on taste. CAP, an angiotensin-converting enzyme inhibitor commonly used to regulate blood pressure, inhibits the production of the angiotensin II hormone. Consequently, CAP can cause weight loss in DIO mice as well as a reversal of obesity-associated metabolic issues (17-19). DIO has been associated with an overexpression of angiotensin II, which regulates fluid balance and plays a role in regulating body weight. It has been hypothesized that CAP leads to weight loss by reducing angiotensin II levels (29, 30). We observed the effectiveness of CAP in the initial weight analyses (Figure 1A). When water +CAP was replaced weekly, the HF +CAP mice began to gain weight. When the water +CAP was exchanged more frequently, that weight was subsequently lost and the mice were comparable to mice on standard chow. While previous work reported that angiotensin II enhanced sweet and suppressed salt responses (31), we found no difference in any measure between CTL ± CAP, suggesting that the CAP concentration used in our study was not sufficient to alter taste.
We measured the effects of DIO and diet on both TRC activity (which initiates taste) and behavior, the final output response to taste stimuli. For each stimulus, we ensured that the behavioral experiments tested a range of concentrations that encompassed the stimulus concentration used in the imaging experiments (shown as a dotted line on the behavior graphs in Figures 2, 3). For some stimuli (AceK and denatonium), the largest separation in the behavior data correlated with the concentrations used in the imaging experiments. However, for other stimuli (saccharin and sucrose), the concentrations used in the imaging experiments were lower than the stimulus concentrations where behavioral differences were recorded. While we used these concentrations in the imaging to avoid any nonspecific effects on the cells, it is possible that there would be more significant differences in the cell responses if a higher stimulus concentration could be used. Our results for denatonium differ from our previous findings (12). Both studies identified differences in denatonium-driven behaviors; however, our previous work reported a nonsignificant reduction in TRC responsiveness for DIO mice. We may have uncovered a significant effect in this study because of the larger sample size.
To identify the potential cellular mechanisms underlying the changes in taste cell activity, we evaluated the expression levels of two proteins with known roles in the transduction of bitter and sweet stimuli. α-gustducin, a G protein, is expressed in 20% to 30% of TRCs and is required for the normal transduction (32). The number of gustducin-expressing cells was reduced in the obese mice but not in HF +CAP mice (Figure 4A, Supporting Information Figure S4A). Conversely, quantitative PCR (qPCR) for gustducin identified a significant reduction in mRNA in TRCs from both groups of HF mice (± CAP). Because there was not a reduction in the number of gustducin-expressing cells in the HF +CAP mice, and loss of gustducin significantly decreases the ability to detect bitter and sweet stimuli (33), we predict that the level of gustducin expression within individual TRCs of these mice may be reduced. Since gustducin is not solely responsible for the transduction of all sweet stimuli (34), the reduction in its expression may contribute to the selective diet effect on the sweet taste responses.
We then evaluated the expression of PLCβ2, which is also required for the transduction of bitter and sweet stimuli (35). PLCβ2 expression was significantly reduced in the obese mice at both the protein and mRNA levels (Figure 4B, Supporting Information Figure S4B). While there appeared to be fewer cells expressing PLCβ2 in the HF +CAP mice, no significant differences were identified at the protein level (P = 0.09) even though there was a significant reduction in the mRNA levels (P = 0.013). The modest reduction of PLCβ2 expression in these taste cells may be a contributor to the selective effects of diet in inhibiting taste responses. These data also suggest that the obesity- and diet-dependent inhibition of TRC activity is not specific to one protein but to many components of the taste pathway.
The strong agreement between our cellular and behavioral data supports the premise that the diet-dependent inhibition of TRC activity is significant enough to impair the animal’s behavior. In the behavior trials, the HF −CAP animals were minimally responsive to all sweet and bitter stimuli tested, licking them similarly to water at all concentrations. The HF +CAP animals were minimally responsive to sucrose and denatonium but responded to the artificial sweeteners with the same concentration-specific responding that we saw in lean animals. Because we saw the effects in both the TRC and the behavioral assay, we do not believe that these differences were due to the more complex taste profile of the artificial sweeteners or postingestive feedback because both of these effects were absent in the cell imaging at the chosen concentration. We are left with the conclusion that diet and obesity alter the taste pathway in complicated ways that remain to be uncovered.
This complexity is further highlighted in the literature. Hajnal et al. reported that obesity developed on a standard chow (in rats lacking the cholecystokinin-1 [CCK-1] receptor) led to increased responding to sucrose (5). Dando and colleagues reported a decrease in taste bud number in obese mice compared with CTL, a finding we failed to replicate (36). However, our work is in agreement with Dando’s in other ways, as we both found a decrease in PLCβ2 expression (37) and no change in taste bud size (36) in DIO mice. Diet, even in the absence of obesity, and perhaps other measures, such as the length of the treatment, may all contribute independently to alterations in the taste system.
Our study highlights that the peripheral taste system can be significantly inhibited by diet, without the onset of obesity, suggesting that diet exposure alone may impair taste responses sufficiently to alter consumption, which can then subsequently induce or maintain obesity.
Acknowledgments
We wish to thank Kristen E. Kay for technical assistance and Alan Siegel/University of Buffalo North Campus Imaging Facility (funded by NSF-MRI Grant DBI 0923133) for confocal images.





