PROP Nontaster Women Lose More Weight Following a Low-Carbohydrate Versus a Low-Fat Diet in a Randomized Controlled Trial
Funding agencies: Funded by a grant-in-aid from the American Heart Association (12GNT12060259; BJT).
Disclosure: The authors declared no conflict of interest.
Author contributions: BJT and HAR conceptualized and designed the research; BJT oversaw the project; BB conducted the research and analyzed the data; BB and BJT wrote the manuscript; HAR critically reviewed the manuscript; all authors approved the final version of the manuscript.
Clinical trial registration: ClinicalTrials.gov identifier NCT01856660.
Abstract
Objective
Taste blindness to 6-n-propylthiouracil (PROP) associates with increased fat preference and intake. No studies have matched a diet to a woman's PROP phenotype to improve weight loss. This study investigated (1) whether PROP nontaster (NT) women would lose more weight following a low-carbohydrate (LC) diet than a low-fat (LF) diet, and (2) whether PROP supertaster (ST) women would lose more weight following a LF diet than a LC diet.
Methods
One hundred seven women (BMI = 34.8 ± 0.5 kg/m2), classified as PROP NTs (n = 47) and STs (n = 60), were randomized to a LC or LF diet within a 6-month lifestyle intervention. Assessments included 4-day dietary recalls and biobehavioral and psychosocial questionnaires.
Results
At 6 months, NTs lost more weight following the LC than the LF diet (−8.5 ± 0.5 kg vs. −6.6 ± 0.5 kg, P = 0.008); there was no difference between STs following either diet (−8.8 ± 0.4 vs. −8.9 ± 0.5, P = 0.35). Dietary self-reports were unrelated to weight loss, and prescription of a LC diet associated with greater self-efficacy.
Conclusions
NT women lost more weight following the LC diet compared to the LF diet. Screening for PROP phenotype may help personalize diet therapy for NT women to optimize their short-term weight loss.
Introduction
Multiple diets are effective for achieving clinically meaningful weight loss in adults from 6 months to 1 year, though individual responses can range from gaining >5 kg to losing > 40 kg (1, 2). Evidence suggests that those who experience early weight loss within this period are more likely to sustain weight loss long term (3). Therefore, identifying which diet works best for a particular person may help to improve both short- and long-term outcomes. Meta-analyses comparing the effectiveness of different diets have revealed that the level of dietary adherence, rather than the type of diet, is the most potent predictor of weight loss (4-6).
Consumers indicate that taste is the primary determinant of their food choices (7), and weight loss investigations have reported that taste strongly impacts diet choice (8) and overall diet satisfaction (9). Accounting for individual taste preferences when prescribing a diet may help promote compliance and enhance weight loss, yet no study has matched an individual to a diet aligned with his/her genetically mediated taste preferences. Variation in TAS238, the gene controlling ability to taste the bitterness of 6-n-propylthiouracil (PROP), has been used as a genetic marker for taste preferences and dietary habits (10). The bitterness of PROP is due to the chemical moiety NCS, which is also a feature of naturally occurring compounds in brassica vegetables. Additionally, PROP nontasters (NTs) perceive less intensity from many oral sensations (e.g., sweetness, chili pepper irritation, fat texture) and prefer foods with higher concentrations of these qualities (10). In contrast, PROP supertasters (STs) find PROP intensely bitter and perceive greater intensity from the aforementioned sensations, preferring more mild-tasting foods. Importantly, lean NT women displayed higher preferences for dietary fat (11) and consumed more energy (11, 12) when exposed to palatable, energy-dense foods in a free-choice setting. Our work has suggested a strong association between PROP NT status in women and increased adiposity (13-15).
Low-fat (LF) (i.e., energy-restricted) diets are the most common diet prescription in lifestyle interventions (16), though this does not align with the high-fat food preferences of NT women.
This study randomized PROP NT and ST women with excess weight and obesity to a LF or low-carbohydrate (LC) diet delivered within a 6-month lifestyle intervention. We hypothesized that NT women following the LC diet would show greater adherence to their diet prescription and lose more weight than NT women following the LF diet, because the former liberalizes fat intake. Conversely, we hypothesized that ST women following a standard LF diet would show greater adherence to their diet prescription and lose more weight than ST women following the LC diet, because the LF diet is consistent with their tolerance for lower-fat foods. A secondary objective was to assess group differences in psychosocial and biobehavioral variables predictive of weight loss.
Methods
Participants
Participants were recruited by advertisements at Rutgers University and the surrounding community from January 2013 to November 2014; the study concluded in June 2015. Eligibility criteria were as follows: (1) being female, (2) aged 18 to 60 years, (3) having BMI between 27 and 40 kg/m2, (4) wanting to lose ≥14 kg, and (5) able to walk two blocks without stopping (17). Those who met these requirements participated in a phone screening to assess general health. Ineligibility criteria were as follows: (1) being vegan or vegetarian, (2) being pregnant or breastfeeding, (3) enrolled in another weight loss program, (4) using weight loss products or medications affecting taste/smell, (5) experiencing >5% weight loss within the past 6 months, (6) suffering from major medical conditions (e.g., heart or kidney disease, schizophrenia, sinusitis), or (7) scoring > 20 on the EAT>26 Questionnaire, which estimates disordered eating behaviors and preoccupation with thinness (18). Qualified women attended an orientation session to receive a lesson on how to keep a 1-day diet record, then submitted one the following week. Those who submitted a plausible 1-day diet record (i.e., reported the amount and timing of each food/beverage consumed, detailed the ingredient breakdown in complex dishes, reported energy intake ≥1,000 kcal/d) were invited to return for anthropometric measurements and screening for PROP taster status. Men were excluded because the PROP phenotype is more strongly related to body weight in women (13-15), and the majority of adults seeking weight loss help through behavioral intervention are female.
Study design
Women were assigned to a LF or LC diet by stratified randomization, using PROP taster status and baseline weight as the cofactors. Personnel not involved in the trial generated the random allocation sequence; odd and even numbers were used to assign the subjects to the diet groups. An allocation ratio of 1:1 created four groups of similar numbers: NT-LF group, ST-LF group, NT-LC group, and ST-LC group.
PROP phenotyping
A filter paper method developed previously (19) and validated in studies (11-15) was used to determine each woman's PROP phenotype. The women received two filter paper disks, one impregnated with 1.0 M NaCl (VWR Scientific, Bridgeport, New Jersey) as a control and one with 50 mM PROP (6-n-propyl-2-thiouracil, #P3755, Sigma-Aldrich, St. Louis, Missouri). Women cleansed their mouths with water, placed the NaCl disk on the tip of their tongue for ∼20 seconds, and rated intensity on a 100-mm labeled magnitude scale (20), anchored from “barely detectable” to “strongest imaginable.” The process was repeated with the PROP disk. Women who marked <15 mm on the scale were classified as NTs, and women who marked >67 mm on the scale were classified as STs. Those who gave ratings between 16 and 66 mm were classified as medium tasters (MTs) and were excluded from participating in order to contrast extremes of the phenotype. NaCl ratings were used to clarify the taster status of women who gave borderline PROP ratings (at 15 mm or 67 mm) according to our method (19). Both the participants and the research staff were blind to the participants' PROP phenotypes until the conclusion of the study.
Sample size
Change in weight at 6 months was the primary outcome of interest. A power calculation revealed that for an effect size of 0.72 at 80% power and P ≤ 0.05, a minimum of 25 participants per subgroup was needed. With the attrition rate estimated at ∼20%, the planned recruitment was 30 participants per subgroup. A power calculation was not conducted for the secondary outcomes. The Institutional Review Board at Rutgers University approved this study, and participants provided written informed consent prior to their participation. The trial was registered at ClinicalTrials.gov (NCT01856660).
Intervention
A 6-month lifestyle intervention was provided. This was conducted in four waves over the course of the 3-year period. Each intervention was separated by ∼2 months.
The intervention was conducted at Rutgers University. It was provided in 60-minute group sessions, delivered weekly during months 1 to 4 and biweekly during months 5 and 6, with the two diet groups meeting on separate nights. At each meeting, the intervention leader (BB) weighed the participants and presented a lesson on diet, physical activity, or cognitive behavioral strategies (e.g., self-monitoring, stimulus control). Participants kept a daily record of their diet, physical activity, and weight in a Keeping Track Booklet that was submitted to BB for weekly review and individualized feedback.
The LF diet followed a modified Dietary Approaches to Stop Hypertension diet plan (21) that allotted 40 to 50 g/d of fat and 1,200 to 1,500 kcal/d (depending on baseline weight), as commonly prescribed in large-scale lifestyle interventions (22, 23). The LC diet was a modified Atkins diet (24) that permitted up to 50 g/d of total carbohydrates. The LC diet did not have an energy limit because a spontaneous reduction in energy intake typically occurs with carbohydrate restriction (25, 26).
Participants were asked to engage in 50 min/wk of unsupervised moderate-intensity physical activity for the first three sessions. This goal increased by 25 min/wk after every three sessions.
Measures
Assessments occurred at baseline and 3 and 6 months by trained research assistants, unless otherwise indicated.
Anthropometric measurements
Women were weighed each week wearing lightweight clothing and no shoes on a scale (BWB-800, Tanita, Arlington Heights, Illinois) to the nearest 0.25 kg.
Compliance and retention
Compliance was measured by frequency of attendance and submission of complete self-monitoring records at weekly meetings. Retention rates were calculated for each cohort at 6 months.
Dietary intake and physical activity
Participants recorded food intake for three weekdays and one weekend day at each time point. Data were entered into the Nutrition Data System for Research (NDSR version 2015; Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota) software program. Major food groups (fruits, vegetables, grains, dairy and nondairy alternatives, proteins, fats, sweets, beverages, and miscellaneous foods) and subgroups of interest (e.g., dark green vegetables, nonnutritive-sweetened foods) were examined. Self-reported means for energy intake, macronutrients (g), percent energy from macronutrients, and food group servings were averaged over 4 days at each assessment. Dietary adherence was assessed by how closely a participant's intake matched her diet prescription (as described previously).
Participants wore an ActiGraph activity monitor (wGT3X-BT model, ActiGraph, LLC, Fort Walton Beach, Florida) for 24 hours over five consecutive days. Daily minutes of moderate-to-vigorous physical activity (MVPA) were calculated for each participant using ActiLife 6.10.2 software. This output was averaged at each assessment.
Psychosocial and biobehavioral questionnaires
We modified the questionnaire developed by Zehle et al. (27) to create diet-specific questionnaires assessing psychosocial factors associated with dietary adherence. This instrument uses a three-point scoring system to evaluate measures of self-efficacy (Not at all = 1, A little = 2, Confident = 3), social support (Never = 1, Sometimes = 2, Often = 3), and perceived barriers to weight loss (Disagree = 1, Unsure = 2, Agree = 3). Self-efficacy refers to a person's belief in his or her competency to change behavior, social support refers to attitudes of friends and family members toward the participant's diet, and perceived barriers to weight loss refers to hindrances to dietary compliance. Survey items are shown in Supporting Information Table S1. Additionally, the Three-Factor Eating Questionnaire (28) was used to measure dietary restraint, disinhibition, and hunger across the study.
Statistical analyses
We used an intent-to-treat model employing a multiple imputation strategy to fill in missing values (29) for participants who dropped out. Briefly, SAS software (SAS Institute Inc., Cary, North Carolina) generated five random variables from a normal distribution with a mean equal to the baseline variable and a variance equal to the estimated variance, based on data from other participants at 3 and 6 months.
Repeated-measures analysis of covariance (ANCOVA), using baseline energy intake and initial body weight as the covariates, probed for differences in weight loss, food intake, physical activity, and scores from the questionnaires. Time was the repeated measure, and diet group, taster group, and diet × taster interaction were the grouping factors. ANCOVA used these same components to assess differences in scores for the diet plan questionnaire and intake of food groups at 6 months. Post hoc comparisons were made using Duncan's multiple range test and a statistical cutoff criterion of P ≤ 0.05 for all tests.
Hierarchical regression analysis determined stepwise effects of the major variables, followed by effects of food intake variables and psychosocial and biobehavioral variables on weight loss at 6 months. Separate models were developed for the two diet groups but not the subgroups because of inadequate sample size in the subgroups. Taster status, attendance, and completion of Keeping Track booklets were entered as steps 1, 2, and 3 in the models, respectively. Exploratory Pearson's correlation coefficients were calculated to assess univariate associations between weight loss, food intake variables, and scores for items from the questionnaires at 6 months. Variables associated with weight loss at P ≤ 0.05 were entered into the models. Variables related to self-efficacy were entered in step 4, those pertaining to social support were entered in step 5, and barriers to weight loss were entered in step 6. All data were analyzed using SAS version 9.4.
Results
Participant characteristics
Altogether 782 women expressed interest in the study, but 46.8% did not meet initial study criteria (Figure 1). In total, 107 women with mean weight = 93.7 ± 1.9 kg, BMI = 34.8 ± 0.5 kg/m2, and age = 45.8 ± 1.1 years were randomized to a LF (n = 21 NTs, n = 29 STs) or LC (n = 26 NTs, n = 31 STs) diet. Baseline characteristics are shown in Table 1. There were no differences in starting weight or BMI for any of the subgroups.
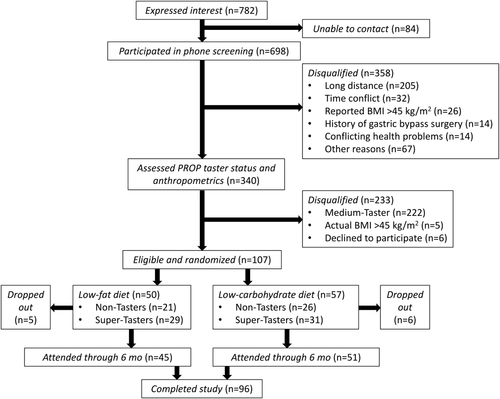
Flowchart of subject recruitment and retention for the intervention.
| Diet | ||||
|---|---|---|---|---|
| Low-fat | Low-carbohydrate | |||
| NT (n = 21) | ST (n = 29) | NT (n = 26) | ST (n = 31) | |
| Race/ethnicity, n | ||||
| Caucasian | 9 | 16 | 16 | 16 |
| African American | 5 | 4 | 8 | 9 |
| Hispanic | 3 | 2 | 1 | 3 |
| Asian | 3 | 5 | 1 | 2 |
| Mixed | 1 | 2 | 0 | 1 |
| Age, y | 48.4 ± 2.4 | 42.1 ± 2.2 | 45.3 ± 2.4 | 47.8 ± 1.6 |
| Weight, kg | 93.8 ± 6.0 | 91.8 ± 3.3 | 95.5 ± 3.4 | 93.8 ± 2.9 |
| BMI, kg/m2 | 34.0 ± 0.8 | 34.3 ± 1.0 | 35.3 ± 1.0 | 35.5 ± 0.9 |
- Mean ± SEM (age, weight, BMI). There were no differences in age, starting weight, or BMI between the groups.
- NT, nontaster; ST, supertaster.
Retention and compliance
The intervention was completed by 96 women, yielding a retention rate of 89.7%. No adverse events were reported and attrition was comparable across the subgroups: NT-LF group (n = 2), ST-LF group (n = 3), NT-LC group (n = 4), and ST-LC group (n = 2). Frequency of attendance at weekly meetings ranged from 79.9% ± 1.8% to 86.2% ± 0.9%, and submission of Keeping Track booklets ranged from 60.1% ± 9.3% to 66.7% ± 2.4%. No differences in compliance measures were observed across the trial.
Weight loss
As expected, the NT-LC group lost more weight than the NT-LF group at 3 months (−6.5 ± 0.3 kg vs. −4.6 ± 0.4 kg, P = 0.0002) and 6 months (−8.5 ± 0.5 kg vs. −6.6 ± 0.5 kg, P = 0.008) (Figure 2). The ST-LC group lost more weight than the ST-LF group at 3 months (−6.4 ± 0.3 kg vs. −5.4 ± 0.3 kg, P = 0.008), but this advantage dissipated by 6 months (−8.9 ± 0.5 kg vs. −8.8 ± 0.4 kg, P = 0.35).
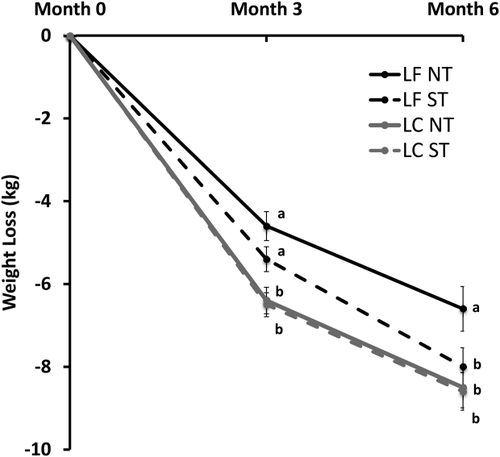
Weight loss by subgroups across 6 months. Intention-to-treat analysis using repeated-measures ANCOVA adjusted for baseline weight was used, followed by Duncan's multiple range test. Values with different superscripts (a, b, etc.) are different at P < 0.05.
LC, low-carbohydrate; LF, low-fat, NT, nontaster; ST, supertaster.
Dietary intake and physical activity
Table 2 shows reported energy and macronutrient intakes. Baseline energy consumption did not differ across study groups. However, the LC diet group consumed more baseline fat and carbohydrates than did the LF diet group (P < 0.0001), and STs consumed more fat than NTs (P = 0.02; all in % energy/d). At both 3 and 6 months, LF diet participants consumed less energy, and less fat and protein (both in % energy/d and g/d), than LC diet participants (P < 0.0001). When assessed at 6 months, the LF diet participants adhered more closely to their diet prescription (consuming ∼54 g/d fat and ∼1,300 kcal/d) than did the LC diet group (consuming ∼68 g/d carbohydrates).
| Diet | Taster status | Diet * Taster status | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-fat | Low-carbohydrate | P | |||||||||
| Low-fat (n = 50) | Low-carbohydrate (n = 57) | NT (n = 47) | ST (n = 60) | NT (n = 21) | ST (n = 29) | NT (n = 26) | ST (n = 31) | Diet | Taster | Diet ×Taster | |
| Energy, kcal | |||||||||||
| Baseline | 2,102.5 ± 37.3 | 2,156.3 ± 34.6 | 2,053.2 ± 38.2 | 2,205.6 ± 33.7 | 1,986.7 ± 56.9 | 2,218.3 ± 48.4 | 2,120.0 ± 51.1 | 2,191.8 ± 46.8 | NS | 0.003 | NS |
| Month 3 | 1,305.2 ± 20.6 | 1,480.0 ± 19.1 | 1,382.1 ± 21.1 | 1,403.0 ± 18.6 | 1,283.3 ± 31.4 | 1,327.1 ± 26.7 | 1,481.0 ± 28.2 | 1,478.8 ± 25.8 | < 0.0001 | NS | NS |
| Month 6 | 1,304.0 ± 19.9 | 1,431.5 ± 18.5 | 1,349.6 ± 20.4 | 1,386.0 ± 18.0 | 1,260.8 ± 30.3 | 1,347.2 ± 25.8 | 1,438.3 ± 27.3 | 1,424.7 ± 25.0 | < 0.0001 | NS | NS |
| Carbohydrate, g | |||||||||||
| Baseline | 245.0 ± 4.6 | 236.2 ± 4.2 | 236.4 ± 4.7 | 244.7 ± 4.1 | 236.4 ± 7.0 | 253.5 ± 5.9 | 236.5 ± 6.3 | 235.9 ± 5.7 | NS | NS | NS |
| Month 3 | 132.1 ± 2.6 | 61.4 ± 2.4 | 101.8 ± 2.7 | 91.8 ± 2.4 | 131.2 ± 4.0c | 133.1 ± 3.4c | 72.4 ± 3.6b | 50.0 ± 3.3a | < 0.0001 | 0.005 | 0.001 |
| Month 6 | 129.7 ± 2.7 | 67.8 ± 2.5 | 102.8 ± 2.7 | 94.6 ± 2.4 | 130.0 ± 4.1c | 129.3 ± 3.5c | 75.6 ± 3.7b | 59.9 ± 3.3a | < 0.0001 | 0.02 | 0.04 |
| Carbohydrate, %-en | |||||||||||
| Baseline | 46.8 ± 0.5 | 44.1 ± 0.4 | 45.8 ± 0.5 | 45.1 ± 0.4 | 47.5 ± 0.7 | 46.1 ± 0.6 | 44.01 ± 0.6 | 44.1 ± 0.6 | < 0.0001 | NS | NS |
| Month 3 | 40.6 ± 0.7 | 17.1 ± 0.7 | 30.3 ± 0.8 | 27.4 ± 0.7 | 40.9 ± 1.1c | 40.4 ± 1.0c | 19.6 ± 1.0b | 14.5 ± 0.9a | < 0.0001 | 0.006 | 0.02 |
| Month 6 | 40.3 ± 0.7 | 18.8 ± 0.7 | 31.1 ± 0.8 | 27.9 ± 0.7 | 41.4 ± 1.1 | 39.1 ± 0.9 | 20.8 ± 1.0 | 16.7 ± 0.9 | < 0.0001 | 0.002 | NS |
| Fat, g | |||||||||||
| Baseline | 86.7 ± 2.0 | 94.8 ± 1.9 | 85.9 ± 2.1 | 95.6 ± 1.8 | 80.4 ± 3.1 | 92.9 ± 3.1 | 91.3 ± 2.8 | 98.3 ± 2.5 | 0.003 | 0.0004 | NS |
| Month 3 | 53.9 ± 1.8 | 94.5 ± 1.7 | 73.3 ± 1.8 | 75.1 ± 1.6 | 54.3 ± 2.8 | 53.6 ± 2.3 | 92.3 ± 2.5 | 96.6 ± 2.3 | <0.0001 | NS | NS |
| Month 6 | 53.9 ± 1.7 | 91.1 ± 1.6 | 72.1 ± 1.8 | 72.9 ± 1.6 | 53.4 ± 2.6 | 54.4 ± 2.2 | 90.8 ± 2.4 | 91.4 ± 2.2 | <0.0001 | NS | NS |
| Fat, %-en | |||||||||||
| Baseline | 36.6 ± 0.4 | 39.1 ± 0.3 | 37.3 ± 0.4 | 38.5 ± 0.3 | 36.0 ± 0.6 | 37.3 ± 0.5 | 38.5 ± 0.5 | 40.0 ± 0.5 | <0.0001 | 0.02 | NS |
| Month 3 | 36.3 ± 0.6 | 56.7 ± 0.6 | 46.3 ± 0.7 | 46.8 ± 0.6 | 37.2 ± 1.0 | 35.5 ± 0.8 | 55.4 ± 0.9 | 58.1 ± 0.8 | <0.0001 | NS | NS |
| Month 6 | 37.1 ± 0.7 | 56.9 ± 0.6 | 46.5 ± 0.7 | 47.4 ± 0.6 | 37.5 ± 1.1 | 36.7 ± 0.9 | 55.5 ± 0.9 | 58.2 ± 0.8 | <0.0001 | NS | NS |
| Protein, g | |||||||||||
| Baseline | 84.7 ± 1.6 | 89.4 ± 1.5 | 83.1 ± 1.6 | 91.0 ± 1.4 | 80.4 ± 2.4 | 89.0 ± 2.0 | 85.8 ± 2.2 | 93.0 ± 2.0 | 0.03 | 0.0003 | NS |
| Month 3 | 71.4 ± 1.4 | 91.0 ± 1.3 | 79.3 ± 1.4 | 83.2 ± 1.2 | 69.7 ± 2.1 | 73.1 ± 1.8a | 88.8 ± 1.9 | 93.2 ± 1.7 | <0.0001 | 0.04 | NS |
| Month 6 | 69.7 ± 1.3 | 85.2 ± 1.2 | 77.1 ± 1.3 | 77.8 ± 1.1 | 67.3 ± 1.9a | 72.1 ± 1.6a | 86.9 ± 1.7c | 83.5 ± 1.6c | <0.0001 | NS | 0.02 |
| Protein, %-en | |||||||||||
| Baseline | 16.4 ± 0.2 | 17.0 ± 0.2 | 16.8 ± 0.2 | 16.6 ± 0.2 | 16.6 ± 0.3 | 16.2 ± 0.3 | 17.0 ± 0.3 | 17.0 ± 0.3 | 0.05 | NS | NS |
| Month 3 | 22.0 ± 0.3 | 24.8 ± 0.3 | 22.9 ± 0.3 | 23.9 ± 0.3 | 21.8 ± 0.4 | 22.1 ± 0.4 | 23.9 ± 0.4 | 25.7 ± 0.4 | <0.0001 | 0.006 | NS |
| Month 6 | 21.6 ± 0.3 | 24.1 ± 0.2 | 22.8 ± 0.3 | 22.9 ± 0.2 | 21.4 ± 0.4 | 21.9 ± 0.3 | 24.3 ± 0.4 | 23.9 ± 0.3 | < 0.0001 | NS | NS |
- All values are mean ± SEM. Values are based on 4-day means at each time point.
- Intention-to-treat analysis using repeated-measures ANCOVA adjusted for baseline weight, followed by Duncan's multiple range test. Values with different superscripted letters are different at P < 0.05.
- NS, not significant; NT, nontaster; ST, supertaster.
Consumption of the food groups did not differ remarkably across the four groups, with a few exceptions. At 6 months, the NT-LC group consumed the greatest number of servings per day of nonnutritive sweeteners; the ST-LF group consumed the lowest number of servings per day of added fats (Table 3).
| Diet | |||||
|---|---|---|---|---|---|
| Low-fat | Low-carbohydrate | P | |||
| NT (n = 21) | ST (n = 29) | NT (n = 26) | ST (n = 31) | Diet × Taster | |
| Fruits | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | NS |
| Vegetables | 3.8 ± 0.2 | 3.8 ± 0.2 | 3.3 ± 0.2 | 3.2 ± 0.2 | NS |
| Dark green vegetables | 2.7 ± 0.2 | 2.7 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.2 | NS |
| Proteins (meat, fish, eggs, nuts, meat alternatives) | 8.3 ± 0.3 | 8.0 ± 0.3 | 11.4 ± 0.3 | 11.0 ± 0.3 | NS |
| Meat | 6.1 ± 3.2 | 6.1 ± 2.1 | 8.4 ± 2.6 | 8.2 ± 2.8 | NS |
| Dairy | 1.7 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | NS |
| Sweetened dairy (yogurt, ice cream) | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | NS |
| Unsweetened dairy (milk, cheese) | 1.1 ± 0.0b | 0.8 ± 0.0a | 1.1 ± 0.0b | 1.1 ± 0.0b | 0.03 |
| Carbohydrates | 3.5 ± 0.2 | 3.1 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | NS |
| All sweets (cakes, snack bars, honey) | – | – | – | – | – |
| Cakes | – | – | – | – | – |
| Sweet snacks (snack bars, candy) | – | – | – | – | – |
| Added sugars (honey, jam, syrup, frosting) | 1.2 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | NS |
| Naturally sweetened foods | 1.8 ± 0.1 | 1.2 ± 1.5 | 0.7 ± 0.1 | 0.4 ± 0.6 | NS |
| Nonnutritive sweeteners and foods with nonnutritive sweeteners | 1.4 ± 0.2a | 1.1 ± 0.2a | 2.6 ± 0.2b | 1.3 ± 0.2a | 0.02 |
| Sweetened beverages | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.6 ± 0.2 | 2.3 ± 0.2 | NS |
| Salty snacks | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | NS |
| Added fats (cream, butter, oil) | 3.8 ± 0.3b | 3.0 ± 0.3a | 4.7 ± 0.3c | 5.2 ± 0.3c | 0.02 |
| Condiments (sauces, pickles) | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | NS |
| Alcohol | – | – | – | – | – |
- All values are mean ± SEM. Values are based on 4-day means at 6 months. ANCOVA adjusted for baseline weight, followed by Duncan's multiple range test. Values with different superscripted letters are different at P < 0.05. Mean values of <0.5 servings per day were considered inconsequential and not included in the data analyses.
- NS, not significant; NT, nontaster; ST, supertaster.
MVPA did not differ across the groups. At baseline, 3 months, and 6 months, MVPA averaged 18.1 ± 1.3 min/d, 21.3 ± 1.7 min/d, and 22.5 ± 1.6 min/d, respectively.
Psychosocial and biobehavioral variables
Supporting Information Table S1 shows scores for measures of self-efficacy, social support, and perceived barriers to weight loss by subgroup at 6 months. Although statistically significant differences were found for several of the survey questions, no pattern emerged for any of these measures that was specific to a particular subgroup.
Scores for factors of the Three-Factor Eating Questionnaire did not differ among the subgroups within each time point, so data were collapsed across all participants. Mean restraint scores increased, and mean disinhibition and hunger scores decreased at 3 and 6 months compared to baseline (Supporting Information Table S2).
Predictors of weight loss
Hierarchical regression models for the diet groups revealed factors that contributed to 6-month weight loss. Attendance at meetings was the single most potent predictor of weight loss for the LF (27%) and the LC (47%) diet groups (Tables 4 and 5, respectively). For the LF diet group, self-efficacy, social support, and perceived barriers to weight loss accounted for ∼3% to 9% of the variance. In contrast, self-efficacy accounted for 34% of the variance for the LC diet group, with “confidence to stay on the diet when in a bad mood” being the largest contributor to weight loss. In total, the models accounted for ∼50% of the variance in weight change for the LF diet participants and ∼89% for the LC diet participants.
| Step | Variables | Parameter estimate | P value | Variance (%) |
|---|---|---|---|---|
| 1 | Taster status | −1.88 | < 0.0001 | 2.77 |
| 2 | Attendance | −13.43 | < 0.0001 | 26.94 |
| 3 | Completing Keeping Track Booklet | −5.38 | < 0.0001 | 4.62 |
| 4 | Confidence to stay on LF diet while in a bad mood | −1.14 | < 0.0001 | 3.40 |
| Confidence to stay on LF diet while eating out | 1.06 | < 0.0001 | ||
| 5 | Receiving encouragement from others | −2.00 | < 0.0001 | 8.42 |
| 6 | Having a busy lifestyle hinders adherence to LF diet | 2.09 | < 0.0001 | 8.61 |
| Finding LF diet too great a change from usual diet | 1.85 | < 0.0001 | ||
| Finding LF diet too expensive | −1.67 | < 0.0001 | ||
| Family members dislike LF foods | 0.38 | 0.031 | ||
| Overall | 49.76% |
- Each step shows percent of variance and P value for the step, with individual parameter estimates for the variables that make a significant contribution to the step.
- Model included intention-to-treat analysis (n = 50).
- LF, low-fat.
| Step | Variables | Parameter estimate | P value | Variance (%) |
|---|---|---|---|---|
| 1 | Taster status | −7.82 | <0.0001 | 3.07 |
| 2 | Attendance | −25.97 | <0.0001 | 46.92 |
| 3 | Completing Keeping Track Booklet | 3.23 | <0.0001 | 1.25 |
| 4 | Confidence to stay on LC diet when preparing LC foods takes a lot of effort | −1.41 | <0.0001 | 34.04 |
| Confidence to stay on LC diet while in a bad mood | −4.75 | <0.0001 | ||
| Confidence to stay on LC diet while eating out | −1.17 | <0.0001 | ||
| 5 | Receiving encouragement from others | −2.64 | <0.0001 | 1.69 |
| 6 | Finding LC diet too expensive | 1.34 | <0.0001 | 1.76 |
| Family members dislike LC foods | 1.57 | 0.031 | ||
| Overall | 88.73% |
- Each step shows percent of variance and P value for the step, with individual parameter estimates for the variables that make a significant contribution to the step.
- Model included intention-to-treat analysis (n = 57).
- LC, low-carbohydrate.
Discussion
This study investigated whether NT women would show greater dietary adherence and weight loss following a LC compared to a LF diet and, alternatively, whether ST women would show greater dietary adherence and weight loss following a LF diet compared to a LC diet in a 6-month lifestyle intervention. As expected, the NT-LC group lost more weight than the NT-LF group at both 3 and 6 months; however, this was unrelated to dietary adherence. Although the ST-LC group lost more weight than the ST-LF group at 3 months, by 6 months, all ST women experienced a nearly identical amount of weight loss, regardless of their level of dietary adherence. It is noteworthy that all women following the LC diet in the present trial lost more weight at 3 months, consistent with the higher initial weight loss typically seen with LC diets (25, 26).
Dietary analyses also failed to reveal differences in energy intake that might explain the weight loss disparities. This is not surprising, as it is well known that self-reported dietary intakes are an imprecise measure of food intake (30), and it is especially common for women with obesity in a lifestyle intervention to underreport food intake (31). To overcome this limitation, future studies should develop strategies to surreptitiously measure food intake in the laboratory, as we did in previous studies documenting food selection patterns among lean PROP-classified women (11, 12).
We observed a few isolated differences in food choices that could be meaningful for understanding dietary adherence. Most notably, the NT-LC diet group consumed more servings per day of nonnutritive sweeteners, and the ST-LF group consumed fewer servings of added fats per day. It is plausible that substituting nonnutritive sweeteners for carbohydrate sweeteners permitted the NT-LC group to maintain sweetness in the diet while adhering to the carbohydrate restriction. In previous studies, NTs perceived less bitterness from nonnutritive sweeteners compared to STs (32, 33). This difference could be related to higher acceptance of nonnutritive sweeteners by NTs and could explain the higher intake of nonnutritive sweetened products by the NT-LC group in the present study. We note, however, that a prior study did not find a relationship between PROP sensitivity and acceptance/rejection of nonnutritive sweeteners (34). The finding of lower fat intakes by the ST-LF group is consistent with our hypothesis that STs exhibit lower dietary preferences for fat compared to NTs and, hence, may be more likely to reduce their consumption of discretionary fats to comply with the dietary fat restriction. Although these interpretations are speculative and should be interpreted with caution, they point to potentially important diet-taste relationships that deserve further investigation.
The role of biobehavioral and psychosocial factors in weight loss has been examined previously (35). However, no study has assessed whether these factors are influenced by a person's PROP taster status and dietary prescription or evaluated their specific contributions to weight loss. For all participants, scores increased for restraint and decreased for disinhibition and hunger in agreement with the literature (36), though this was unrelated to weight loss in our study. Additionally, we did not observe trends in scores for self-efficacy, social support, or perceived barriers to weight loss between the diet group, taster group, and taster × diet subgroups. Nevertheless, hierarchical regression revealed diet-specific differences in predictors of weight change. Most notably, meeting attendance and self-efficacy contributed more to weight loss in women following the LC diet than the LF diet (regardless of taster status). Why the LC diet associated with greater self-efficacy in our study is unknown. However, a prior study (37) examining the public's understanding of LC diets reported that high-socioeconomic-status women were more likely to embrace LC diets than women in other demographic groups. Our cohort was predominantly college educated with high household income; hence, they may have exhibited higher awareness of and confidence in following the LC diet. We note, however, that PROP status made a very modest contribution to each of the models.
The study had strengths and weaknesses. A strength was that the attrition rate (12%) was lower than other weight loss investigations (22, 23) and unrelated to diet or PROP taster status. With respect to weaknesses, our study only addressed weight loss in women, and the sample size was modest and not representative of a wider demographic, as only middle-income women were studied. We were also unable to link reported food intake or dietary adherence to differences in weight loss; a larger sample size might reveal differences.
Conclusion
For decades, the US Dietary Guidelines have advised the public to control daily fat intake to maintain a healthy weight and reduce the risk of chronic diseases (38). Current guidelines have abandoned restrictions on daily fat intake, focusing more specifically on added sugars (among other components) (39). LC diets are more effective for short-term weight loss; however, they may not be appropriate for everyone in the long term (2). There is an ongoing need to customize lifestyle interventions to reduce large disparities in weight loss outcomes and better address individual treatment needs (2). In a 6-month trial, we showed that PROP NT women lost more weight following a LC diet that does not restrict fat intake and matches their preferences for high-fat foods, according to our previous research (11, 12). Screening for PROP phenotype among women seeking help with weight loss may be a valuable tool for identifying the subset of NT women who presumably would benefit more from a LC as compared to a LF diet.
Acknowledgments
We thank the women who participated in our study and Rocco Paluch, who assisted with data analysis.





