Insulin Access to Skeletal Muscle is Preserved in Obesity Induced by Polyunsaturated Diet
Funding: This work was supported by NIH grants DK29867 and DK27619 and by the Society in Science Branco Weiss Fellowship, administered by the Swiss Federal Institute of Technology Zürich (to JLB).
Disclosure: CMK and RNB have a grant from AstraZeneca outside of the submitted work. The authors declared no conflict of interest.
Author contributions: JLB and CMK conceived and conducted experiments and analyzed data. IAB, RLP, and MSI conducted experiments. All authors were involved in writing the paper and had final approval of the manuscript. CMK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abstract
Objective
Diets high in saturated fat induce obesity and insulin resistance and impair insulin access to skeletal muscle, leading to reduced insulin levels at the muscle cell surface available to bind insulin receptors and induce glucose uptake. In contrast, diets supplemented with polyunsaturated fat improve insulin sensitivity (SI) and reduce the risk for type 2 diabetes. It was hypothesized that a diet high in polyunsaturated fat would preserve SI and insulin access to muscle, as compared with a diet high in saturated fat.
Methods
After 12 weeks of control, saturated (LARD), or polyunsaturated (salmon oil [SO]) high-fat diet feeding, muscle SI and insulin access to skeletal muscle were measured by using lymph, a surrogate of skeletal muscle interstitial fluid.
Results
Both high-fat diets induced similar weight gain, yet only LARD impaired SI. Hyperinsulinemia in the LARD group did not induce an increase in basal interstitial insulin, suggesting reduced insulin access to muscle after LARD, but not after SO.
Conclusions
A diet high in polyunsaturated fat does not impair insulin access to muscle interstitium or induce insulin resistance as observed with a saturated fat diet, despite similar weight gain. Future studies should determine whether dietary SO supplementation improves impairments in insulin access to skeletal muscle.
Introduction
Endothelial dysfunction is suggested to be an early complication of obesity (1-3), and it is associated with metabolic syndrome (4) and prediabetes (5) and is present in people with a family history of diabetes (6). The endothelium is involved in insulin action, as insulin must cross the capillary endothelium to access the interstitial space where it can bind receptors to initiate insulin signaling (7, 8). Therefore, factors that affect endothelial function and thus insulin delivery to the surface of the cell may directly impact insulin sensitivity (SI). In the case of women with obesity, higher circulating levels of insulin have been reported as compared with lean women, yet no differences exist in interstitial insulin levels in adipose tissue and skeletal muscle, suggesting impaired insulin access to the interstitial space (9). In addition, men with obesity have slower transcapillary transport of insulin than lean men (10), further suggesting a reduced ability of insulin to cross the interstitial space to act on peripheral tissues during obesity. Our results in the canine model support these human findings and demonstrate that impaired insulin access under basal insulin levels develops with long-term fat feeding (11) but that this effect is overcome at higher insulin levels.
Dietary fat can induce endothelial dysfunction (12). In contrast, polyunsaturated fats, including omega-3s, have been shown to have beneficial effects on endothelial function in a variety of different models (13, 14). To test the hypothesis that insulin access to skeletal muscle is preserved in a model of obesity induced by a diet high in polyunsaturated fat, we performed hyperinsulinemic euglycemic clamps to assess SI and measured interstitial insulin in dogs fed a normal diet, a diet high in saturated fat, or a diet high in polyunsaturated fat. We observed marked differences among these diets in their effects on insulin action as well as insulin movement across the capillary endothelial barrier.
Methods
Animals
Male mongrel dogs (Antech, Barnhart, Missouri; > 1 year old) were housed in the Cedars-Sinai Medical Center vivarium under controlled kennel conditions (12:12 light/dark cycle). Animals were accepted into the study following physical examination and a comprehensive panel of blood tests and were included in the study only if judged to be in good health as determined by visual observation, body temperature, and hematocrit. Protocols were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee.
Diet
The control group (CON, n = 8) was fed a standard ad libitum diet, consisting of dry chow (40% carbohydrate, 26% protein, 14% fat, and 3% fiber [mixture of Laboratory HDL Canine Diet and Prolab Canine 2000; LabDiet, Richmond, Indiana]).
For the high-fat diet groups, food was presented from 9:00 am to 10:00 am each day. Animals were fed a daily diet of one can of Hill's Prescription Diet (415 g; 10% carbohydrate, 9% protein, 8% fat, 0.3% fiber, and 73% moisture [Hill's Pet Nutrition, Topeka Kansas]) and 825 g of dry chow (40% carbohydrate, 26% protein, 14% fat, and 3% fiber [mixture of Laboratory HDL Canine Diet and Prolab Canine 2000; LabDiet]), supplemented with either 6 g/kg of rendered pork fat (LARD, n = 8) or salmon oil (SO, n = 8). These alternative diets contained identical macronutrient content consisting of 21,025 kJ/day comprised of 27% carbohydrate, 19% protein, and 53% fat. The added lard consisted of 38.5% saturated fatty acid, 10.8% polyunsaturated fatty acid (PUFA) (10% ω-6; 0.8% ω-3), 44.6% monounsaturated fatty acid, and 0.1% cholesterol. The salmon oil addition consisted of 19.9% saturated fatty acid and 40.4% PUFA (1.5% ω-6; 35.5% ω-3).
The acute effects of 6 weeks of LARD and SO diets on SI and cardiac function have been reported previously (15). The present study reports data on extended diet durations of 3.0 ± 0.3 months of LARD feeding and 3.9 ± 0.1 months of SO feeding.
Hyperinsulinemic euglycemic clamp
Clamps were done under anesthesia; animals were fasted overnight (for ad lib fed animals, food was removed at noon), then at 8 am were sedated with acepromazine maleate (Prom-Ace, Aueco, Fort Dodge, Iowa; 0.22 mg/kg) and atropine sulfate (Western Medical, Arcadia, California; 0.11 mL/kg). Anesthesia was induced with sodium pentobarbital (Western Medical; 0.5 mL/kg) or propofol (Western Medical; 6mg/kg) and maintained with inhaled isofluorane or sevoflurane (Western Medical). Dogs were placed on heating pads to maintain body temperature. Intracatheters were inserted into the left cephalic vein for variable glucose infusion (GINF) and the right cephalic vein for insulin, somatostatin, and tracer infusion. Indwelling catheters were placed into the left femoral artery and vein for sampling. The hindlimb lymphatic vessel was cannulated by placing a polyethylene catheter (PE10) into the afferent lymphatic vessel of the deep inguinal lymph node. Lymph was collected by gently massaging the leg directly above the popliteal area, which has been shown to instantaneously increase lymph drainage without affecting lymph or plasma oncotic pressures (16). Blood pressure (cuff on opposite hind leg), heart rate, oxygen saturation, and carbon dioxide saturation were monitored continuously. The surgery was completed at approximately 10 am, at which time the experiment began.
Immediately after the completion of the surgical procedures and sampling of fasting lymph and plasma, somatostatin was infused to inhibit endogenous insulin secretion (1 μg/min/kg; Bachem, Bubendorf, Switzerland) (at time = −180 min), and basal insulin was replaced systemically (0.2 mU/min/kg; Novo Nordisk, Bagsvaerd, Denmark) and continuously for the remainder of the study. Exogenous 20% glucose was infused into the left cephalic vein at variable rates to clamp arterial glucose to basal levels throughout the experimental period. Plasma samples were taken from the femoral artery and femoral vein every 10 to 15 minutes. Lymph vessels were sampled by gently massaging the hindlimb distal to the site of catheterization. After 180 minutes of insulin replacement (time = 0 min), the insulin concentration was increased to 1.2mU/min/kg, which was continued for the remainder of the study. Insulin levels from the artery, vein, and lymph were averaged over the last 30 minutes of the clamp to examine differences in insulin access at steady state. At the conclusion of these experiments, animals were euthanized with an overdose of sodium pentobarbital (Eutha-6, Western Medical; 65 mg/kg).
Assays
Arterial, venous, and lymph samples were collected in microtubes precoated with lithium-heparin (Becton Dickinson, Franklin Lakes, New Jersey). Arterial and venous tubes also contained 50 µL of EDTA (Sigma Chemicals, St Louis, Missouri). Blood samples were centrifuged immediately and the supernatant was transferred. Plasma and lymph samples were immediately assayed for glucose with a YSI 2700 autoanalyzer (Yellow Springs Instrument Co., Yellow Springs, Ohio) before freezing at −20°C until further analysis. Insulin was measured in plasma and lymph with an ELISA developed for dog plasma (Alpco, Salem, New Hampshire).
Tissue SI
Tissue SI (SItissue) reflects the insulin-mediated glucose uptake in response to the local, rather than systemic, insulin concentration and thus indicates the ability of the muscle to respond to interstitial insulin. SItissue was calculated from the arteriovenous glucose difference, the change in interstitial insulin, and the glucose concentration at steady state: change in arteriovenous glucose / (change in interstitial insulin × glucose concentration).
Statistical analyses
Experimental data are shown as means ± SEM. Statistical analyses were performed with paired or unpaired Student's t tests, or one-way or two-way ANOVAs with Tukey's pairwise comparisons, as appropriate (GraphPad Prism version 5.04 for Windows, GraphPad Software, San Diego, California). An interquartile test for outliers was used. All differences were considered statistically significant when P < 0.05.
Results
Baseline characteristics of diets
Dietary fat supplementation for 3 months induced an increase in weight of 6.4 ± 3.4% and 8.3 ± 1.5% in LARD- and SO-fed dogs, respectively, which was not a statistically significant difference between fat-fed groups (P = 0.7). Glucose levels assessed in arterial plasma under anesthesia prior to the clamp did not differ between groups (CON: 99.7 ± 3.4, LARD: 97.3 ± 2.0, SO 103.6 ± 4.1 mg/dL) and, similarly, no changes in lymph glucose levels were noted (CON: 109.6 ± 4.9, LARD: 103.4 ± 2.0, SO: 110.9 ± 6.0 mg/dL).
Previously published findings after six weeks in each diet condition reported that plasma insulin was significantly increased from baseline from 50.4 ± 7.7 to 60.4 ± 10.7 pmol/L in LARD animals, whereas the SO intervention caused no significant changes in insulin (34.6 ± 6.8 to 31.0 ± 4.3 pmol/L) (15). Similarly, in these longer studies, we found that LARD feeding, but not SO feeding, induced plasma hyperinsulinemia at basal insulin levels in both artery and vein (Figure 1A-1B). However, elevated plasma insulin levels in the LARD group did not translate to an elevation in interstitial insulin levels, in contrast to chow-fed lean animals (Figure 1C), indicating reduced insulin access to the interstitium.
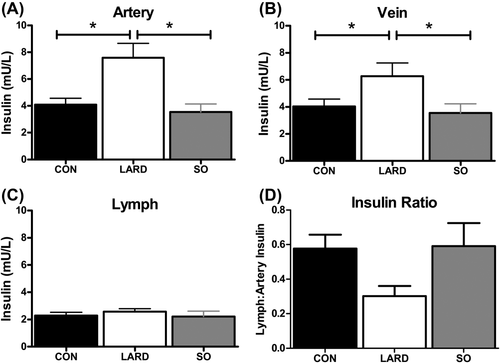
Insulin levels in the (A) artery, (B) vein, and (C) lymph and (D) ratio of lymph to artery insulin under fasting conditions in CON, LARD, and SO. Statistics were performed by using one-way ANOVA. *Represents significance at P < 0.05.
SI measures
To ensure that animals were not differentially stressed at the start of the experiment as compared with the end, we confirmed no significant difference between heart rate, blood pressure, or blood flow at baseline compared with steady state (Table 1), and oxygen saturation was maintained at 98% to 99% in all groups. Plasma glucose concentrations during the clamp were maintained at euglycemia and did not differ between groups (Figure 2A). However, to maintain euglycemia, a lower GINF rate was required for LARD animals compared with CON animals (Figure 2B). In contrast, GINF in SO animals did not differ from that for CON animals. The peripheral glucose rate of disappearance (Rd) (Figure 2C) was also reduced in the LARD group, indicating impaired SI.
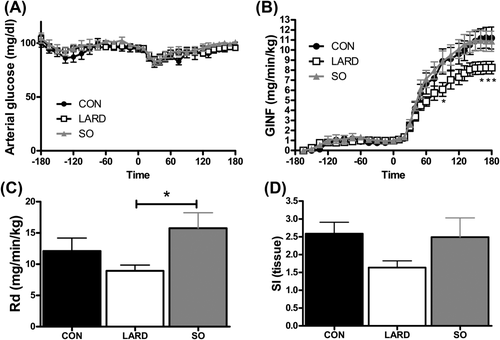
(A) Glucose levels in the artery were maintained by (B) infusing exogenous glucose at variable rates (GINF). Insulin infusion began at time 0 and induced changes in (C) Rd and (D) SItissue in CON, LARD, and SO. Statistics were performed by using one-way or two-way repeated measures ANOVA, as appropriate, *P < 0.05, compared to SO.
| CON | LARD | SO | |
|---|---|---|---|
| Baseline heart rate | 95.8 ± 7.4 | 108.9 ± 5.5 | 95.6 ± 4.3 |
| Baseline blood pressure | 100/50 (70 ± 4) | 108/60 (72 ± 5) | 106/54 (75 ± 2) |
| Baseline femoral blood flow | 155.6 ± 29.8 | 132.3 ± 26.0 | 147.3 ± 18.0 |
| Clamp heart rate | 107.5 ± 6.5 | 103.8 ± 5.0 | 107.5 ± 3.8 |
| Clamp blood pressure | 95/47 (67 ± 2) | 102/48 (64 ± 4) | 102/51 (72 ± 1) |
| Clamp femoral blood flow | 202.1 ± 15.8 | 179.6 ± 23.2 | 174.0 ± 21.2 |
| SIGINF (dL/min/kg per µU/mL × 104) | 18.2 ± 2.8 | 10.5 ± 1.5 * | 14.6 ± 1.8 |
| SIRd (dL/min/kg per µU/m × 104) | 17.4 ± 3.6 | 8.1 ± 5.4 | 17.4 ± 4.0 |
| SIEGP (dL/min/kg per µU/mL × 104) | −0.4 ± 3.0 | −0.4 ± 1.1 | 1.1 ± 1.4 |
| MCR (mL/min/kg) | 16.9 ± 1.6 | 13.8 ± 0.8 | 15.0 ± 1.0 |
| FFA (arterial) (mmol/L) | 0.14 ± 0.04 | 0.14 ± 0.02 | 0.14 ± 0.04 |
| FFA (lymph) (mmol/L) | 0.62 ± 0.28 | 0.30 ± 0.03 | 0.21 ± 0.06 |
- Means ± SEM. Statistics were performed by using one-way ANOVA. Significance is assumed at P < 0.05.
- EGP, endogenous glucose production; MCR, metabolic clearance rate.
SItissue, calculated from the arteriovenous glucose removal across the leg and the interstitial insulin concentration, showed a trend to be impaired in LARD animals only (P = 0.09) (Figure 2D).
In contrast to fasting plasma hyperinsulinemia in conjunction with unchanged interstitial insulin levels, exogenous insulin infusion at both stages of the clamp elevated interstitial insulin levels (Figures 3, 4. Plasma insulin levels during the clamp were significantly higher in LARD animals at low insulin levels (Figure 3) and showed a similar trend at high insulin levels (Figure 4). However, the same insulin doses (0.2 and 1.2 mU/min/kg) were administered to all animals, suggesting a trend for a decrease in the metabolic clearance rate of insulin in the LARD group (Table 1).
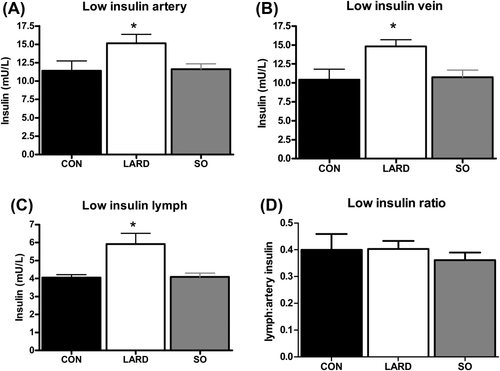
Insulin levels at steady state in the (A) artery, (B) vein, and (C) lymph after infusion of low insulin (0.2 mU/min/kg insulin) in CON, LARD, and SO. (D) The insulin ratio of lymph to artery is also shown. Significance is calculated by using one-way ANOVA and is assumed when P < 0.05. *P < 0.05 versus CON.
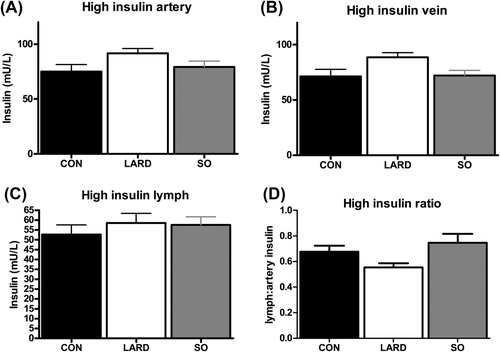
Insulin levels at steady state in the (A) artery, (B) vein, and (C) lymph after infusion of high insulin (1.2 mU/min/kg insulin) in CON, LARD, and SO. (D) The insulin ratio of lymph to artery is also shown.
LARD animals displayed a significantly reduced SI compared with CON animals, as measured by GINF (SIGINF) during the clamp, whereas there was no difference in SIGINF in SO compared with CON animals (Table 1). A similar, but nonsignificant trend was seen for reduced SI as measured by the Rd (SIRd). There was no effect of diet on endogenous glucose production by the liver, which is consistent with previous findings in anesthetized animals. We also observed no significant effects of any diet on plasma or lymph free fatty acid (FFA) levels.
Discussion
Diets rich in PUFA have been proposed to have beneficial effects on the endothelium. In this study, we found that a PUFA diet, although inducing a significant weight gain, did not induce insulin resistance or impair insulin movement into the interstitial space under basal insulin levels, as compared with the LARD diet. Our previously published results also demonstrate that a LARD diet induces insulin resistance and impairs insulin access to the skeletal muscle interstitium under basal, noninsulin stimulated conditions (11), but not during higher levels of exogenously infused insulin. Thus, the present results support the concept that PUFA diets do not have the detrimental effects on insulin action seen with saturated fatty acid diets, even in the context of comparable obesity.
We have extensive experience using a well-established canine model to demonstrate that diets supplemented with lard induce insulin resistance (15, 17) within a relatively short time frame (18). We have also shown that a diet supplemented with SO induces weight gain similar to that caused by a lard diet, but is not associated with impairments in SI (15). In mouse studies, a diet high in saturated fat and subsequently enriched with omega-3 PUFAs reversed glucose intolerance and vascular dysfunction and improved insulin signaling (19, 20).
Omega-3 supplementation may improve vascular function in part through changes in fatty acid composition (14) and activation of adenosine monophosphate-activated protein kinase (21). Conversely, hyperlipidemia is normally thought to impair vascular function (1, 22). As we and others have shown, insulin must cross the endothelial barrier to bind to insulin receptors on the muscle cells to initiate insulin-mediated glucose uptake. However, prior to crossing the endothelium, capillary recruitment may also occur to more fully perfuse the muscle with blood, and more efficiently deliver insulin and glucose to the muscle cell. Blocking capillary recruitment can impair insulin and glucose delivery as well. FFA levels affect capillary recruitment, which may contribute to some of the insulin resistance induced by elevated circulating FFA (19). However, we did not see changes in FFA levels in our study, which indicates that elevated plasma FFA does not directly impair insulin access in this model. The lard-based diet has been extensively studied in our laboratory, and we have shown that although there are no changes in fasting or fed FFA levels, we do detect a nocturnal increase in plasma FFA content (23). Although it is possible that elevated nocturnal FFA may induce endothelial dysfunction and therefore impair capillary recruitment, we did not measure nocturnal FFA in this study. Further studies are needed to determine whether saturated fat indeed restricts capillary function as it relates to insulin access in this model.
One mechanism by which omega-3s may preserve glucose tolerance has been suggested to be by preventing accumulation of lipid intermediates that interfere with mitochondrial function (24). In skeletal muscle, mitochondria can adapt to a saturated fat diet by reducing proton leak, whereas proton leak is increased with diets high in omega-3 PUFAs (25), resulting in differential effects of these dietary fats on energy conservation and expenditure. In addition, there are distinct effects of dietary fat on mitochondrial enzyme activity, fission proteins, and apoptotic signaling (25); however, we did not investigate these mechanisms of insulin resistance in the current study.
Hyperinsulinemic compensation has been hypothesized to be a normal response to insulin resistance (26). Hyperinsulinemia can be driven by changes in insulin secretion and/or insulin clearance (18). Indeed, insulin resistance develops in LARD animals and, therefore, hyperinsulinemic compensation achieved by increased insulin secretion (15) and/or decreased metabolic clearance rate of insulin is necessary to produce adequate glucose disposal. Reduced insulin clearance in LARD animals is supported by our finding that the same amount of insulin infused into each group of animals led to higher plasma insulin concentrations in LARD animals only. SO feeding did not induce insulin resistance at the whole-body (SIGINF), peripheral (SIRd), or tissue level (SItissue); as such, hyperinsulinemic compensation was not required.
Lymph is generally accepted to be an appropriate measure of interstitial fluid, and results are similar to other methods of interstitial sampling (27). The lymph vessel is in fact very similar in structure to blood vessels; thus, things that induce endothelial dysfunction may also have effects on lymph function. In fact, recent studies have implicated lymphatic dysfunction in metabolic syndrome (28-30) in addition to endothelial dysfunction. In future studies, we plan to further confirm the use of lymph sampling with microdialysis.
Another limitation of our study is the use of the hyperinsulinemic euglycemic clamp. Although this is the gold standard for assessing SI in vivo, there are limitations to its physiological relevance, as an individual is normally not exposed to a sustained, supraphysiological level of insulin. Thus, assessing insulin access under these conditions may not be representative of the shorter-term elevations that occur when an animal is exposed to a glucose challenge, and further studies investigating insulin access under a more dynamic glucose and insulin environment, such as an intravenous or oral glucose tolerance test, may clarify whether impaired insulin access occurs in a physiological setting. However, under these high arterial insulin levels, the vein insulin concentrations are also high, and insulin levels are slow to respond and much lower than plasma levels.
Conclusion
We have demonstrated that obesity induced by a diet high in PUFAs is not associated with the development of insulin resistance or impairments in insulin access to skeletal muscle. We have confirmed that the insulin level in the interstitial space in animals is approximately half that of plasma, suggesting a barrier to insulin transport across the endothelium that appears to be exacerbated by a saturated fat, but not by a polyunsaturated fat, diet. Further research is needed to determine whether dietary supplementation with polyunsaturated fat is protective against insulin resistance, plasma hyperinsulinemia, reduced insulin clearance, and impaired insulin access to muscle associated with saturated fat diets.
Acknowledgments
The authors would like to thank Rita Thomas, CSMC, for performing the insulin assay and the Cedars-Sinai Medical Center Comparative Medicine staff for their assistance with and care for our animals.





