Weight Gain Over 6 Years in Young Adults: The Study of Novel Approaches to Weight Gain Prevention Randomized Trial
Abstract
Objective
The study objective was to determine whether two self-regulation interventions that reduced 3-year weight gain in young adults remain effective at 6 years.
Methods
A randomized trial was conducted in two academic settings in 599 young adults, aged 18 to 35 years, with normal weight or overweight; 504 (84%) reconsented for a 6-year extension (Study of Novel Approaches to Weight Gain Prevention-Extended [SNAP-E]) with ongoing intervention and assessments. Weight gain over 6 years was compared for all assigned to Control, Large Changes (LC; lose 5-10 pounds initially), and Small Changes (SC; make small daily changes in intake and activity).
Results
Weight change from baseline to 6 years did not differ significantly among the three groups (Control = 3.9 kg, SC = 4.1 kg, and LC = 2.8 kg). However, there was a significant age-by-treatment interaction (P = 0.002). Among those < 25 years old, weight gain from baseline to 6 years averaged 7.3 kg in the Control group and was reduced by almost 50% in LC and SC. LC also significantly reduced mean weight gain (area under the curve) over 6 years compared with Control or SC.
Conclusions
Although the interventions did not reduce weight gain at 6 years for the full cohort, they were effective in those < 25 years old. Future efforts should focus on young adults aged 18 to 24.9 and test more intensive interventions with more diverse participants.
Study Importance
What is already known?
- Young adults are the age group at greatest risk of significant weight gain.
- Interventions to prevent weight gain are often effective initially, but few have documented benefits at 2 years or longer.
- We previously tested two self-regulation interventions: Large Changes, which taught participants to lose 5 to 10 pounds initially to create a weight gain buffer, and Small Changes, which stressed small daily changes in intake and activity. Both interventions significantly reduced weight gain in young adults, relative to a control condition, over a mean follow-up of 3 years.
What does this study add?
- This study examined whether the effects of the two self-regulation interventions on weight gain prevention were still seen at 6 years.
- Weight changes from baseline to 6 years did not differ significantly among groups. However, relative to the control condition, both interventions significantly decreased weight gain in the subgroup of participants aged 18 to 25 at baseline.
- This study suggests that those aged 18 to 25 are especially vulnerable to weight gain, but this weight gain can be significantly reduced through self-regulation interventions.
Introduction
Recently, there has been increased attention to prevention of weight gain in young adults (1). This derives from the difficulties of producing sustained weight loss in individuals with obesity and the recognition that the rate of weight gain is greatest at this time in life (2-4). Individuals aged 20 to 35 years experience weight gain of 1 to 2 pounds per year, with worsening cardiovascular risk factors in those who gain the most weight (5). There have been several studies designed to identify predictors of weight gain (6-8) and to test interventions to reduce or prevent this weight gain (9). Frequently, these interventions have yielded positive short-term effects, but there have been few studies with longer follow-up (10-14) and most were unsuccessful (15).
We have reported positive results from the Study of Novel Approaches to Weight Gain Prevention (SNAP), a randomized clinical trial in 599 young adults comparing two self-regulation interventions and a control on weight-gain prevention over a mean follow-up of 3 years (16). Both self-regulation approaches involved frequent self-weighing; the Large Changes (LC) approach focused on losing 5 to 10 pounds during the first 4 months to provide a buffer against expected weight gains, and the Small Changes (SC) approach taught participants to make small daily changes (approximately 100 calories) in both intake and activity. As previously reported (16), mean weight gain averaged across the 3 years of follow-up was 0.26 kg, −0.56 kg, and −2.37 kg in the control group, SC, and LC, respectively, with significant differences among all three groups.
Given these positive results, SNAP was extended (SNAP-E) to test the hypothesis that there would be less weight gain from baseline to 6 years in both intervention groups than in the control group. To our knowledge, 6 years is the longest follow-up to date of a weight-gain prevention intervention trial.
Methods
Participants
SNAP was conducted at two clinical sites (University of North Carolina, Chapel Hill, North Carolina; Miriam Hospital, Providence, Rhode Island), with Wake Forest School of Medicine, Winston-Salem, North Carolina, as the Coordinating Center. Eligibility criteria for SNAP have been described in detail (16, 17), and they included being between 18 and 35 years old and having BMI between 21.0 and 30.9 kg/m2. SNAP recruitment was via mailings and emails from August 2010 to February 2012. After completing 4 years in SNAP, all participants (N = 599) were invited to reenroll in SNAP-E; 504 (84%) consented for SNAP-E. All three Institutional Review Boards approved the initial study and extension.
Interventions
At the start of SNAP, participants were randomly assigned to one of three treatment groups. Randomization was implemented through a Web-based data management system, using variable block lengths, stratified by clinical site, sex, and race/ethnicity (16, 17). The interventions have been described in detail elsewhere (16), and they are briefly described here. Both interventions, LC and SC, attended 10 meetings over the first 4 months (weekly for 8 weeks, then monthly). Using a self-regulation framework (18, 19), both intervention groups were taught to weigh themselves frequently (daily weighing was recommended) and to use these weights to know when changes in diet and physical activity were needed. The SC approach involved making daily changes of approximately 100 calories in both intake and physical activity. The LC group was given calorie, fat, and activity goals to produce a 5- to 10-pound initial weight loss during the first 4 months to act as a buffer against expected weight gains. After the initial 4 months, participants in the intervention groups were instructed to report their weight weekly and to either reinforce themselves for remaining below baseline weight, be cautious if small weight gains occurred, or return to the LC or SC approaches if weight gain was > 5 pounds. The Control group (referred to as the Self-Guided group) attended one group session, and they were introduced to the two different approaches and encouraged to follow whichever seemed most appropriate.
After the first 4 months, all groups received quarterly newsletters, and the two intervention groups were sent monthly feedback related to their current color zone. Twice a year, participants in the two intervention groups were invited to participate in optional 4-week refresher programs, offered online, which encouraged a return to the core principles of the SC or LC approach.
Those who enrolled in SNAP-E were provided with “smart scales” (BT-003; BodyTrace, Inc., Union City, California) and Fitbits (San Francisco, California) to encourage participation and allow remote data collection. In addition, all groups continued to receive newsletters, and the two intervention groups were provided with quarterly feedback regarding their color zones and invited to two 4-week online refreshers each year similar to those during SNAP (16).
Assessments
In SNAP, weights were measured by blinded assessors at in-clinic visits at 4 months and annually thereafter during years 1 through 4. Participants who had moved or were unwilling to come in to the clinic were sent a smart scale, which automatically transmitted data to the clinic. During the extension (SNAP-E), weights were collected via smart scale at year 4.5, 5, and 5.5 years and in the clinic at year 6 (or remotely for those who were not willing or had moved out of state). Because of the timing of the start of funding for the extension, only some participants could be approached for the 4.5- and 5-year assessments, whereas all could be approached for the 5.5-year and 6-year assessments. Height was assessed with a stadiometer at baseline and year 6 and was used to calculate BMI.
Statistical analysis
We examined whether there were differences in the characteristics at SNAP enrollment between participants who reconsented to extended follow-up compared with those who did not using χ2 tests.
Analyses were guided by the statistical plan prespecified in the SNAP-E protocol (Supporting Information Figure S1). The primary outcome for SNAP-E was weight change from the SNAP baseline to year 6, as estimated with a linear contrast from mixed-effects models applied to weights at times corresponding to the following clinic visits: at 4 months and at years 1, 2, 3, 4, and 6. Intent-to-treat analysis, including all initially randomized participants, was used. Covariate adjustment was made for whether weights were obtained at clinics or via smart scale and for the stratification factors used in randomization: clinic site, gender, and race/ethnicity (Non-Hispanic white vs. others). Overall differences among the three treatment groups were assessed with two-tailed type I error set at 0.05; three linear contrasts comparing pairwise group differences (LC vs. Control, SC vs. Control, and LC vs. SC) in the mean weight change from baseline to year 6 were used to compute 98.3% confidence intervals (Bonferroni adjustment for the three comparisons) for mean differences. Sensitivity analyses were conducted including only those who consented to SNAP-E and using inverse probability weighting to gauge the impact of missing data. The N of 504, observed standard error (SE), and Bonferroni adjustments provided 80% post hoc power to detect mean pairwise differences of 1.6 kg for the primary outcome.
Prespecified secondary analyses included weight gain averaged over the 6 years (referred to as “weight gain exposure”). This outcome was calculated using linear contrasts from a mixed-effects model across all time points and summing the areas (both positive and negative) traced by the trajectory of means from 0 (or area under the curve; see Supporting Information Figure S1); positive values represented the accumulated exposure to weight gain over time. Area under the curve was used in preference to simply averaging across time points in order to account for the difference in time intervals between the assessments (4 months and years 1, 2, 3, 4, 4.5, 5, 5.5, and 6). We conducted parallel analyses in which all SNAP participants were included and, separately, limited to SNAP-E participants. An additional prespecified secondary outcome was the odds that participants transitioned to a greater weight class from baseline to year 6, i.e., from having normal weight to having overweight or obesity or from having overweight to having obesity, as well as an exploratory analysis of transitions to obesity. These analyses were based on logistic regression and were limited to participants with BMI < 30.0 at baseline, with BMI calculated at year 6 using the most recent measured height.
Three subgroup comparisons were prespecified, based on gender, age (18.0-24.9; 25.0-35.0), and BMI at SNAP enrollment. Interaction terms were used to assess whether intervention effects varied among subgroups.
Results
Study participants
Table 1 shows that the 599 original SNAP participants and the 504 (84%) who reconsented for extended follow-up were similar at baseline. Of note, the proportion of participants in the three conditions did not differ in SNAP-E versus SNAP. Figure 1 indicates the number who completed each of the clinic visits. Retention at year 6 was comparable across the three groups (92%-94%), as were the proportions of weights collected remotely (24.1%, 24.2%, and 27.2% for Control, LC, and SC; P = 0.77).
| Baseline characteristic | SNAP enrollment, N = 599 | SNAP-E enrollment, N = 504 | Differential follow-up, P value |
|---|---|---|---|
| Age group, y | |||
| 18.0 to 24.9 | 169 (28.2%) | 135 (26.8%) | 0.07 |
| 25.0 to 35.0 | 430 (71.8%) | 369 (73.2%) | |
| BMI | |||
| 21 to 24.9 | 277 (46.2%) | 229 (45.4%) | 0.36 |
| 25.0 to 30.0 | 322 (53.8%) | 275 (54.6%) | |
| Sex | |||
| Females | 469 (78.3%) | 390 (77.4%) | 0.21 |
| Males | 130 (21.7%) | 114 (22.6%) | |
| Clinic site | |||
| Chapel Hill, NC | 307 (51.2%) | 264 (52.4%) | 0.20 |
| Providence, RI | 292 (48.8%) | 240 (47.6%) | |
| Race/ethnicity | |||
| African American | 66 (11.0%) | 51 (10.1%) | 0.27 |
| Non-Hispanic white | 438 (73.1%) | 372 (73.8%) | |
| Other | 95 (15.9%) | 81 (16.1%) | |
| Intervention assignment | |||
| Control | 202 (33.7%) | 172 (34.1%) | 0.73 |
| Large Changes | 197 (32.9%) | 167 (33.1%) | |
| Small Changes | 200 (33.4%) | 165 (32.7%) |
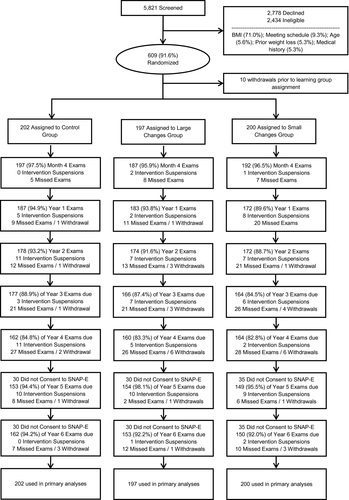
Weight changes
As seen in Table 2, differences among the intervention groups for the primary outcome of weight change from baseline to year 6 were not statistically significant (P = 0.11). Participants in the Control group gained a mean (SE) of 3.92 (0.49) kg over the 6 years, whereas those in the LC group gained 2.80 (0.50) kg and SC participants gained 4.16 (0.49) kg. None of the pairwise comparisons was significant. Analyses including only those who consented to the extension and using inverse probability weighting provided similar results as did analyses using percent weight change (5.57% [0.67%], 4.18% [0.69%], 5.65% [0.68%] for Control, LC, and SC, respectively; P = 0.23).
| Intervention assignment, mean (SE) | Overall P | Bonferroni-adjusted 95% CI | |||||
|---|---|---|---|---|---|---|---|
| Control | Large Changes | Small Changes | Large Changes minus/vs. Control | Small Changes minus/vs. Control | Large Changes minus/vs. Small Changes | ||
| Primary outcome: weight changes from baseline to year 6, kg | 3.92 (0.49) | 2.80 (0.50) | 4.16 (0.49) | 0.11 | −1.12 (0.69) [−2.78 to 0.54] | 0.24 (0.69) [−1.41 to 1.90] | −1.36 (0.69) [−3.03 to 0.305] |
| Primary outcome (sensitivity): weight changes from baseline to year 6, kg: SNAP-E participants only | 3.78 (0.50) | 2.65 (0.51) | 4.32 (0.50) | 0.06 | −1.14 (0.71) [−2.84 to 0.57] | 0.54 (0.71) [−1.16 to 2.24] | −1.67 (0.71) [−3.39 to 0.04] |
| Primary outcome (sensitivity): inverse probability weighting | 3.68 (0.48) | 2.69 (0.50) | 4.25 (0.49) | 0.08 | −0.99 (0.69) [−2.65 to 0.67] | 0.57 (0.69) [−1.09 to 2.23] | −1.56 (0.70) [−3.23 to 0.11] |
| Secondary outcome: difference in weight exposure (AUC) over follow-up a | 8.06 (2.12) | −0.53 (2.10) | 4.08 (2.10) | 0.003 | −8.59 (2.51) [−14.61 to −2.57]* | −3.98 (2.50) [−9.98 to 2.02] | −4.61 (2.51) [−10.64 to 1.41] |
| Secondary outcome: difference in weight exposure (AUC) over follow-up: SNAP-E participants only a | 7.05 (2.24) | −1.54 (2.25) | 4.90 (2.25) | 0.004 | −8.59 (2.67) [−14.99 to −2.19]* | −2.15 (2.66) [−8.55 to 4.24] | −6.44 (2.68) [−12.86 to −0.01]* |
| Secondary outcome: transition to overweight or obesity at year 6 b | 31.0% | 26.5% | 27.7% | 0.66 | OR 0.80 [0.44 to 1.48] | OR 0.84 [0.46 to 1.55] | OR 0.95 [0.51 to 1.79] |
| Exploratory outcome: transition to obesity at year 6 | 19.0% | 14.6% | 27.7% | 0.46 | OR 0.73 [0.35 to 1.52] | OR 1.04 [0.52 to 2.08] | 0.70 [0.33 to 1.47] |
- a Includes weight changes at 4 months and at years 1, 2, 3, 4, 4.5, 5, 5.5, and 6; metric for outcome is kg-years.
- b Based on current height for in-clinic measures and most recent measured height for smart scale weights. Limited to participants without obesity at baseline.
- * Bonferroni-adjusted 95% confidence interval (CI) excludes 0.
- AUC, area under the curve; OR, odds ratio.
However, groups differed significantly for the secondary outcome of weight-gain exposure (P = 0.003) (Figure 2A). In analyses including all SNAP participants, this was largely driven by less weight-gain exposure in LC versus Control: there was a mean difference of 8.59 kg-years of exposure; the Bonferroni-adjusted confidence interval for this difference excluded 0. In analyses limited to SNAP-E participants, differences among groups were also statistically significant (P = 0.004), and in this analysis, the LC group differed significantly from both SC and Control. The secondary outcome of transition to a greater weight class at year 6 did not differ among groups (P = 0.66), nor did the proportion who transitioned to obesity (P = 0.46).
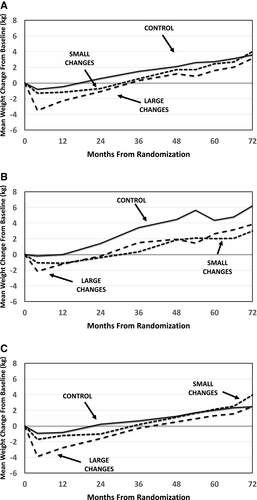
Subgroup analyses
We observed a significant interaction (P = 0.002) between participants’ age and treatment group for the primary outcome of weight change from baseline to year 6 (Table 3). Figure 2B-2C depicts the weight at each assessment for participants grouped by age. Individuals aged 18 to 24.9 (Figure 2B) assigned to the Control group (n = 41) gained, on average, 7.30 kg over the 6 years, which differed significantly from similarly aged individuals in LC (n = 48) or SC (n = 46), who gained 3.86 and 3.18 kg, respectively (P = 0.008). In older individuals (Figure 2C), the differences among treatment groups were also significant (P = 0.026), with greater weight gain in the SC group (4.52 kg) than in LC or Control (2.44 and 2.97 kg, respectively).
| Intervention assignment, mean (SE) | Overall P | |||
|---|---|---|---|---|
| Control | Large Changes | Small Changes | ||
| Primary outcome | ||||
| Age 18.0-24.9 | 7.30 (1.02) | 3.86 (1.01) | 3.18 (0.95) | 0.008 |
| Age 25.0-35.0 | 2.97 (0.55) | 2.44 (0.57) | 4.52 (0.57) | 0.026 |
| Difference [95% CI] | 4.32 [1.55 to 7.09]* | 1.42 [−1.35 to 4.21] | −1.34 [−3.99 to 1.32] | 0.002** |
| Females | 3.79 (0.54) | 3.14 (0.56) | 3.79 (0.56) | 0.631 |
| Males | 4.66 (1.15) | 1.63 (1.08) | 5.39 (1.00) | 0.029 |
| Difference [95% CI] | −0.87 [−3.92 to 2.19] | 1.51 [−1.40 to 4.43] | −1.60 [−4.35 to 1.15] | 0.156** |
| Normal weight | 3.46 (0.71) | 2.27 (0.71) | 2.54 (0.76) | 0.46 |
| Overweight | 4.34 (0.67) | 3.29 (0.69) | 5.29 (0.64) | 0.10 |
| Difference [95% CI] | −0.88 [−3.22 to 1.46] | −1.02 [−3.40 to 1.35] | −2.75 [−5.13 to −0.37]* |
0.33** |
| Secondary outcome a | ||||
| Age 18.0-24.9 | 15.47 (3.79) | 6.95 (3.45) | 4.71 (3.47) | 0.06 |
| Age 25.0-35.0 | 5.43 (2.30) | −3.68 (2.42) | 3.63 (2.37) | 0.005 |
| Difference [95% CI] | 10.04 [2.06 to 18.02]* | 10.64 [2.88 to 18.40]* | 1.08 [−6.51 to 8.68] | 0.15** |
| Females | 8.88 (2.17) | 1.77 (2.19) | 5.91 (2.18) | 0.04 |
| Males | 9.72 (4.08) | −3.76 (3.90) | 1.36 (3.99) | 0.04 |
| Difference [95% CI] | −0.84 [−9.52 to 7.83] | 5.53 [−2.85 to 13.91] | 4.55 [−3.99 to 13.09] | 0.54** |
| Normal weight | 4.98 (2.91) | 0.07 (2.85) | 3.84 (2.98) | 0.37 |
| Overweight | 11.11 (2.65) | −0.87 (2.70) | 4.40 (2.58) | 0.003 |
| Difference [95% CI] | −6.14 [−13.20 to 0.92] | 0.95 [−6.15 to 8.05] | −0.56 [−7.65 to 6.53] | 0.33** |
- a Change in kg-years over follow-up including weight changes at 4 months and at years 1, 2, 3, 4, 4.5, 5, 5.5, and 6.
- * 95% confidence interval (CI) excludes 0.
- ** Interaction P value.
- AUC, area under the curve.
The interaction between age and treatment arm was not statistically significant for the secondary outcome of weight-gain exposure (P = 0.15), but the pattern paralleled that seen for the primary outcome (Table 3). Younger individuals had the greatest weight-gain exposure when assigned to Control (15.5, 6.9, and 4.7 kg-years for Control, LC, and SC, respectively, P = 0.06). Among older individuals, those assigned to LC averaged below their baseline across the 6 years (−3.68 kg-years), whereas those in the SC or Control groups averaged above their baseline weights (3.63 and 5.43 kg-years, respectively), with the difference among the three significant at P = 0.005. The interaction between age and treatment condition was not significant for the proportion that transitioned to a higher BMI category.
Although formal tests for interactions of treatment group with gender and baseline weight did not reach statistical significance, weight-gain exposure for males and participants who had overweight at baseline was greatest in Control and lowest in LC participants.
Discussion
In contrast to our primary hypothesis, we found no significant differences in weight gain from baseline to 6 years among the three groups. However, the secondary outcome of weight-gain exposure differed significantly among the three groups: the LC approach, which taught young adults to lose 5 to 10 pounds to buffer the expected subsequent weight gains, was effective in reducing weight-gain exposure over the 6 years relative to the Control group. Moreover, for those who entered SNAP-E, the LC approach reduced weight-gain exposure relative to both the Control and the SC intervention.
Second, there was a significant age-by-treatment-group interaction for the primary outcome of 6-year weight change from baseline. This resulted primarily from differences in the younger participants (aged 18-24.9): those in the Control group gained 7.3 kg over the 6 years, or more than 1 kg per year, and both the LC and the SC self-regulation interventions were effective in reducing the average weight gained over 6 years by more than 50% relative to the Control group. In the older participants, weight changes from baseline to 6 years were greatest in SC.
Weight gain is an important health problem for young adults, with 98% of men and 92% of women shown to experience upward-sloping weight trajectories over an 18-year follow-up (20). Weight gain in this age group has stronger associations with critical outcomes such as cancer risk and mortality than weight gain at other ages (21, 22). Moreover, young adults who experience early or rapid weight gain were most likely to be on a steeper long-term weight-gain trajectory (20), which is associated with a greater likelihood of reporting hypertension, diabetes, and arthritis in middle age. Thus, the finding that those in the Control group aged 18 to 24.9 gained more than 1 kg per year is of concern and strengthens the argument for weight-gain prevention approaches in this age group. Moreover, evidence that the LC intervention delayed weight gain initially and reduced weight gain exposure over 6 years suggests that this approach may yield positive health benefits longer term. Weight-gain exposure, like pack years in smoking or obese-years (23), provides a measure of cumulative exposure to the risk factor. Such measures are used frequently in long-term observational studies such as the Framingham Heart Study and the Bogalusa Heart Study (24, 25) because they can capture the cumulative damage of these conditions on health outcomes.
The results presented here extend prior findings from SNAP. The primary outcome in SNAP was weight-gain exposure over an average of 3 years, which differed significantly among the three treatment groups as did the secondary outcomes of weight change from baseline to 2 years (the last time point reached by all cohorts) and the proportion that transitioned to obesity (16). At 6 years in the current analyses, weight gain from baseline no longer differed significantly among groups, suggesting that the intensity of the intervention may not have been sufficient to prevent weight gain in later years. In contrast, weight-gain exposure continued to differ significantly. Over the 6 years of follow-up, the LC participants had a negative exposure to obesity, indicating that their trajectory of weight changes over the 6 years averaged below their baseline level. The beneficial effects of the LC intervention on weight-gain exposure occurred as the result of the 3.5-kg weight loss at 1 year; subsequently, all three groups gained weight, and the difference among groups narrowed. Given the long-term effects of creating the initial buffer in the LC intervention, future efforts at weight-gain prevention should consider the possibility of periodically (e.g., on an annual basis) encouraging participants to lose 5 to 10 pounds to reinstate the buffer. Thus far in SNAP, we have found no negative effects of creating an initial weight-loss buffer (26, 27), although future studies should continue to evaluate positive and potential negative effects of this strategy.
As already noted, we found evidence of important differences among subgroups. For both the primary and secondary outcomes, younger individuals who were assigned to the Control group, and thus received very limited intervention, gained the greatest amount of weight and had the greatest exposure to weight gain over the 6 years. Previous studies focusing on emerging adults (i.e., those aged 18-25) have confirmed their large weight gains over time and noted their poor outcomes in weight-loss trials (28-31). Only 25% of the participants entering SNAP were in the younger age group, and there was a trend (P = 0.07) for poorer retention rates in this age group. In addition, among those who were older or heavier at baseline, and among males, the LC intervention led to negative levels of weight-gain exposure (i.e., the average weight for these subgroups over 6 years was below their baseline weight). In all other subgroups, weight-gain exposure was greater than zero. These results suggest that those who already had overweight at baseline or were older may particularly benefit from an approach focused on modest weight losses.
Strengths of this study include the large sample size, excellent retention, and extended follow-up. Given the mobility of this population, our intervention was delivered primarily via online methods in out-years. Smart scales allowed us to capture frequent weight data and maintain high levels of retention. We and others have demonstrated the validity of smart scale weights in intervention trials (32, 33). This study is limited, however, by concerns about the generalizability of findings. All participants had joined SNAP, a trial that specifically focused on weight-gain prevention; moreover, the sample had limited diversity (73% were non-Hispanic white and 78% were female) and recruited in only two geographic areas. Future studies should include more men, more racial/ethnic minorities, and more geographic diversity. In addition, the provision of scales and activity trackers (i.e., self-monitoring tools) could have affected the weight changes seen in SNAP-E.
In conclusion, there were no significant effects of the interventions on weight gain from baseline to 6 years. However, relative to Control, the LC approach, in which participants are encouraged to lose weight initially to buffer expected weight gains, was effective in reducing weight gain exposure over 6 years. Future studies should consider this approach particularly for those who already have overweight and examine the efficacy of intermittently encouraging participants to lose small amounts of weight to create this buffer. In addition, our findings strengthen the imperative to develop weight-gain prevention interventions for young adults, aged 18 to 25. Without such interventions, this group is at high risk of weight gain and future weight-related health problems. The two interventions tested here were both effective in reducing the amount of weight gained over 6 years in this subgroup and should receive further study.
Acknowledgments
Data availability: All deidentified data collected during the trial will be available from the NHLBI Data Repository (https://biolincc.nhlbi.nih.gov/home/) by January 2020. This will include the data, data dictionary, and informed consent documents. Data will be made available to others according to NHLBI policies, which will determine the types of analyses and with whom the data will be shared.
The SNAP Research Group members are listed below, alphabetically by site unless otherwise specified.
Miriam Hospital/Brown Medical School: Rena R. Wing, PhD (principal investigator); Elissa Jelalian, PhD (coinvestigator); Erica Ferguson Robichaud, MSW, RD (program coordinator); Jose DaCruz; Kaitlyn Dahlborg, BS; Caitlin Egan, MS; Denise Fernandes Pierre, BS; Chelsea Pimentel, BA; Wilza Rodrigues, BS; Samantha Williams, MSc. University of North Carolina at Chapel Hill: Deborah Tate, PhD (principal investigator); Kristen Polzien, PhD (program coordinator); Candice Alick, MS; Loneke Blackman, MS, RD; Molly Diamond, MPH; Karen E. Hatley, MPH; Brett Meager; Julianne Power, MS; Brooke Tompkins Nezami, MA; Paige Trexler; Carmina Valle, PhD, MPH. Wake Forest School of Medicine: Mark A. Espeland, PhD (principal investigator); Judy L. Bahnson, BA, CCRP (coinvestigator); Letitia H. Perdue, MS (program coordinator); Cheryl Bentley; Patty Davis, BS; Katelyn Garcia, MS; Rebecca H. Neiberg, MS; Julia Robertson, BS; Greg Russell, MS. Consultant coinvestigators: Cora E. Lewis, MD, MSPH, University of Alabama at Birmingham; Amy A. Gorin, PhD, University of Connecticut; Jessica G. LaRose, PhD, Virginia Commonwealth University School of Medicine.





