How can we approach preoperative frailty and related factors in patients with cancer? A scoping review
Abstract
Aim
To identify factors related to preoperative frailty in patients with cancer and map the tools that measure frailty.
Design
A Scoping review.
Methods
This scoping review based on Arksey and O'Malley's framework. Articles from CINAHL, PubMed, EMBASE, and PsycINFO databases published between January 2011 and April 2021. The searched keywords were concepts related to ‘cancer’, ‘frailty’ and ‘measurement’.
Results
While 728 records were initially identified, 24 studies were eventually selected. Research on frailty was actively conducted between 2020 and 2021. Factors related to preoperative frailty were age (22.9%), sex (11.4%), body mass index (11.4%) and physical status indicators (54.3%). The most common result of preoperative frailty was postoperative complications (35.0%). 24 instruments were used to measure frailty.
Implications for Patient Care
Selecting an appropriate preoperative frailty screening tool can help improve patient postoperative treatment outcomes.
Impact
There are many instruments for assessing preoperative frailty, each evaluating a multi-dimensional feature. We identified the frailty screening tools used today, organized the factors that affect frailty, and explored the impact of frailty. Identifying and organizing frailty measurement tools will enable appropriate evaluation.
Reporting Method
PRISMA-ScR.
Patient Contribution
No patient or public contribution.
1 INTRODUCTION
For patients with solid cancer, surgery is often an essential component of treatment (American College of Surgeons' Command on Cancer, 2022). However, treatment outcomes vary among patients with cancer undergoing similar modalities, and frailty is a considerable contributor in patients with cancer who have undergone surgery (Ness & Wogksch, 2020; Uslu & Canbolat, 2021; Zhang et al., 2017). Frailty is a status of elevated vulnerability to stress associated with reduced homeostatic capacity across multiple physiological systems (Fried, 2001), requiring a comprehensive understanding of the factors influencing frailty and the available measurement tools. Although frailty in patients with cancer has been studied (Komici et al., 2022; Nishijima et al., 2021; Wang et al., 2022), there is a notable lack of an integrated review that specifically identifies the factors that influence preoperative frailty in patients with cancer and the measurement tool used.
2 BACKGROUND
Surgery is the primary treatment in 35.5% of cancer cases, and this proportion rises to 59.5% when systemic treatment and radiotherapy are included (American College of Surgeons' Command on Cancer, 2022). By 2030, it is estimated that there will be 45 million yearly surgeries for patients with cancer worldwide (Sullivan et al., 2015). Various factors including age, sex, body mass index (BMI), race, smoking, pathologic factors, and physical status are influencing postoperative outcomes in patients with cancer (Cheong et al., 2022; Foukakis & Bergh, 2022; Sebesta & Anderson, 2017). Frailty, specifically, is known to affect postoperative outcomes in older patients with cancer (Ness & Wogksch, 2020; Uslu & Canbolat, 2021; Zhang et al., 2017); however, patients with cancer could experience frailty regardless of age (Boakye et al., 2018; Ethun et al., 2017; Komici et al., 2022; Wang et al., 2022). More than 40% of people diagnosed with cancer and undergoing treatment such as surgery, chemotherapy, and radiation therapy reported frailty (Komici et al., 2022; Wang et al., 2022). Frailty in patients with various cancer types predicts negative treatment outcomes such as prolonged hospital stay, adverse effects, and unplanned admissions (Boakye et al., 2018; Dai et al., 2021; Momota et al., 2020; Zhang et al., 2017), as well as survival/mortality rates (Handforth et al., 2015; Huisingh-Scheetz & Walston, 2017; Molina-Garrido & Guillén-Ponce, 2017). However, most studies of preoperative frailty in patients with cancer focused on older adults (Ness & Wogksch, 2020; Uslu & Canbolat, 2021; Zhang et al., 2017) or used mixed treatment modalities (Komici et al., 2022; Wang et al., 2022). This limits our understanding of the impact of preoperative frailty in the adult cancer population as a whole.
Many tools have been developed to evaluate frailty in older adults; currently, the Comprehensive Geriatric Assessment (CGA) is the gold standard (Overcash et al., 2018). It has demonstrated its usefulness in predicting cancer treatment resistance (Hamaker et al., 2014), as well as survival and adverse events in hospitalized patients (Antonio et al., 2017). The CGA involves multi-dimensional investigations of frailty status by experts; however, it is considerably time-consuming and limited in its universal applicability (Chen et al., 2016; Kenig et al., 2020; Nishijima et al., 2021). To overcome the limitations of the CGA, several tools have been developed to help medical staff easily identify frailty before referring the patient to an expert (Lu et al., 2018; Nishijima et al., 2021). Consequently, several tools have been used to measure preoperative frailty in patients with cancer (Lu et al., 2018; Ness & Wogksch, 2020; Nishijima et al., 2021; Uslu & Canbolat, 2021; Zhang et al., 2017).
The prevalence of frailty in patients with cancer is associated with treatment modality, frailty scale, and age (Wang et al., 2022). In addition, intervening in preoperative frailty could reduce the negative treatment outcomes experienced by patients with cancer after surgery and ultimately contribute to improved survival rates (Ommundsen, Wyller, et al., 2018). Despite the above-mentioned literature, the evidence for measurement tools related to preoperative frailty in patients with cancer is scattered, with many studies focusing on older adults.
In conclusion, understanding the factors and measurement tools associated with preoperative frailty in adult patients with cancer, including older patients with cancer, is necessary to effectively intervene in preoperative frailty.
3 AIMS
This study aimed to bridge existing gaps by identifying factors that influence preoperative frailty in patients with cancer across age groups and by mapping the evidence for tools used to assess frailty in this population.
4 METHODS
4.1 Research questions
- What tools have been used to measure preoperative frailty in patients with cancer and what are their characteristics?
- What has been the association between preoperative frailty and postoperative outcomes in patients with cancer?
- What factors are associated with preoperative frailty in patients with cancer?
4.2 Design
This scoping review was guided by the six steps of the scoping review framework proposed by Arksey and O'Malley (2005): specifying the research questions, identifying relevant studies, selecting studies, extracting data, and summarizing and reporting the data. The PRISMA-ScR (Tricco et al., 2018) checklist was used to map the search process and report the results. Data were summarized and reported using the PAGER framework (Bradbury-Jones & Aveyard, 2021).
4.3 Search methods
We searched for literature published in the last decade (between January 2011 and April 2021) using the following databases: CINAHL, EMBASE, PubMed, and PsycINFO. We searched for relevant literature using the keywords—cancer, frailty or vulnerability, and measurement—in conjunction with Boolean operators, proximity locators, and MeSH terms. As subject headings varied in the databases, the terminology modification was repeated in each database. As we defined frailty as a state of increased vulnerability in multiple physiological systems (Fried, 2001), we used “frailty” and “vulnerability” together as search terms.
4.4 Inclusion and exclusion criteria
The inclusion criteria were studies that (1) included patients with cancer; (2) used frailty or vulnerability, or frailty-related terms, as a description of the condition of patients with cancer; (3) assessed preoperative frailty in patients with cancer; (4) assessed postoperative outcomes; and (5) were published in English. The exclusion criteria were (1) studies exploring the use of tools to measure preoperative frailty (i.e. use frailty measurement tools and report the degree of frailty); (2) determine the effect of preoperative frailty on outcomes (i.e. frailty is measured before surgery and used as an independent variable in the outcome analysis); (3) identify factors that are associated with preoperative frailty (i.e. analyse the factors that patients with cancer have that are associated with preoperative frailty); (4) systematic reviews, meta-analysis, protocols, reviews, letters, or grey literature; and (5) when the full text was not available.
In an initial search with “surgery” as a search term, we did not find studies that only mentioned the name of the surgical procedure. Therefore, in the second screening process, we selected reports of preoperative frailty in patients with cancer.
4.5 Search outcomes
The research team developed the search and selection strategies. The team comprised four researchers with a master's degree or higher and practical and research experience in oncology nursing. Figure 1 presents our search process. The initial database screening resulted in 728 studies. We excluded 548 studies that did not include keywords in the abstract or title. We also excluded 48 duplicated studies. For the 132 remaining studies, three researchers conducted the primary screening process by independently reviewing the titles and abstracts. Accordingly, 91 studies that were not relevant to preoperative patients with cancer were excluded. Then, two researchers independently reviewed the full text of the remaining 41 studies in the second screening process and identified 24 studies (Ahmed et al., 2017; Bras et al., 2015; Chen et al., 2016; Choe et al., 2017; Courtney-Brooks et al., 2012; Goldstein et al., 2020; Harland et al., 2020; Ho et al., 2017; Kaneda et al., 2021; Kenig et al., 2020; Korkmaz Toker et al., 2020; Lu et al., 2018; Miller et al., 2020; Misawa et al., 2020; Nishijima et al., 2021; Ommundsen et al., 2014; Ommundsen, Nesbakken, et al., 2018; Ommundsen, Wyller, et al., 2018; Pitts et al., 2019; Richards et al., 2021; Rønning et al., 2016; Sastry et al., 2020; Vermillion et al., 2017; Xu et al., 2020) that met the selection criteria. Disagreements among reviewers regarding the selection of eight studies were resolved through a consensus process within the research team, which included the senior researcher.
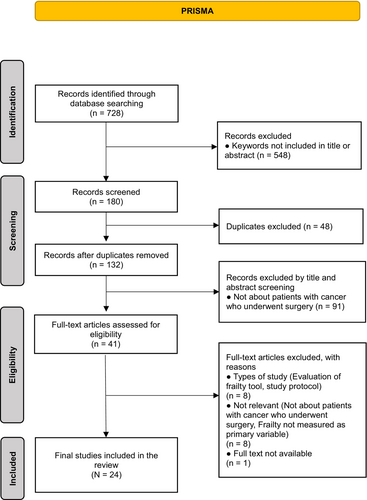
In the context of quality appraisal and considering the existing evidence that does not necessitate quality appraisal in scoping reviews (Pollock et al., 2021), we opted to forego this step while maintaining the rigorous procedure of scoping review process.
4.6 Data charting
Following the recommendations of Tricco et al. (2018), we used the data charting form. As data charting is an iterative process, the form was further developed by the research team, pre-tested on three sample studies, and employed. Two researchers completed the data charting and extraction of general information about participants' characteristics (e.g. type of cancer, surgical procedure, sex, and age), frailty measures, and outcome factors that demonstrated a significant relationship to frailty, using Microsoft Excel version 2020. To ensure accuracy, the data charting form was reviewed by one other researcher.
4.7 Data analysis
Data analysis followed Arksey and O'Malley's (2005) guidelines. The extracted data were assigned to the study characteristics using descriptive statistics. In the following step, we categorized the data into themes to answer the research questions. In the measurement analysis, we derived themes from each measurement domain. We provided a comprehensive description and critique of the literature included in this scoping review using the PAGER framework (Bradbury-Jones & Aveyard, 2021). Based on the research questions, we structured the key themes into patterns. Patterns, progress, and gaps were used to provide contextualized evidence for practice and research recommendations.
5 RESULTS
5.1 Overview of study characteristics
Of the 24 selected studies, about half (45%) were conducted 2 years preceding this report (2020 and 2021). Cancer types included colorectal cancer (29.2%), stomach cancer (16.7%), head and neck cancer (12.5%), and different primary cancers (12.5%). One-third of the studies were conducted in North America (i.e. the United States and Canada; 37.5%), Europe (i.e. Norway, Turkey, Poland, Netherlands, and New Zealand; 37.5%), and Asia (i.e. China and Japan; 25%). In North America, the most studied type of cancer was head and neck cancer (30%), followed by brain tumour (20%) and rectal cancer (20%). In Europe, colorectal cancer was the most studied (75%) type of cancer. Gastric cancer was the most studied type of cancer in Asia (66.7%). All studies employed quantitative designs, including prospective (50%), retrospective (45.8%), and randomized controlled trials (RCTs; 4.2%). Most studies (95.8%) were exploratory or observational. Table 1 summarizes the patterns, progress, gaps, evidence for practice, and research recommendations emerging from this review based on the PAGER framework (Bradbury-Jones & Aveyard, 2021).
| Subcategory | Patterns | Advance | Gaps | Evidence for practice | Research recommendations |
|---|---|---|---|---|---|
| Frailty measures | Thirty-four tools identified, including similar subscales with different names | Demonstrates advancement in identifying and using a variety of frailty measurement tools | Reveals the lack of a prominently used tool for preoperative frailty in patients with cancer | The most commonly used tools to assess preoperative frailty in patients with cancer were the Fried's frailty phenotype and the modified Frailty Index | Suggests further research to establish consensus on the most effective frailty measurement tool for preoperative patients with cancer |
| Association with physical conditions | Only 10 out of 24 studies investigated the association between frailty and physical status in preoperative patients with cancer | Advances understanding of the association between frailty and physical conditions | Identifies a limited number of studies investigating the association between frailty and physical status | Recognizes associations between frailty and preoperative physical conditions assessed by the ASA-PS and ECOG-PS | Recommends additional studies to investigate the association between frailty and physical status in diverse populations of preoperative patients with cancer |
| Association with postoperative outcomes | Frailty is significantly associated with negative treatment outcomes, including complications; longer length of hospital stays; higher mortality, morbidity, and readmission rates; and lower survival | Demonstrates consistent associations between frailty and several adverse postoperative outcomes | Identifies potential gaps in understanding of the relationship between frailty and specific postoperative outcomes | Provides evidence to support the use of frailty assessment to predict negative postoperative outcomes | Encourages future research to examine specific aspects of the relationship between frailty and postoperative outcomes, considering different cancer types and patient demographics |
5.2 Frailty measures
Thirty-four tools were used to measure frailty; these, together with the domains, are presented in Table 2. Although the names of the tools differ, they used the same subscales. The Fried's frailty phenotype (Courtney-Brooks et al., 2012; Goldstein et al., 2020; Kaneda et al., 2021), Hopkins Frailty Scale (Harland et al., 2020), and Phenotype Model modified in the Japanese version (Kaneda et al., 2021) have originated from Fried's Phenotype of Frailty (Fried, 2001). They use the same subscales and scoring scheme for frailty: weight loss, grip strength measurement, self-reported health status, low mobility, and slow gait. The tools using these domains were used the most in five studies for measuring frailty in patients with cancer before surgery. The next most used tool was the modified Frailty Index (mFI; Korkmaz Toker et al., 2020; Pitts et al., 2019; Vermillion et al., 2017).
| Measurement | Item# (score range) | Cut-off for assessing frailty | ADL, IADL (n = 14) | Functional performance (n = 13) | Self-reported health condition (n = 6) | Comorbidity or medication intake (n = 13) | Cognitive function (n = 12) | Psychological status (n = 5) | Nutrition (n = 9) | Loss of weight (n = 7) | BMI (n = 2) | Risk of fall (n = 5) | Social support (n = 3) | Incontinence (n = 4) | Age (n = 4) | ETC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G8 (Kenig et al., 2020; Nishijima et al., 2021) | 8 (0–17) | ≤14 | o | o | o | o | o | o | o | o | o | o | ||||
| Groningen Frailty Index (Bras et al., 2015; Kenig et al., 2020; Rezende et al., 2022) | 15 (0–15) | >4 | o | o | o | o | o | o | o | o | o | o | ||||
| Edmonton Frail Scale (Nishijima et al., 2021; Richards et al., 2021) | 9 (0–17) | ≥8 | o | o | o | o | o | o | o | o | ||||||
| Balducci Frailty Criteria (Kenig et al., 2020) | 4 (0–4) | ≥1 | o | o | o | o | o | o | o | |||||||
| Modified Frailty Index (mFI)a (Ho et al., 2017) | 10 (0–10) | ≥5 | o | o | o | o | o | Transfer to facility | ||||||||
| mFIb (Korkmaz Toker et al., 2020; Pitts et al., 2019; Vermillion et al., 2017) | 11 (0–1) | >0.27 | o | o | ||||||||||||
| mFIb-5 (Miller et al., 2020; Sastry et al., 2020) | 5 (0–1/0–5) | >0.2/≥3 | o | o | ||||||||||||
| Robinson Frailty Score (Nishijima et al., 2021) | 7 (0–7) | ≥4 | o | o | o | o | o | o | ||||||||
| Triage Risk Screening Tool (Kenig et al., 2020) | 5 (0–5) | >2 | o | o | o | o | o | ED | ||||||||
| Vulnerable Elderly Survey-13 (Kenig et al., 2020; Ommundsen et al., 2014) | 13 (0–13) | ≥3 | o | o | o | o | ||||||||||
| Vulnerable Elderly Survey-13 in combination with clinical criteria (Ommundsen, Wyller, et al., 2018) | 5 (0–5) | ≥1 | o | o | o | o | o | o | o | o | o | |||||
| Fried's frailty phenotype (Courtney-Brooks et al., 2012; Goldstein et al., 2020; Harland et al., 2020; Kaneda et al., 2021; Kenig et al., 2020) | 5 (0–5) | ≥4 | o | o | o | |||||||||||
| Study of Osteoporotic Fractures Frailty Index (Choe et al., 2017) | 3 (0–3) | ≥2 | o | o | o | |||||||||||
| Spinal Tumour Frailty Index (Ahmed et al., 2017) | 9 (0–9) | >3 | o | o | ||||||||||||
| Preoperative Modified Frailty Index (Lu et al., 2018) | 3 (0–3) | ≥3 | o | |||||||||||||
| Abbreviated Comprehensive Geriatric Assessment (Kenig et al., 2020) | 15 (0–15) | GDS >1, ADL >1, IADL >2, MMSE <6 | o | o | ||||||||||||
| The Frailty Scale (Kenig et al., 2020; Rockwood, 2005) | 3 (0–3) | NA | o | o | o | |||||||||||
| Sarcopenia (Chen et al., 2016) | 3 | NA | o | Skeletal muscle mass by CT | ||||||||||||
| Clinical Frailty Scale (Misawa et al., 2020) | By expert evaluation (1–9) | ≥4 | o | o | o | o | ||||||||||
| Geriatric Assessment (Ommundsen et al., 2014) | By expert evaluation | NA | o | o | o | o | ||||||||||
| Johns Hopkins Adjusted Clinical Groups frailty-defining diagnosis indicator (Pitts et al., 2019; Xu et al., 2020) | By calculator program | NA | o | o | o | o | o | o |
- Note: ADL, IADL, results of functional independence measures, including ability to perform activities of daily living, etc.; Functional performance, degree of physical functioning, including grip strength measurements, low mobility, slow gait, or American Society of Anesthesiologists performance scale, etc.; Self-reported health condition, patient-reported health condition; Comorbidity or medication intake, comorbidity and history of diabetes, lung disease, cardiovascular disease or cerebrovascular accident, or medication to treat any of the above conditions; Cognitive function, cognitive impairment; Psychological status, mood assessment including depression, etc.; Nutrition, malnutrition identification indicators calculated through blood tests or physical measurements; Incontinence, urinary or faecal incontinence; risk of fall, assessment of risk of falling, including a history of cerebrovascular accidents with neurological deficits, sensory impairment, etc.
- Abbreviations: ADL, activities of daily living; BMI, body mass index; CT, computed tomography; ED, unplanned use of emergency department in last month; IADL, instrumental activities of daily living; mFIa, modified Frailty Index; mFIb, modified Frailty Index of the American College of Surgeons National Surgical Quality Improvement Program; mFIb-5, modified Frailty Index of the American College of Surgeons National Surgical Quality Improvement Program-5.
Although the mFI-5 (Sastry et al., 2020) and the simplified five-item Frailty Index (sFI; Miller et al., 2020) have different names, they are the same tool derived from the mFI. They were developed by the American College of Surgeons National Surgical Quality Improvement Program (NSQIP; Chimukangara et al., 2017) and encompass the same subscales, which include diabetes history, functional status, obstructive pulmonary disease history, congestive heart failure history, and needs for treatment in the presence or absence of a history of hypertension. By contrast, two tools with the same name—the mFI—had different subscales. One of them is defined by 11 preoperative variables within the NSQIP (Korkmaz Toker et al., 2020; Pitts et al., 2019; Vermillion et al., 2017). Patients were grouped according to the following variables: functional status; diabetes; chronic obstructive pulmonary disease or pneumonia; congestive heart failure; history of myocardial infarction or angina; history of prior percutaneous coronary intervention or previous coronary surgery; hypertension requiring medication; impaired sensorium; peripheral vascular disease or rest pain; history of either transient ischemic attack or cerebrovascular accident; or history of a cerebrovascular accident with neurologic deficit. The other mFI assesses performance on the preoperative American Society of Anesthesiologists Performance Scale (ASA-PS), functional status, dyspnoea, impaired sensorium, weight loss, transfer from care facility (including outside hospital, nursing home, and chronic care facility), prealbumin, creatinine, and haematocrit to identify patients with frailty (Ho et al., 2017).
Additionally, two studies measured frailty based on healthcare providers' judgement of specific criteria such as vitality, mobility, and cognitive impairment (Kenig et al., 2020; Misawa et al., 2020) and one by including other physiological indicators along with a frailty measurement tool (Lu et al., 2018). One measurement was a program that used its own calculation formula to determine frailty status (Pitts et al., 2019; Xu et al., 2020).
The domains of frailty were categorized as functional performance, daily living ability, self-reported health condition, prevalence or maintenance of drug treatment, cognitive function, psychological status, nutritional condition, weight loss, BMI, risk of fall, the status of social support, incontinence, and age. Fourteen out of 24 tools measured physical functional status the most, including ability to perform activities of daily living, functional performance, and self-reported health condition, among the domains. In addition, 13 tools measured frailty based on the prevalence or maintenance of drug treatment. Eleven tools were used to classify frailty based on cognitive function, and nine tools were used to measure nutritional status. Four tools were used to collect information on participants' age. In addition, a subdomain measured musculoskeletal mass using computed tomography (Chen et al., 2016) when referral to another hospital was required or when the emergency room was used in the preceding month (Table 2).
5.3 Association between preoperative frailty and physical conditions
Table 3 describes the preoperative factors significantly related to frailty and the tools used to measure them. Of the 24 studies, only 10 investigated the association between preoperative frailty and physical status in patients with cancer, and 70% of studies reported a significant association between the two variables. Physical status was assessed using the ASA-PS (five studies) and Eastern Cooperative Oncology Group Performance Status (ECOG-PS; two studies). Five of the eight studies reported that frailty and high scores on the ASA-PS were significantly associated with each other (Korkmaz Toker et al., 2020; Misawa et al., 2020; Pitts et al., 2019; Richards et al., 2021; Vermillion et al., 2017). Additionally, two studies showed that high scores on the ECOG-PS were significantly associated with preoperative frailty (Choe et al., 2017; Richards et al., 2021). Three studies did not find a significant relationship between physical status and preoperative frailty. One study used the “Study of Osteoporotic Fractures” to determine whether the postoperative outcome of patients with gastric cancer can be predicted (Choe et al., 2017). One study used the presence of sarcopenia to confirm the relationship between postoperative nutritional status and frailty in patients with gastric cancer (Chen et al., 2016). Another study used the Fried's frailty phenotype to investigate postoperative complications in older patients with gynaecological cancer (Courtney-Brooks et al., 2012).
| Categories | Variable | Measurement of frailty | Number of studies/number of studies with significant association (%) |
|---|---|---|---|
| Physical status | Higher ASA-PS | CFS (Misawa et al., 2020), mFIb (Korkmaz Toker et al., 2020; Pitts et al., 2019; Vermillion et al., 2017), EFS (Richards et al., 2021) | 8/5 (63%) |
| Higher ECOG-PS | SOF (Choe et al., 2017), EFS (Richards et al., 2021) | 2/2 (100%) | |
| Clinical characteristics | Higher BMI | mFIb-5 (Miller et al., 2020), mFIb (Vermillion et al., 2017), Fried's frailty phenotype (Courtney-Brooks et al., 2012) | 9/4 (44%) |
| Lower PNI | CFS (Misawa et al., 2020) | 1/1 (100%) | |
| Non-elective operation | mFIb-5 (Miller et al., 2020), mFIa (Ho et al., 2017) | 3/2 (67%) | |
| Lower CCI | CFS (Misawa et al., 2020) | 1/1 (100%) | |
| Higher cancer stage | SOF (Choe et al., 2017) | 4/1 (25%) | |
| General characteristics | Older age | SOF (Choe et al., 2017), CFS (Misawa et al., 2020), Fried's frailty phenotype (Goldstein et al., 2020), mFIb-5 (Miller et al., 2020), EFS (Richards et al., 2021), ACG (Xu et al., 2020), mFIa (Ho et al., 2017), mFIb (Pitts et al., 2019) | 10/8 (80%) |
| Sex | Women: SOF (Choe et al., 2017), ACG (Xu et al., 2020), Fried's frailty phenotype (Courtney-Brooks et al., 2012) | 9/5 (56%) | |
| Men: mFIb-5 (Miller et al., 2020), mFIb (Vermillion et al., 2017) | |||
| Race | White: ACG (Xu et al., 2020) | 2/2 (100%) | |
| Non-white: mFIb-5 (Miller et al., 2020) | |||
| Current smoking | mFIb-5 (Miller et al., 2020) | 2/1 (50%) | |
| A history of smoking | mFIb (Korkmaz Toker et al., 2020) | 1/1 (100%) |
- Abbreviations: ACG, Johns Hopkins Adjusted Clinical Groups Frailty Index; ASA-PS, American Society of Anesthesiologists Performance Scale; BMI, body mass index; CCI, Charlson Comorbidity Index; CFS, Clinical Frailty Scale; ECOG-PS, Eastern Cooperative Oncology Group Performance status; EFS, Edmonton Frail Scale; mFIa, modified Frailty Index; mFIb, modified Frailty Index of the American College of Surgeons National Surgical Quality Improvement Program; mFIb-5, modified Frailty Index of the American College of Surgeons National Surgical Quality Improvement Program-5; PNI, Prognostic Nutritional Index; SOF, Study of Osteoporotic Fractures Frailty Index.
Some clinical characteristics of patients with cancer were significantly associated with preoperative frailty. Higher BMI (Courtney-Brooks et al., 2012; Miller et al., 2020; Vermillion et al., 2017) and lower prognostic nutritional index (Misawa et al., 2020) were associated with preoperative frailty. There was a significant association between undergoing non-elective surgery and preoperative frailty (Ho et al., 2017; Miller et al., 2020). Additionally, a higher Charlson Comorbidity Index was associated with preoperative frailty (Misawa et al., 2020). Moreover, of the four studies that confirmed a relationship between cancer stage and preoperative frailty, only one reported a significant relationship between advanced cancer and frailty (Choe et al., 2017).
Preoperative frailty was related to patients' demographic characteristics. Age, sex, race, and smoking were significantly related to frailty. Eight studies found a significant relationship between old age and preoperative frailty (Choe et al., 2017; Goldstein et al., 2020; Ho et al., 2017; Miller et al., 2020; Misawa et al., 2020; Pitts et al., 2019; Richards et al., 2021; Xu et al., 2020). Nine studies explored the relationship between sex and preoperative frailty. Among them, three cases reported a significant association among women (Choe et al., 2017; Courtney-Brooks et al., 2012; Xu et al., 2020), and two reported a significant association among men (Miller et al., 2020; Vermillion et al., 2017). Two studies reported a significant relationship between race and preoperative frailty: one among White people (Xu et al., 2020) and one among non-White people (Miller et al., 2020). Smoking-related factors were also significantly associated with preoperative frailty in two studies. One study exploring the relationship between current smoking status and frailty reported a significant relationship (Miller et al., 2020). The other study reported a significant association between current or past smoking history and preoperative frailty (Korkmaz Toker et al., 2020).
5.4 Association between preoperative frailty and postoperative outcomes
Table 4 shows that preoperative frailty was significantly associated with negative treatment outcomes after surgery. The negative treatment outcomes included in this review were increased postoperative complications; higher hospital mortality, length of hospital stay, morbidity, and hospital readmission rates; and lower survival rates. Twenty-five studies explored the relationship between postoperative complications and preoperative frailty, and 16 of them (Ahmed et al., 2017; Chen et al., 2016; Courtney-Brooks et al., 2012; Goldstein et al., 2020; Harland et al., 2020; Ho et al., 2017; Korkmaz Toker et al., 2020; Lu et al., 2018; Miller et al., 2020; Misawa et al., 2020; Nishijima et al., 2021; Ommundsen, Wyller, et al., 2018; Richards et al., 2021; Sastry et al., 2020; Vermillion et al., 2017; Xu et al., 2020) reported a significant relationship. The most common postoperative complications in 11 studies were based on the Clavien–Dindo classification (Chen et al., 2016; Courtney-Brooks et al., 2012; Goldstein et al., 2020; Ho et al., 2017; Korkmaz Toker et al., 2020; Lu et al., 2018; Misawa et al., 2020; Nishijima et al., 2021; Ommundsen, Wyller, et al., 2018; Richards et al., 2021; Vermillion et al., 2017), wherein the more severe the frailty, the higher the Clavien–Dindo classification grade. The Clavien–Dindo classification is a widely used system for grading the severity of surgical complications, ranging from Grade I (least severe) to Grade V (most severe; Clavien et al., 2009). Eight out of 11 studies reported longer hospital stays with increasing preoperative frailty severity (Ahmed et al., 2017; Goldstein et al., 2020; Harland et al., 2020; Korkmaz Toker et al., 2020; Pitts et al., 2019; Richards et al., 2021; Vermillion et al., 2017; Xu et al., 2020). Additionally, eight out of 11 cases showed a higher mortality rate with preoperative frailty (Goldstein et al., 2020; Ho et al., 2017; Kenig et al., 2020; Miller et al., 2020; Pitts et al., 2019; Sastry et al., 2020; Vermillion et al., 2017; Xu et al., 2020). Mortality included events within 30 days of operation, within 1 year after surgery, or more than 5 years after surgery. All three studies reported a statistically significant positive correlation between morbidity and preoperative frailty (Kenig et al., 2020; Miller et al., 2020; Pitts et al., 2019). In four out of six studies, the greater the frailty, the higher the readmission rate (Choe et al., 2017; Pitts et al., 2019; Richards et al., 2021; Sastry et al., 2020). The readmission rate includes readmission within 30 days (Pitts et al., 2019; Sastry et al., 2020) or 1 year (Choe et al., 2017) of surgery, as well as unplanned readmissions after surgery (Richards et al., 2021). Six studies showed that the greater the preoperative frailty, the lower the survival rate. In these studies, a 3-year survival rate (Lu et al., 2018; Misawa et al., 2020), 5-year survival rate (Ommundsen et al., 2014), and recurrence-free survival (Lu et al., 2018) were all significantly associated with the severity of preoperative frailty. Other factors related to preoperative frailty included rate of discharge to another medical facility (Richards et al., 2021; Sastry et al., 2020), rate of home discharge (Harland et al., 2020), length of intensive care stay required after surgery (Richards et al., 2021), and postoperative quality of life (Rønning et al., 2016). Moreover, one study not included in Table 4 reported no significant relationship between preoperative frailty and postoperative complications (Courtney-Brooks et al., 2012). One of the two studies that confirmed the association with perioperative complications using the mFIa also reported a significant relationship (Pitts et al., 2019).
| Treatment outcomes | Subcategory | Measurement of frailty | Number of studies/number of studies with significant association (%) |
|---|---|---|---|
| Postoperative complication | Clavien–Dindo classification | mFIb (Korkmaz Toker et al., 2020; Vermillion et al., 2017), mFIa (Ho et al., 2017), Fried's frailty phenotype (Courtney-Brooks et al., 2012; Goldstein et al., 2020), PMFI (Lu et al., 2018), EFS (Richards et al., 2021), RFS (Nishijima et al., 2021), GA (Ommundsen, Wyller, et al., 2018), CFS (Misawa et al., 2020), Sarcopenia (Chen et al., 2016) | 25/16 (64%) |
| Non-use of classification criteria | Fried's frailty phenotype (Harland et al., 2020), mFIb-5 (Miller et al., 2020; Sastry et al., 2020), ACG (Xu et al., 2020), STFI (Ahmed et al., 2017) | ||
| Mortality | Within 30 days | mFIb (Vermillion et al., 2017), mFIb-5 (Miller et al., 2020; Sastry et al., 2020) | 11/8 (73%) |
| Within 1 year | G8 (Kenig et al., 2020) | ||
| Over 5 years | mFIa (Ho et al., 2017), Fried's frailty phenotype (Goldstein et al., 2020), ACG (Xu et al., 2020), mFIb (Pitts et al., 2019) | ||
| Length of hospital stay | Fried's frailty phenotype (Goldstein et al., 2020; Harland et al., 2020), mFIb (Korkmaz Toker et al., 2020; Pitts et al., 2019; Vermillion et al., 2017), EFS (Richards et al., 2021), ACG (Xu et al., 2020), STFI (Ahmed et al., 2017) | 11/8 (73%) | |
| Morbidity | Within 30 days | G8 (Kenig et al., 2020), mFIb (Pitts et al., 2019) | 4/4 (100%) |
| Within 1 year | G8 (Kenig et al., 2020) | ||
| Overall | mFIb-5 (Miller et al., 2020) | ||
| Readmission | Within 30 days | mFIb (Pitts et al., 2019), mFIb-5 (Sastry et al., 2020) | 6/4 (67%) |
| Within 1 year | SOF (Choe et al., 2017) | ||
| Unplanned | EFS (Richards et al., 2021) | ||
| Survival | Recurrence-free survival | PMFI (Lu et al., 2018) | 6/4 (67%) |
| Within 3 years | CFS (Misawa et al., 2020), PMFI (Lu et al., 2018) | ||
| Within 5 years | GA (Ommundsen et al., 2014) | ||
| Discharge to another medical facility | EFS (Richards et al., 2021), mFIb-5 (Sastry et al., 2020) | 5/2 (40%) | |
| Home discharge | Fried's frailty phenotype (Harland et al., 2020) | 1/1 (100%) | |
| Intensive care after operation | EFS (Richards et al., 2021) | 1/1 (100%) | |
| Quality of life | GA (Rønning et al., 2016) | 1/1 (100%) |
- Abbreviations: ACG, Johns Hopkins Adjusted Clinical Groups Frailty Index; CFS, Clinical Frailty Scale; EFS, Edmonton Frail Scale; GA, geriatric assessment; mFIa, modified Frailty Index; mFIb, modified Frailty Index of the American College of Surgeons National Surgical Quality Improvement Program; mFIb-5, modified Frailty Index of the American College of Surgeons National Surgical Quality Improvement Program-5; PMFI, Preoperative Modified Frailty Index; RFS, Robinson Frailty Score; SOF, Study of Osteoporotic Fractures Frailty Index; STFI, Spinal Tumour Frailty Index.
6 DISCUSSION
This scoping review demonstrated the research trends on preoperative frailty in patients with cancer. About half of the included studies were conducted in 2020 and 2021. Frailty was significantly associated with several patient demographic and clinical characteristics, including the level of physical performance and nutritional condition before surgery. Further, preoperative frailty status was significantly related to postoperative treatment outcomes. In particular, preoperative frailty was significantly associated with postoperative complications, length of hospital stay, and mortality rate. Many tools were used to measure frailty in these studies. However, no tool was prominently used to measure preoperative frailty in patients with cancer.
The cumulative deficit model of frailty suggests that accumulating medical, social, and functional deficits over an individual's lifetime leads to nonspecific, age-related vulnerability or frailty (Robinson et al., 2015). In this review, physical status indicators (ASA-PS, ECOG-PS) and age were identified as factors associated with preoperative frailty in patients with cancer. For factors other than physical status indicators and age, a significant relationship with preoperative frailty was reported in less than 50% of the studies, or the opposite result was seen. In addition, among the physical status indicators, the ASA-PS was most frequently reported to be significantly related to preoperative frailty. Most of the studies including the ASA-PS were retrospective studies, suggesting that interpretations should be made considering the limitations of a retrospective study using an already written electronic medical record. The ASA-PS is a traditional preoperative screening tool (Davenport et al., 2006) that has been used for several decades to determine a patient's suitability for surgery. As the ASA-PS is an essential electronic health record element before surgery for the past years, we inferred that it could confirm the relationship with frailty more often than other physical status indicators. This suggests that more studies on the predictors of preoperative frailty in patients with cancer must be conducted based on the factors identified in previous studies.
Twenty-four tools were used to measure frailty in the included studies. Although the names of the tools differed, the Fried's frailty phenotype (Fried, 2001) was the most used. This tool is an early measure of frailty and focuses on identifying diminishing physical abilities alone. The frailty assessment tools developed later include functional aspects, including the maintenance of daily living ability and symptoms of geriatric syndromes (e.g. depression, cognitive impairment, incontinence, and risk of fall), social support, and comorbidities. Frailty is often diagnosed by integrating the findings of experienced healthcare providers or from other physiological indicators rather than a single assessment tool. Among the 24 tools, functional performance, comorbidity or medication intake, cognitive function, and nutrition condition were the most frequently studied domains to determine frailty. This can support the trend of the broadening concept of frailty. Frailty does not only simply imply weakness because of ageing but is a dynamic condition physically or psychosocial or a combination of both factors. It can be improved or worsened over time. Our results support the argument that the physical activity capacity and psychosocial status are also important (Fried, 2001).
However, as the importance of screening preoperative frailty in clinical practice has been emphasized, simple measurement tools that include subjective symptoms have been developed to screen patients for frailty within a short period (Kenig et al., 2020). A recently developed tool to measure frailty includes age, mobility, cognitive ability, psychosocial functioning, nutrition, and the presence of a disease (Nishijima et al., 2021). However, given the scarcity of studies using the recently developed tool, determining its suitability is difficult. Further, tools with identical measurement items but with different names (Fried's frailty phenotype, Hopkins Frailty Scale, Phenotype Model modified in the Japanese version; mFI-5, sFI), as well as those with the same name but different measurement items (mFI) exist. This reflects the difficulty in developing a tool to measure frailty within a brief period. Therefore, future studies on frailty must select a tool suitable for the research topic by confirming the tool domains in detail. This also indicates the need for studies evaluating the reliability and accuracy of tools in diverse populations to determine the feasibility of frailty screening.
Outcome indicators related to preoperative frailty can be divided into short-term outcomes within 30 days of surgery and long-term outcomes beyond 30 days of surgery (Myles, 2020). These findings support the idea that frailty in patients with cancer who have undergone surgery is associated with adverse treatment outcomes such as poor survival rates and postoperative complications (Ethun et al., 2017). This supports a previous finding that preoperative frailty screening has a positive effect on postoperative outcomes and economic burden (Lin et al., 2016). This also implies that frailty can have fatal effects on patients with cancer after surgery. Preoperative frailty affects the long-term outcomes of patients with cancer. Accordingly, preoperative frailty in patients with cancer is a predictor of negative outcomes related to the quality of life and postoperative survival rate. This study confirmed that frailty is related to the health-related quality of life, consistent with previous studies (Williams et al., 2019) and one study (Richards et al., 2021) included in this review. However, Courtney-Brooks et al. (2012) found that the degree of frailty did not make a significant difference in postoperative outcomes such as complications. This suggests that further research is needed to determine the appropriateness of the current threshold level for robust, pre-frail, and frail conditions. In view of the several national and international efforts to systematically describe and manage key indicators of long-term care services (e.g. Description and Evaluation of Services and Directories in Europe for Long Term Care; Mencía Ruiz et al., 2011), there is also a need for a systematic strategy for the standardized description and classification of frailty in patients with cancer.
While our review revealed a predominant use of quantitative designs in the included studies, the proportion of RCTs was surprisingly low, accounting for only 4.2% of studies. RCTs are widely regarded as the gold standard for evaluating the effectiveness of interventions (Gray et al., 2016) and provide robust evidence for clinical decision-making. Given the significant impact of preoperative frailty on outcomes in patients with cancer, there is a clear need to prioritize and facilitate more RCTs and intervention trials in this population. The limited presence of RCTs in the current literature highlights the need for further research that not only identifies factors associated with frailty but also investigates and evaluates interventions aimed at mitigating the adverse effects of frailty.
In this review, BMI had a significant relationship with preoperative frailty in patients with cancer. Scores on the ASA-PS, which measures physical status, also had a significant relationship with frailty. Nutritional imbalance and muscle loss can be predicted by BMI (Cederholm et al., 2015). As the body's energy levels decrease, the frequency and intensity of physical activity could also decrease and affect ASA-PS scores. Frailty causes nutritional imbalance and musculoskeletal system decline from ageing-related cellular and physiological changes, leading to reduced body energy levels (Fried, 2001; Morley et al., 2013; Rockwood, 2005). These results suggest the need for an intervention study to improve the nutritional status of patients with cancer.
This scoping review has several limitations. First, the included studies were retrieved from four major health databases over a 10-year period. Articles in databases other than these four, as well as other grey literature, have been overlooked, as well as studies from 10 years ago. Therefore, the generalizability of the findings is limited. Second, the search strategy was limited to studies published in English within the last decade. Although clear inclusion and exclusion criteria were established, the aforementioned limitations could have introduced a bias. Despite these limitations, our findings are significant in that we identified factors affecting preoperative frailty in patients with cancer and focused on the domains of screening frailty measurements.
Appropriate preoperative frailty assessment is important to identify patients more vulnerable to postoperative complications and improve treatment outcomes. The present review could be used to conduct more studies on the appropriate time point to measure preoperative frailty status and develop interventions for improving frailty status. In addition, follow-up studies on the effects of preoperative frailty on psychosocial and physical outcomes after surgery are needed.
7 CONCLUSION
Preoperative frailty in patients with cancer is related to not only their physical condition but also their physical activity capacity and psychosocial status, similar to other populations with frailty. Moreover, preoperative frailty in patients with cancer can result in poor treatment outcomes and negatively affect quality of life. Although several tools have been used to assess frailty, none has emerged as the dominant choice, including consensus on a simple initial screening tool that can detect preoperative frailty in patients with cancer. Further research on frailty in patients with cancer through a prospective study of the influencing factors, outcomes, and tools is necessary.
AUTHOR CONTRIBUTIONS
Misun Jeon: Conceptualization (lead), Methodology (lead), Data Curation (lead), Formal Analysis (lead), Writing–Original Draft (lead), Writing–Review and Editing (lead). Sanghee Kim: Conceptualization (equal), Supervision (lead), Methodology (supporting), Resources (lead), Writing–Review and Editing (equal). Sang Hwa Lee and Ji Yoon Jang: Conceptualization (supporting), Methodology (supporting), Data Curation (supporting), Formal Analysis (supporting), Writing–Original Draft (supporting), Writing–Review and Editing (supporting).
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No.2020 R1A6A1A03041989). Misun Jeon received a scholarship from the Brain Korea 21 FOUR Project funded by the National Research Foundation of Korea, Yonsei University College of Nursing. No funding was received for this study. All data collection and analysis fees were paid by the authors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available upon request to the corresponding author.




