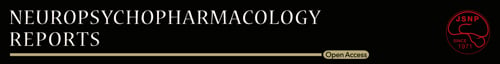Bidirectional brain-gut interactions: Involvement of noradrenergic transmission within the ventral part of the bed nucleus of the stria terminalis
Funding information
This study was supported by Challenging Research (Exploratory [M.M., 17K19469]) from Japan Society for the Promotion of Science and an interdisciplinary project for psychosomatological research at Hokkaido University.
Abstract
Introduction
Although the important roles of bidirectional interactions between the brain and gut in stress and emotional responses have long been recognized, the underlying neuronal mechanisms remain unclear. The bed nucleus of the stria terminalis (BNST) is a limbic structure involved in stress responses and negative affective states, such as anxiety and depression. We have previously demonstrated that noradrenergic transmission within the ventral part of the BNST (vBNST) plays a crucial role in anxiety-like behaviors and pain-induced aversion.
Objectives
This study aimed to examine the involvement of noradrenergic transmission via β-adrenoceptors within the vBNST in bidirectional brain-gut interactions.
Methods
We measured the gastric distention (GD)-induced noradrenaline release within the vBNST of freely moving rats using an in vivo microdialysis technique. Gastric emptying and intestinal transit were examined following intra-vBNST injections of isoproterenol, a β-adrenoceptor agonist, in the absence or presence of the coadministration of timolol, a β-adrenoceptor antagonist.
Results
Gastric distention at a higher pressure (45 mm Hg) but not at a lower pressure (25 mm Hg) resulted in a significant increase in extracellular noradrenaline levels within the vBNST. Intra-vBNST injections of isoproterenol (30 nmol/side) induced significant reductions in gastric emptying and small intestinal transit, both of which were reversed by the coadministration of timolol (30 nmol/side).
Conclusion
Noradrenergic transmission via β-adrenoceptors within the vBNST was involved in bidirectional brain-gut interactions. These findings suggest that gastric dysfunction may induce negative affective states via the enhanced release of noradrenaline within the vBNST which, in turn, may cause gastrointestinal impairments.
1 INTRODUCTION
The important roles of bidirectional brain-gut interactions in stress and emotional responses have long been recognized. Gastrointestinal problems can affect mental health conditions, such as anxiety and depression, and, conversely, anxiety is closely correlated with the symptom intensities of functional gastrointestinal disorders.1, 2 Functional dyspepsia (FD) is a gastrointestinal syndrome defined by the presence of symptoms such as epigastric pain, epigastric burning, and postprandial fullness in the absence of any organic or metabolic disease that is likely to explain the symptoms.3 Patients with FD are hypersensitive to gastric distention (GD)4-6 which is thought to activate the dorsal-vagal complex, including the nucleus of the solitary tract (NST), via vagal nerves. In the human brain, GD-induced changes in neuronal activities have been observed in a number of brain regions, such as the insula, thalamus, anterior cingulate cortex, and amygdala, using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) scans.7-9 On the other hand, pharmacological manipulations of various brain regions in rodents have been shown to affect gastric functions.10-15 Thus, the dysfunction of neurotransmission within these brain regions could be a possible mechanism underlying the pathology of FD.
The extended amygdala, which consists of the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST), is involved in stress responses and the regulation of negative affective states such as anxiety and depression. The BNST, particularly its ventral part (vBNST), is densely innervated by noradrenergic fibers that primarily originate in the NST (including the A2 cell group) and caudal ventrolateral medulla (including the A1 cell group).16, 17 We have previously demonstrated that noradrenergic transmission via β-adrenoceptors within the vBNST mediates the negative affective states induced by inflammatory pain.18-20 Furthermore, the activation of β-adrenoceptors within the vBNST induces anxiety-like behaviors and reduction in food intake.21 However, the involvement of noradrenergic transmission via β-adrenoceptors within the vBNST in gastrointestinal pain and functions remains unclear. Thus, this study aimed to examine GD-induced alterations in noradrenaline release within the vBNST and the effects of β-adrenoceptor activation within the vBNST on gastric emptying and intestinal transit in rats.
2 MATERIALS AND METHODS
2.1 Animals
Male Sprague Dawley rats weighing 180-290 g (Japan SLC; Hamamatsu, Japan) were used. The rats were housed 3-4 per cage in a room maintained at a constant ambient temperature (23 ± 1°C) under a 12/12-h light/dark cycle with food and water available ad libitum. Following surgeries to implant the balloon or the microinjection guide cannulae, the rats were individually housed in cages.
2.2 Drugs
Isoproterenol (β-adrenoceptor agonist) and timolol (β-adrenoceptor antagonist) were purchased from Sigma (St Louis, MO). Both drugs were dissolved in phosphate-buffered saline (PBS).
2.3 Measurement of GD-induced noradrenaline release within the vBNST
After fasting for at least 16 hours with water available ad libitum, the rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal [ip]). A left lateral epigastric incision that was approximately 1 cm long was made to expose the stomach, and a latex balloon (long diameter: 4 cm, short diameter: 2 cm) attached to a polyethylene catheter (inner diameter: 1.6 mm) was placed in the stomach through a small incision made in the forestomach. The catheter's free end was exteriorized at the back of the neck. Subsequently, the rats were individually housed in cages and provided with free access to food and water. After 3-5 days of recovery, each rat was again anesthetized with sodium pentobarbital and unilaterally implanted with a microdialysis guide cannula (outer diameter: 0.5 mm, AG-7; Eicom; Kyoto, Japan) at 1.0 mm above the left vBNST (−0.3 mm rostral, 1.6 mm lateral, and 6.7 mm ventral to the bregma).22 After this surgery, the rats were returned to their individual cages and allowed to recover for 1-2 days.
After fasting for 16 hours with water available ad libitum, the microdialysis experiments were performed in freely moving rats. A microdialysis probe (dialysis membrane: length 1.0 mm, outer diameter: 0.22 mm, A-I-7-01; Eicom) was inserted through the guide cannula and continuously perfused with Ringer's solution (Na+ 147 mmol/L, K+ 4 mmol/L, Ca2+ 2.3 mmol/L, Cl− 155.6 mmol/L) at a constant flow rate of 1 μL/min. The tip of the microdialysis probe protruded 1.0 mm from the tip of the guide cannula to reach the vBNST. The rats were placed in a Plexiglas chamber (width × length × height: 30 × 30 × 35 cm) for testing. After a stabilization period (>2 hours), the catheter of the distending balloon was connected to a pressure controller-timing device (Barostat Model Distender IIR, Star Medical; Tokyo, Japan), and three 15-minute dialysate fractions were collected as baseline samples in the absence of distention. After collecting the baseline samples, each rat was given the GD stimulus for 30 minutes (either 25 or 45 mm Hg for 4.5 minutes with 0.5-min interstimulus intervals × 6 times). Each dialysate sample was separated by a liquid chromatography column (Eicompak CAX, inner diameter: 2.0 mm × length: 200 mm; Eicom) with 0.1 mol/L ammonium acetate buffer (pH 6.0) containing 0.05 mol/L sodium sulfate, 50 mg/L ethylenediaminetetraacetic acid, and 30% methanol at a constant flow rate of 0.25 mL/min. The column was maintained at 35°C.
The noradrenaline contents of the dialysate samples were measured using an electrochemical detector (HTEC-500; Eicom) with a working electrode set at +450 mV vs an Ag/AgCl reference electrode, and the chromatogram peaks were analyzed using the PowerChrom data recording system (Eicom). Behavioral responses were counted before and during the GD stimulus according to the method of Liu et al.23, 24 with modifications because of microdialysis probe connection to the rat's head. It has been reported that behavioral responses to the GD stimulus are well correlated with electromyographic responses to the GD stimulus.23 Thus, in this study, behavioral responses were evaluated by well-trained investigators who were blind to the pressure conditions and quantified the responses using a rating scale method by assigning weights to abnormal behaviors as follows: normal body posture (category 0), abdominal contraction and vomiting-like behavior (category 1), and writhing (category 2). The abnormal behavior score was calculated using the following formula: abnormal behavior score = (the number of behaviors in category 1) × 1 + (the number of behaviors in category 2) × 2.
2.4 Microinjections
Under sodium pentobarbital anesthesia, each rat was bilaterally implanted with 25-gauge stainless steel guide cannulae 1.5 mm above the vBNST (−0.3 mm rostral, 1.6 mm lateral, and 6.0 mm ventral to the bregma).22 The rats were allowed to recover for at least 5 days and were handled for 1-2 minutes each day for habituation during the recovery period. Microinjections were performed using 33-gauge stainless steel injection cannulae inserted bilaterally into the guide cannulae. The injection cannulae protruded 1.5 mm from the tip of the guide cannulae to reach the vBNST and were attached to a microinjection pump (CMA; Stockholm, Sweden) via PE 8 tubing. Either the drugs or a vehicle was bilaterally administered into the vBNST in a volume of 0.5 μL/side at a rate of 0.5 μL/min, and the injection cannulae were left in place for 1 minute after the injection to prevent backflow. The doses of isoproterenol (30 nmol/side) and timolol (30 nmol/side) for intra-vBNST injection were determined in reference to our previous reports in which intra-vBNST injection of isoproterenol at a dose of 30 nmol/side, but not 10 nmol/side, significantly induced aversive responses in a conditioned place aversion test and anxiety-like behaviors in an elevated plus maze test, and the anxiety-like behaviors were suppressed by a coadministration of timolol at a dose of 30 nmol/side.20, 21
2.5 Gastric emptying and intestinal transit
After 24 hours of fasting with water available ad libitum, the intra-vBNST injections were carried out. Five minutes after the microinjection, 1.5 mL of Evans blue (50 mg/mL in 0.9% NaCl containing 0.5% methylcellulose) was administered per os (p.o.), and the animals were sacrificed 20 minutes later under deep anesthesia with isoflurane. To prevent leakage of Evans blue, the stomach was ligated at 2 sites at the lower esophageal sphincter and the pylorus before the stomach was cut outside of the ligatures. The stomachs were then frozen at −80°C until the measurement of gastric emptying. The small intestine between the pylorus and the cecum was resected, and the intestinal transit of Evans blue was measured from the pylorus to the most distal point of migration. The total length of the small intestine was measured, and small intestine transit was expressed as a percentage: the distance of Evans blue migration compared with the total length of the small intestine.25
Gastric emptying was spectrophotometrically determined according to a slightly modified version of the methods of De Winter et al.25 Briefly, the stomachs were cut immediately inside of the ligatures, minced, placed in 15 mL of 0.1 N NaOH, and homogenized for 30 seconds. Each sample was diluted to 30 mL with 0.1 N NaOH and then kept for 1 hour at room temperature. Next, 5 mL of supernatant was centrifuged at 1356 g for 20 minutes at 4°C. The supernatant was further diluted (1/50) with 0.1 N NaOH, and the absorbance of the solution was measured at a wavelength of 565 nm (A565) with a spectrophotometer. The stomach obtained from a rat sacrificed immediately after the p.o. administration of Evans blue served as the standard (reference stomach). The percentage of gastric emptying was calculated as follows: [(A565 reference–A565 sample)/A565 reference] × 100.
2.6 Histology
Following the experiments, histological analyses were performed to examine the placements of the injection cannulae and the microdialysis probes. Briefly, each rat was decapitated, the brain was rapidly removed, and it was then frozen in powdered dry ice. Coronal sections (50 μm) were prepared on a cryostat, stained with thionin, and examined by light microscopy (40x). Data from the rats with correct placements of the microdialysis probe (Figure 1A) and the bilateral microinjection cannulae (Figure 1B–E) were used for the statistical analyses.
2.7 Statistical analyses
All data were analyzed with an analysis of variance (ANOVA) followed by Holm-Sidak's post hoc tests for multiple comparisons. Differences with P < .05 were considered statistically significant.
3 RESULTS
3.1 GD-induced noradrenaline release within the vBNST
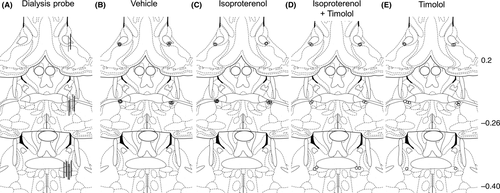
Behavioral responses were evaluated before and during the GD stimulus (Figure 2A). Abnormal behavior scores were significantly increased by 25 and 45 mm Hg GD stimuli (11.3 ± 4.0 and 39.3 ± 4.1, respectively) in a tension-dependent manner, compared with the score before GD stimulus (0.1 ± 0.1) (Figure 2A, 1-way ANOVA, F2,22 = 33.88, P < .0001).
Using an in vivo microdialysis technique in freely moving rats, we examined GD-induced changes in extracellular noradrenaline levels within the vBNST (Figure 2B). A 2-way repeated-measures ANOVA revealed a significant effect of the GD stimulus on extracellular noradrenaline levels within the vBNST (GD stimulus: F1,15 = 6.97, P = .019; time: F10, 150 = 8.78, P < .001; interaction: F10,150 = 4.93, P < .001). According to Holm-Sidak's post hoc test, there were significant increases in noradrenaline between 15 and 60 minutes after the initiation of the 45 mm Hg GD stimulus compared with the last baseline sample (−15 to 0 minutes). However, there were no significant increases in noradrenaline either during or after the 25 mm Hg GD stimulus.
3.2 Effects of intra-vBNST β-adrenoceptor activation on gastric emptying and small intestinal transit
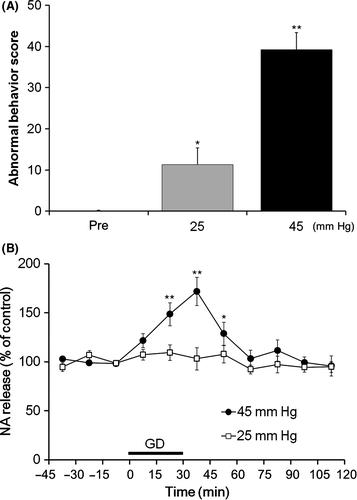
The effects of intra-vBNST injections of isoproterenol (30 nmol/side) and timolol (30 nmol/side) on gastric emptying were examined (Figure 3A). A 2-way ANOVA revealed a significant main effect of isoproterenol (F1,22 = 4.44, P < .05) and a significant interaction between the effects of isoproterenol and timolol (F1,22 = 4.68, P < .05). Intra-vBNST injections of isoproterenol significantly reduced gastric emptying (14.5 ± 6.7%, P < .05, Holm-Sidak's post hoc test) compared with injections of vehicle (38.4 ± 5.7%), and this reduction was significantly reversed by the coadministration of timolol (38.6 ± 5.7%, P < .05, Holm-Sidak's post hoc test). There were no significant effects of timolol alone (38.3 ± 3.0%) compared with vehicle injections.
The effects of intra-vBNST injections of isoproterenol (30 nmol/side) and timolol (30 nmol/side) on small intestinal transit were also examined (Figure 3B). A 2-way ANOVA revealed a significant main effect of isoproterenol (F1,22 = 4.32, P < .05) and a significant interaction between the effects of isoproterenol and timolol (F1,22 = 4.33, P < .05). Intra-vBNST injections of isoproterenol significantly reduced small intestinal transit (54.3 ± 2.6%, P < .05, Holm-Sidak's post hoc test) compared with vehicle injections (74.0 ± 5.6%), and this reduction was significantly reversed by the coadministration of timolol (73.1 ± 5.9%, P < .05, Holm-Sidak's post hoc test). There were no significant effects of timolol alone (73.1 ± 4.2%), compared with vehicle injections.
4 DISCUSSION
The present study utilized an in vivo microdialysis technique in freely moving rats to demonstrate that GD induced an increase in noradrenaline release within the vBNST. Additionally, intra-vBNST injections of isoproterenol, a β-adrenoceptor agonist, reduced gastric emptying and small intestinal transit, and these reducing effects were reversed by the coadministration of timolol, a β-adrenoceptor antagonist. Thus, the present findings demonstrated important roles of noradrenergic transmission via β-adrenoceptors within the vBNST in the bidirectional brain-gut interactions (Figure 4).
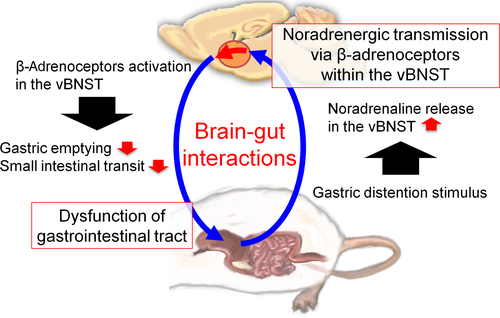
Noradrenergic transmission within the BNST is critical for the mediation of negative emotions such as anxiety, fear, and aversion. Exposure to trimethylthiazoline, which is a component of fox odor, increases noradrenaline release in rats, and the intra-vBNST administration of clonidine, an α2-adrenoceptor agonist, suppresses the enhanced release of noradrenaline as well as the trimethylthiazoline-induced potentiation of freezing behavior.26 Noradrenaline release within the BNST is elevated following acute restraint stress in rats, and intra-vBNST injections of a β1- and β2-adrenoceptor antagonist cocktail block stress-induced anxiety-like behaviors.27 Furthermore, microinjections of β-antagonists or an α2-agonist into the BNST markedly attenuate opiate withdrawal-induced aversive responses in rats.28 We have previously demonstrated that noradrenaline release within the vBNST is increased following exposures to somatic and visceral inflammatory pain and that β-adrenoceptors are involved in pain-induced aversion.19, 20 Furthermore, we found that the intra-vBNST administration of clonidine attenuates pain-induced noradrenaline release and aversive responses.18 Taken together, these findings strongly indicate that aversive stimuli increase noradrenaline release within the vBNST, and enhanced noradrenergic transmission via β-adrenoceptors within the vBNST mediates the generation of negative emotions. In the present study, we demonstrated the increase in extracellular noradrenaline levels within the vBNST following exposures to GD stimulus. It is well known that the vagal afferent fibers activated during GD stimulus terminate in the NTS. It could be likely that the vagal nerve-mediated activation of noradrenergic cells within the NTS following the GD stimulus significantly increases a noradrenaline release within the vBNST, the region which is densely innervated by noradrenergic fibers arising from the NTS16, to induce GD stimulus-induced negative emotion.
Pharmacological manipulations, particularly the activation of G protein-coupled receptors (GPCRs) in various brain regions, have been reported to affect gastric functions. The microinjection of nesfatin-1 into the CeA14 or the arcuate nucleus of the hypothalamus (Arc)13 reduces gastric motility in rats. On the other hand, the administration of ghrelin into the Arc,15 lateral septum,10 or lateral hypothalamic area (LHA)11 increases gastric motility in rats. Furthermore, the activation of α1-adrenoceptors within the NST causes a reduction in gastric tone in rats.12 The present findings indicate that the activation of β-adrenoceptors, which are GPCRs, within the vBNST also plays a role in the regulation of the gastric functions.
In the present study, we demonstrated that intra-vBNST injection of isoproterenol reduced gastric emptying. We previously reported that intra-vBNST injection of isoproterenol also reduced food intake.21 The BNST provides neuronal input to various brain regions, including the LHA which is known to play an important role in the regulation of feeding and gastric motility.29-31 Recently, Jennings et al.32 have demonstrated that inhibitory synaptic inputs from the BNST to the LHA control the activity of glutamatergic neurons in the LHA, which in turn regulates food intake. It is possible that the activation of β-adrenoceptors within the vBNST suppresses gastric function and food intake via the activation of the neuronal pathway from the BNST to the LHA. Further studies are needed to elucidate the detailed neuronal mechanisms regulating gastric function and food intake.
It is well known that psychological factors have an important influence on functional gastrointestinal disorders (FGIDs) and that there is a high comorbidity of FGIDs with psychiatric disorders. The BNST is well characterized as one of the important brain regions mediating stress responses and negative emotional states such as anxiety, fear, and depression. However, the roles of the BNST in the neuronal mechanisms underlying the relationship between negative emotional states and gastrointestinal functions remain to be elucidated. The present findings demonstrated the important roles of the noradrenergic transmission via β-adrenoceptors within the vBNST in the bidirectional brain-gut interactions. Further studies investigating the brain regions involved in emotional regulation, such as the vBNST, may illuminate the mechanisms underlying the bidirectional interactions between emotional states and gastrointestinal functions as well as the mechanisms underlying the high comorbidity between FGIDs and psychiatric disorders.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Yoshihiro Keto for teaching the methods of gastric distention.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article. [Correction added on 5 March 2018, after first online publication: The word, ‘The’, has been added to the start of the conflict of interest statement.]
ANIMAL STUDIES
All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Hokkaido University.
AUTHOR CONTRIBUTIONS
SI, HT, and MM involved in the conception and design of the experiments. SI and RY performed the experiments and statistical analyses and wrote the manuscript. HT and MM finalized the manuscript. All authors read and approved the final manuscript.