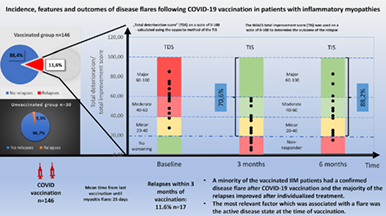
Incidence, features, and outcome of disease relapse after COVID-19 vaccination in patients with idiopathic inflammatory myopathies
Melinda Nagy-Vincze and Tibor Béldi contributed equally.
Abstract
Introduction/Aims
Vaccination against coronavirus disease 2019 (COVID-19) is relatively safe in patients with idiopathic inflammatory myopathies (IIM); however, myositis flares following vaccination have been poorly studied. We aimed to evaluate the frequency, features, and outcomes of disease relapses in patients with IIM following COVID-19 vaccination.
Methods
A cohort of 176 IIM patients were interviewed after the third wave of the COVID-19 pandemic and followed prospectively. Relapses were determined using the disease state criteria and the outcome of the flares with myositis response criteria, calculating the total improvement score (TIS).
Results
A total of 146 (82.9%) patients received a vaccination, 17/146 (11.6%) patients had a relapse within 3 mo, and 13/146 (8.9%) patients within 1 mo. The relapse rate of unvaccinated patients was 3.3%. Three months after the post-vaccination relapses, 70.6% of the patients (12/17) achieved an improvement of disease activity (average TIS score: 30 ± 15.81; seven minor, five moderate, and zero major improvements). Six months after flares improvement was detected in 15/17(88.2%) of relapsed patients (average TIS score: 43.1 ± 19.53; 3 minimal, 8 moderate, and 4 major). Forward stepwise logistic regression analysis revealed that the active state of myositis at the time of injection (p < .0001; odds ratio, 33; confidence interval, 9–120) was significantly associated with the occurrence of a relapse.
Discussion
A minority of the vaccinated IIM patients had a confirmed disease flare after COVID-19 vaccination and the majority of the relapses improved after individualized treatment. An active disease state at the time of vaccination probably contributes to the increased risk of a post vaccination myositis flare.
Graphical Abstract
Abbreviations
-
- ACA
-
- anti-centromere antibodies
-
- ACR
-
- American College of Rheumatology
-
- ADE
-
- adverse drug event
-
- ANA
-
- antinuclear antibodies
-
- anti-EJ
-
- anti-aminoacyl-transfer-ribonucleic-acid synthetase antibody
-
- anti-HMGCR
-
- anti-3-hydroxy-3-methylglutaryl coenzyme A reductase
-
- anti-Jo1
-
- anti-histidyl-transfer ribonucleic acid synthetase
-
- anti-SSA
-
- anti–Sjögren's-syndrome-related antigen A
-
- anti-TIF1 gamma
-
- transcription intermediary factor 1-gamma
-
- B2GPI
-
- anti-beta-2-glycoprotein I
-
- CI
-
- confidence interval
-
- CK
-
- creatine kinase
-
- COVAD study
-
- COVID-19 Vaccination in Autoimmune Diseases
-
- COVAX
-
- COVID-19 Vaccines Global Access
-
- COVID 19
-
- coronavirus disease 2019
-
- CSA
-
- cyclosporine-A
-
- CSM
-
- Core Set Measures
-
- DM
-
- dermatomyositis
-
- DMARD
-
- disease-modifying antirheumatic drugs
-
- dsDNA
-
- anti-double-stranded deoxyribonucleic acid antibodies
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- EMG
-
- electromyogram
-
- ENA
-
- extractable nuclear antigen
-
- EULAR
-
- European Alliance of Associations for Rheumatology
-
- HAQ
-
- Health Assessment Questionnaire
-
- HRCT
-
- high-resolution computed tomography
-
- IIM
-
- idiopathic inflammatory myopathies
-
- ILD
-
- interstitial lung disease
-
- IMACS
-
- International Myositis Assessment and Clinical Studies Group
-
- IRD
-
- inflammatory rheumatic diseases
-
- IVIG
-
- intravenous immunoglobulin therapy
-
- LDH
-
- lactate dehydrogenase
-
- MDA5
-
- anti-melanoma differentiation-associated gene 5
-
- MDAAT
-
- Myositis Disease Activity Assessment Tools
-
- MMF
-
- mycophenolate mofetil
-
- MMT-8
-
- Manual Muscle Test
-
- OR
-
- odds ration
-
- PM
-
- polymyositis
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- TDS
-
- total deterioration score
-
- TIS
-
- total improvement score
-
- VAS
-
- visual analogue scale
1 INTRODUCTION
Based on an increasing amount of evidence,1 the recent European Alliance of Associations for Rheumatology (EULAR) recommendations support the proposal that patients with rheumatic musculoskeletal diseases should be strongly advised to receive a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination with any of the vaccines approved in their country.2 However, despite the significant morbidity and mortality associated with a SARS-CoV-2 infection, studies have shown that many patients with inflammatory rheumatic diseases (IRD) remain hesitant about getting vaccinated due to the increased risk of a disease flare.3 Fortunately, there is increasing evidence in the literature that a coronavirus disease 2019 (COVID-19) vaccination does not increase the risk of disease relapses of autoimmune diseases.4-11 Nevertheless, some of these reports were based on patient surveys, which were completed by self-selecting participants whose responses were not validated by physicians. Moreover, the patient populations of these large cohorts are heterogeneous, so the different autoimmune diseases might show different results.
Idiopathic inflammatory myopathies (IIM) are chronic immune mediated systemic disorders affecting the proximal muscles and frequently multiple extramuscular organs, such as lungs, joints and skin. Due to the rarity and heterogeneity of the disease, studies about vaccination related side effects and disease relapses for large sample sizes are scarce. There are several case reports in the literature demonstrating occurrence of newly diagnosed myositis after COVID-19 vaccination,12-15 but a direct causal relationship was not established. In a recent study, fully vaccinated dermatomyositis (DM) patients were more likely to report worsening skin symptoms after receiving the vaccine (22.7%) than fully vaccinated lupus erythematosus patients (8.6%).16 Recently, a case series of seven anti-melanoma differentiation-associated gene 5 (anti-MDA5) positive DM was reported a few days after a COVID-19 vaccination, raising the possibility of a relationship between vaccination and pathogenesis of MDA5 DM.17 However, retrospective and epidemiological studies failed to ascertain an association between myositis and vaccines, ie, no significant increase in the incidence of myositis was reported after large vaccination campaigns18; consequently, the importance of vaccination during a worldwide pandemic has been emphasized.
The relationship between vaccination and myositis is poorly understood and data regarding the incidence, severity and outcome of disease flares in IIM patients is needed. Therefore the aims of this study were (1) to determine the frequency, outcome, and features of myositis relapses after COVID vaccination in a Hungarian tertiary myositis center; and (2) to identify relevant factors associated with an increased risk of relapse.
2 METHODS
2.1 Patients
In this prospective cohort study, IIM patients under the care of the Department of Clinical Immunology, University of Debrecen, Hungary were interviewed at the end of the third wave of the COVID-19 pandemic (May–June 2021). The vaccination of the patients started March 1, 2021. At the time of survey every patient had been given the opportunity to receive the vaccines. The vaccination rate, the type of administered vaccines, and the adverse reactions experienced were assessed by questionnaires. These patients were followed prospectively at the clinic or in outpatient departments until July 2022. A loss of follow-up was avoided by conducting telephone interviews with all patients at the end of the study. IIM disease activity, comorbidities, COVID-19 infection data, later vaccination data (booster vaccination started in August 2021), immunosuppressive therapy, and laboratory results were also recorded.
All patients had a definite or probable diagnosis of IIM (muscle weakness, high muscle enzyme levels, plus abnormal electromyogram (EMG) with myopathic motor unit potentials, fibrillation potentials, positive sharp waves, increased insertional activity, and/or muscle biopsy features of inflammatory infiltration in polymyositis (PM) or typical skin symptoms in DM) according to Bohan and Peter,19 or probable/definite IIM according to the EULAR/American College of Rheumatology's (ACR) myositis criteria.20 Patients with inclusion body myositis were excluded. Interstitial lung disease (ILD) was defined as present by radiographic findings (high-resolution computed tomography [HRCT]) and pulmonary function tests (spirometry, diffusing capacity for carbon monoxide). Dysphagia was diagnosed by barium radiography of the esophagus. Informed consent was obtained from the subjects. This study was carried out in compliance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of the University of Debrecen (Ethical permission number: DE RKEB/IKEB-5723-2021).
2.2 Evaluation of myositis disease status
Disease activity was evaluated using the International Myositis Assessment and Clinical Studies Group (IMACS) core set measures (CSM)21: the physician global activity visual analogue scale (VAS), the Manual Muscle Test (MMT-8), patient global activity VAS, a health assessment questionnaire (HAQ), laboratory values of muscle enzymes (CK, creatine kinase; LDH, lactate dehydrogenase), and Myositis Disease Activity Assessment Tools (MDAAT) were assessed at each visit. Active disease at/prior vaccination was defined by physician global activity ≥2 cm of VAS.
Myositis relapse was defined as any of the following: (1) physician-assessed global worsening by ≥2 cm on a 10-cm VAS and worsening on MMT-8 by ≥20%; OR (2) extramuscular organ disease activity worsening by ≥2 cm on a 10-cm VAS; OR (3) any three of six IMACS CSM worsening by ≥30%.21, 22
The criteria for improvement used the six IMACS CSM, combining the absolute percentage change in each with varying weights to obtain a total improvement score (TIS) on a scale of 0–100 using a Web-calculator.23 Different thresholds of improvement were set for minimal (20–39 points), moderate (40–59 points) and major responses (≥60 points).24
The severity of myositis relapse was graded as minimal, moderate and major. This was determined by a total deterioration score (TDS), which was calculated using the opposite method of the TIS calculation, that is, the absolute percentage worsening change in each IMACS CSM was scored and the sum of the six scores determined the TDS. The relapse category was minimal, moderate, or major based on TDS 20–39, 40–59, or ≥60, respectively.
2.3 Laboratory tests
Routine laboratory markers such as erythrocyte sedimentation rate, total blood count, and chemistry including renal and liver function, ion levels and muscle enzymes (CK, LDH) were tested. Immunological analyses included tests for the following autoantibodies: antinuclear antibodies (ANA), anti-centromere antibodies (ACA), anti-histone antibodies, and anti-cytoplasmic antibodies were determined by indirect immunofluorescence on HEp-2 cells (Viro-Immun Labor-Diagnostika GmbH, Oberursel, Germany); ANA positivity was assessed at 1:40 dilution. Titers of the antibodies against extractable nuclear antigen (ENA) complex, anti-SS-A (Ro) and anti-Jo-1 antibodies, were measured (HYCOR Biomedical Inc., CA, USA) using this latter method. Myositis specific antibodies were detected by membrane-fixed line blots according to the manufacturer's instructions (Euroline Myositis Antigen Profile4, EuroImmun, Lübeck, Germany). Anti-HMGCR antibodies were detected by indirect immunofluorescence assay and confirmed by in-house enzyme-linked immunosorbent assay (ELISA; the cutoff value was ≥40 U/mL). ELISA was used for the measurement of anti-double-stranded deoxyribonucleic acid (dsDNA), anti-beta-2-glycoprotein I (B2GPI), anti-cardiolipin, and antiphospholipid antibodies (ORGENTEC Diagnostica GmbH, Mainz, Germany). These commercially available methods were used following the manufacturer's protocol.
2.4 Determination of COVID-19 infection
COVID-19 disease diagnosis was made by (1) positive rapid antigen test and/ or positive polymerase chain reaction testing performed with oral/nasopharyngeal swabs and/or (2) anti-N and anti-S protein antibody positivity without previous vaccination.
2.5 Statistical analysis
The statistical analysis was performed using the SPSS version 29 (IBM, Armonk, NY). The normality of distributions in the case of continuous variables was tested using the Shapiro–Wilk test. Normally distributed continuous variables were described by mean and SD values. Categorical variables were described using frequencies (case numbers) and percentages. To compare several matched groups, the Friedman test was used since data were not normally distributed (Shapiro–Wilk test: p > .05). Two related scale variables were compared, either by the paired t-test, or the Wilcoxon test, depending on the distribution. The contingency tables presenting the connection of two categorical variables were analyzed by Fisher's exact test. To identify the significant independent predictors of the relapse of myositis, forward stepwise (likelihood ratio) logistic regression analysis was applied, using the following candidate variables: age, duration of the disease, gender, disease subtype and state (PM/DM, activity at the time of vaccination), autoantibody profile, comorbidities (chronic obstructive pulmonary disease, ILD, diabetes mellitus, hypertension, ischemic heart disease, asthma), medications (glucocorticoids, methotrexate, leflunomide, cyclosporine-A (CSA), azathioprine, mycophenolate mofetil (MMF), intravenous immunoglobulin, hydroxychloroquine, cyclophosphamide, rituximab), and vaccination features (number of vaccination, manufacturers). We also calculated the odds ratios (ORs) for the predictors found. A p value less than .05 was regarded as statistically significant.
3 RESULTS
3.1 Demographic data, vaccinations, and relapse incidence
One hundred and eighty-one patients were contacted, 176 of whom finally participated in the study. The epidemiologic data of the interviewed study population are summarized in Table 1. By May 1, 2022, 82.9% (146/176) of the patients had received an anti-COVID-19 vaccination, 67% (99/146) of whom were given the Pfizer-Biontech type (BNT162b2). One hundred and thirty nine patients received both the first and the second dose, while seven patients received only one vaccination. No major immediate adverse events, such as hospitalization or anaphylaxis were reported. 11.6% (17/146) of patients who were vaccinated experienced a relapse (based on the IMACS criteria) within 3 mo of the vaccination. The general characteristics of the unvaccinated population (n = 30) showed distinct similarities and differences in comparison with the vaccinated group (Table 1). We found that the relapse rate of the unvaccinated patients was 3.3% during the follow-up period, which was not different in comparison with the relapse rate of the vaccinated group (Fisher: p = .32; OR, 3.82; confidence interval [CI], 0.49–29.9). However, due to small sample size a significant effect in either direction cannot be confidently excluded.
| Variable | Study population (n = 176) | Study population with vaccination (n = 146) | Study population without vaccination (n = 30) | Fisher test | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | CI | n (%) | CI | p | |||
| PM subset | 106 (60%) | 82 (56%) | 48 | 65 | 24 (80%) | 64 | 96 | .015 |
| DM subset | 70 (40%) | 64 (44%) | 35 | 52 | 6 (20%) | 4 | 36 | |
| Mean age ± SD (y) | 57.6 ± 13.7 | 58.6 ± 13.7 | 53 ± 13.3 | |||||
| Female | 117 (66.5%) | 106 (73%) | 65 | 80 | 11 (36%) | 18 | 56 | <.001 |
| Male | 59 (34%) | 40 (27%) | 20 | 35 | 19 (63%) | 44 | 82 | |
| Average disease duration (y) | 12.9 | 12.9 | 12 | |||||
| ILD frequency | 37 (21%) | 32 (22%) | 15 | 29 | 5 (17%) | 2 | 32 | >.1 |
| Relapse rate | 18 (10%) | 17 (12%) | 6 | 17 | 1 (3%) | 0 | 11 | >.1 |
| Autoantibodies | ||||||||
| Any MSA | 77 (44%) | 57 (39%) | 31 | 47 | 20 (67%) | 48 | 85 | .008 |
| Anti-Jo1 | 26 (15%) | 24 (16%) | 10 | 23 | 2 (7%) | 0 | 17 | >.1 |
| Anti-Mi2 | 10 (6%) | 6 (4%) | 1 | 8 | 4 (13%) | 0 | 27 | .069 |
| Anti-SRP | 7 (4%) | 2 (1%) | 0 | 4 | 5 (17%) | 2 | 32 | .002 |
| Anti-HMGCR | 4 (2.2% | 4 (3%) | 0 | 6 | 0 | 0 | 2 | >.1 |
| Anti-NXP2 | 3 (2%) | 3 (2%) | 0 | 5 | 0 | 0 | 2 | >.1 |
| Anti-MDA5 | 4 (2%) | 3 (2%) | 0 | 5 | 1 (3%) | 0 | 11 | >.1 |
| Anti-TIF1gamma | 8 (5%) | 5 (3%) | 0 | 7 | 3 (10%) | 0 | 22 | >.1 |
| Anti-SAE | 8 (5%) | 6 (4%) | 0 | 8 | 2 (7%) | 9 | 44 | <.001 |
| Anti-PL7 | 3 (2%) | 2 (1%) | 0 | 4 | 1 (3%) | 0 | 11 | >.1 |
| Anti-PL12 | 2 (1%) | 2 (1%) | 0 | 4 | 0 | 0 | 2 | >.1 |
| Anti_EJ | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Anti-OJ | 2 (1%) | 0 | 0 | 0 | 2 (7%) | 0 | 17 | .028 |
| Other (Anti-Ro52, anti-Pm-scl, anti-Ku, etc) | 43 (24%) | 35 (24%) | 17 | 31 | 8 (27%) | 9 | 44 | >.1 |
| Vaccination | ||||||||
| BNT162b2 (Pfizer/BioNTech) | 99 (56%) | 99 (68%) | ||||||
| mRNA-1273 (Moderna) | 14 (8%) | 14 (10%) | ||||||
| ChAdOx1-S (AstraZeneca) | 17 (10%) | 17 (12%) | ||||||
| Sinopharm | 13 (7%) | 13 (9%) | ||||||
| Sputnik | 2 (1%) | 2 (1%) | ||||||
| Janssen | 1 (1%) | 1 (1%) | ||||||
- Note: CIs are continuity-corrected.
3.2 Features and outcomes of post-vaccination disease relapses
The major characteristics of the relapses are shown in Table 2. No patient died during their relapse, but two patients needed hospitalization, with one patient requiring intensive care because of breathing insufficiency. The average time from last vaccination to the patient reporting a worsening of symptoms was 25 days. Thirteen patients (8.9%) experienced a disease worsening within 1 mo of the last vaccination. The severity of the relapses was minimal in three (17.6%), moderate in six (35.3%), and major in eight (47.1%) patients. The CK levels were significantly different during follow-up (p = .022). In 3/17 cases, new auto-antibodies were detected after vaccination. One patient had high titer anti-aminoacyl-transfer-ribonucleic-acid synthetase antibody (anti-EJ) positivity, and two patients had an occurrence of anti-SSA antibody as a second autoantibody in addition to anti-Jo1 and anti-TIF1 gamma respectively.
| Factor | Results |
|---|---|
| Mean age at the time of study ± SD (y; min–max) | 59.05 ± 12.96 (32–78) |
| Gender: F/M | 13/4 (76.5%/25.5%) |
| IIM subset: PM/DM | 8/9 (47%/53%) |
| Myositis disease duration ± SD (y; min–max) | 11.82 ± 9.31 (2–31) |
| ILD presence: n (%) | 8 (47%) |
| MSA presence: n (%) | 13 (76.5%) |
| Time of worsening ± SD (days after vaccination) | 24.76 ± 25.44 (4–90) |
| BNT162b2 vaccine: n (%) | 14 (82.35%) |
| Active disease at the time of vaccination: n (%) | 12 (70%) |
| TDS: points ± SD (min–max) | 56.91 ± 15.29 (27.5–85) |
| At least minimal improvement 3 mo after relapses: n (%) | 12 (70.6%) |
| TIS 3 mo after relapse ± SD (min–max) | 30 ± 15.81 (5–57.5) |
| At least minimal improvement 6 mo after relapses: n (%) | 15 (88.2%) |
| TIS 6 mo after relapse ± SD (min–max) | 43.1 ± 19.53 (15–82.5) |
| Mean CK (U/L) before vaccination ± SD (min–max) | 336.94 ± 604.73 (33–2191) |
| Mean CK (U/L) at relapse diagnosis ± SD (min–max) | 929.29 ± 1807.84 (27–6395) |
| Mean CK (U/L) 3 mo after relapse ± SD (min–max) | 308.94 ± 773.61 (16–3273) |
| Mean CK (U/L) 6 mo after relapse ± SD (min–max) | 261.88 ± 397.57 (30–1590) |
- Note: Main clinical data of patients with IIM relapse within 3 mo after vaccination.
- Abbreviations: F, female; IIM M, male; MSA, myositis specific antibody.
After the relapses, all of the patients required different individual therapeutic interventions, such as corticosteroids, an increase in dose of a disease modifying antirheumatic drug (DMARD), administration of a new DMARD, intravenous immunoglobulin (IVIG), treatment with a biological agent or a combination of the above. The details of each relapses (symptoms, treatment schedule, scores, etc.) are presented in Table S1. Three months after the relapses, 70.6% of the patients achieved at least a minimal improvement of disease activity based on the TIS calculation (seven minor, five moderate, and zero major improvements), whereas 29.4% did not respond to the treatment. Six months after the relapses improvement was detected in 88.2% of patients (three minimal, eight moderate, and four major improvements), while in two patients disease activity did not decrease despite the treatment. One patient who did not achieve at least a minimal improvement after the treatment escalation had a mild relapse, while another patient did not respond to a change in DMARD therapy.
3.3 Breakthrough COVID infections
In the current study, we could detect a confirmed COVID-19 breakthrough infection after vaccination in 18 patients (12.3%), 17 of whom (94.4%) had a mild course of infection without hospitalization. The mean duration between vaccination and infection was 22 wk (range 2–38 wk). One of our vaccinated patients with cancer associated myositis (colorectal carcinoma with multiple liver and pulmonary metastases) without post vaccination seroconversion (undetectable antibodies against spike protein) died because of tumor progression and terminal COVID-19 infection. The COVID infection rate in the follow-up period was 20% (6/30) of the unvaccinated group. All fatal COVID-19 infections in the whole study group (n = 3) occurred only in patients whose serum antibodies against spike protein were undetectable, regardless of whether or not they had been vaccinated.
3.4 Relevant factors that could contribute to post vaccination IIM relapses
When comparing each variable individually between the patients with and without relapse, several parameters were significantly different in the two populations: vaccination status, active disease at vaccination, the number of vaccinations, double vaccination (first and second dose), BNT162b2 vaccination, the presence of any myositis specific antibodies, ILD, and treatment with methylprednisolone, MMF, and CSA. However, forward stepwise logistic regression analysis revealed that only two factors were significantly associated independently with the occurrence of relapse: the active state of myositis at time of injection and the use of the BNT162b2 vaccine (Table 3). When restricting the logistic regression analysis to those patients who were not infected with COVID (eliminating the confounding effect of COVID-19 infection), in addition to active myositis at vaccination, a diagnosis of DM was associated with relapse in logistic regression analysis. The wide CI for the OR of relapse for DM showed that our limited data set cannot confirm or exclude an effect of vaccination on DM relapse. By ordinal regression analysis, we could not find any factor associated significantly with the severity of relapse.
| Factor | p-Value | OR | CI | p if removed | |
|---|---|---|---|---|---|
| All patients | |||||
| Active state of myositis | <.0001 | 33 | 9 | 120 | <.0001 |
| BNT162b2 vaccination | .040 | 4.62 | 1.07 | 19.94 | .026 |
| Patients without COVID infection | |||||
| Active state of myositis | <.0001 | 122 | 14 | 1098 | <.0001 |
| Type: DM versus PM | .054 | 8.57 | 0.96 | 76.39 | .021 |
4 DISCUSSION
We can summarize our recent work as follows: (1) a minority of the vaccinated patients experienced a confirmed disease flare of IIM after a COVID-19 vaccination, (2) the majority of these relapses were easily treatable, and (3) the most relevant factor which was associated with a flare was the active disease state at the time of vaccination.
The approved COVID-19 vaccines have shown clear safety and efficacy in the reduction of severe SARS-CoV-2 disease. Recently, a multicenter Italian study focusing on post vaccination relapses (defined almost identically to our study) revealed that the flare rate was 6.1% within 1 mo of vaccination and the development of a flare was mostly influenced by the number of organs involved.25 No flares occurred after the third dose of a vaccination. Our relapse rate was higher in comparison with the Italian data, but we assessed the flares within 3 mo of a COVID-19 vaccination. We decided to extend the assessment time to 3 mo because the majority of the patients had a regular visit more than 1 mo after vaccination and the disease status assessment required multiple physical and laboratory examinations. Restricting our relapse rate to those patients whose symptoms worsened within 1 mo of administration of the last vaccine, the post vaccination relapse rate was 8.9% (13/146) in our cohort, which is quite similar to the Italian results. Furthermore, the relapse rate in the unvaccinated patients (3.3%) was comparable to the vaccinated patients, but due to small sample size a significant effect of vaccination in either direction cannot be confidently determined.
Importantly, no firm causal conclusions regarding vaccination and the development of flares can be drawn from this dataset. The results of our study are similar to COVAX registry data,7 where the percentage of post vaccination flares was slightly higher in patients with moderate/high disease activity compared with patients in remission/low disease activity. Our study results are in line with the previous EULAR recommendation that vaccination in patients with autoimmune rheumatic diseases should preferably be administered during quiescent disease.26 The second factor that was significantly associated with disease relapse, was the use of the BNT162b2 vaccine. However, the availability of the vaccines, the higher proportion of patients vaccinated with the BNT162b2 vaccine and the lower confidence level might limit comparisons between different vaccine types.
We determined the relapse rate based on IMACS defined criteria21, 22 and during the follow-up we used the myositis response criteria24 to define the outcome of the flare. The advantage of this method in contrast to patient reported surveys is that it requires laboratory parameters, physical examination, a physician global opinion, and the patient's opinion simultaneously. Our study showed that myositis response criteria are useful and easy to use to achieve objective, comparable, and reproducible measurements of a patients' disease activity in everyday clinical practice.
Another interesting issue regarding myositis flare after vaccination is the severity of the relapse. We strongly believe that there would be significant relevance in developing criteria for a definition of disease relapse severity, or at least, differentiating them as minor or major, since this likely would lead to different treatment recommendations. We tried to grade the deterioration of the disease using the opposite method of calculation to that used in the myositis improvement criteria (TDS vs. TIS), but the consequences deriving from these calculations should be interpreted carefully. The threshold determination and exact calculation of disease deterioration to assess a mild, or a major relapse requires standardized development with the consensus of an international group of myositis experts. We could not detect any factors that were associated significantly with the severity of relapse or hospitalization, but this could be due to the low number of patients with relapses.
The breakthrough COVID infections were rare and milder than in unvaccinated patients and all fatal COVID infections occurred in patients without any vaccination, or without post vaccination seroconversion. These facts argue strongly for considering the administration of SARS-CoV-2 vaccination and booster vaccinations, if no anti-spike antibody is detected.
The possible limitations of this study should be acknowledged. This work was a single center study from a national myositis unit in Hungary, some of the data were self-reported and the number of participants (especially the unvaccinated group) in the study was relatively low.
In conclusion, the rate of flares after COVID vaccination was low and based on myositis response criteria, the majority of the patients responded well to individualized treatment. Active disease state at vaccination might be an important factor in the development of post vaccination relapse. Clinicians should promote vaccination in most patients with idiopathic inflammatory myopathies. Vaccination during the active disease state should be carefully considered, and patients should be monitored frequently. Further evaluation of larger multi-center cohorts with graded outcome results are required to assess more detailed features of flares after COVID-19 vaccinations.
AUTHOR CONTRIBUTIONS
Melinda Nagy-Vincze: Data curation; investigation; project administration; writing – original draft; writing – review and editing. Tibor Béldi: Data curation; project administration; writing – original draft; writing – review and editing. Katalin Szabó: Data curation. Anett Vincze: Data curation. Balázs Miltényi-Szabó: Data curation. Zsófia Varga: Data curation. Jozsef Varga: Methodology; writing – review and editing. Zoltan Dr. Griger: Conceptualization; data curation; investigation; project administration; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
This article is dedicated to the memory of Dr. Katalin Dankó for her dedication and contribution to the field of myositis. The authors thank George Seel for the language editing and proof reading of the manuscript.
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.