Risdiplam in non-sitter patients aged 16 years and older with 5q spinal muscular atrophy
Abstract
Introduction/Aims
Risdiplam has been approved for the treatment of patients with 5q spinal muscular atrophy (SMA), but data from type 2 non-sitter patients are lacking. In this study we describe our experience regarding the use of risdiplam in a series of type 2 non-sitter patients.
Methods
Type 2 SMA patients over 16 years of age were administered risdiplam through the expanded access program (NCT04256265). Patients were followed-up with a battery of scales and clinical measures.
Results
Six non-sitter patients (17 to 46 years old) were treated with risdiplam. One patient reported mild adverse events (dyspepsia and headache). After 1 year of treatment, all patients showed clinically meaningful improvements in at least one scale and none of them showed any clinically meaningful deterioration. Two patients showed a clinically meaningful increase in body mass index (>5%) and two others scored higher on the Revised Upper Limb Module (>2 points). Moreover, five patients had clinically meaningful improvements on the Egen Klassifikation 2 scale (>2 points), including the motor (axial and upper limbs), bulbar (speech and swallowing), and respiratory (coughing) domains. Four subjects achieved at least one of the goals set with the Goal Attainment Scale (GAS).
Discussion
This series suggests that risdiplam is safe and may be effective in non-sitter SMA patients older than 16 years of age. In these patients, functional scales and the GAS would be more sensitive than motor scales to detect changes, because they include axial, bulbar, and respiratory domains. Larger studies are needed to confirm these results.
Abbreviations
-
- AE
-
- adverse event
-
- ALSFRS-R
-
- Amyotrophic Lateral Sclerosis Functional Rating Scale—Revised
-
- BMI
-
- body mass index
-
- C-GIC
-
- clinical impression of change
-
- EAP
-
- expanded access program
-
- EK2
-
- Egen Klassifikation 2 scale
-
- FVC%
-
- percent predicted forced vital capacity
-
- GAS
-
- Goal Attainment Scale
-
- NIV
-
- noninvasive ventilation
-
- PEG
-
- percutaneous endoscopic gastrostomy
-
- P-GIC
-
- patients’ global impression of change
-
- RULM
-
- Revised Upper Limb Module
-
- SMA
-
- spinal muscular atrophy
-
- SMN
-
- survival of motor neuron
1 INTRODUCTION
Risdiplam is an oral medication approved for the treatment of patients with spinal muscular atrophy (SMA) types 1, 2, and 3 in the United States and Europe.1 It modifies SMN2 pre-mRNA splicing to promote the inclusion of exon 7 and increases the production of functional SMN protein.2 Its approval for type 2 and 3 patients was based on the results of the SUNFISH study part 2, which showed efficacy in nonambulant patients, including a small subgroup of adults.3 However, non-sitters were excluded from that study.3
There are other approved treatments for SMA patients, including onasemnogene abeparvovec and nusinersen. The first is an SMN1 gene replacement therapy that uses a nonreplicating adeno-associated virus capsid (scAAV9) to deliver wild-type the SMN1 gene to motor neurons and is mainly indicated for use in patients under 2 years of age. The second is an antisense oligonucleotide administered intrathecally, but its use in non-sitter patients is often challenging due to their complex spines.4
Consequently, the efficacy of disease-modifying therapies in non-sitter patients is not well known and, despite their poor prognosis, access to such treatments may be limited in some countries.4
The aim of this report is to describe our experience regarding the use of risdiplam in a series of non-sitter patients over 16 years of age.
2 METHODS
Six type 2 patients over the age of 16, who were consecutively evaluated in Hospital la Fe (Valencia, Spain) between October and December 2020 and fulfilling the inclusion and exclusion criteria of the expanded access program (EAP; NCT04256265), were given risdiplam in a single daily dose of 0.25 mg/kg for those under 20 kg and 5 mg for those over 20 kg.3 All patients had achieved the motor milestone of sitting without support but had subsequently lost this ability.
Four of these patients had contraindications to intrathecal administration of nusinersen due to severe scoliosis or vertebral fusion with extensive instrumentation. The other two had been treated previously with nusinersen. For these two patients, risdiplam was started at least 4 months after the last nusinersen dose.
For this study, only non-sitter patients (not able to maintain the seated position for 5 seconds, with support of one or both upper limbs) were considered.
The following outcome measures were prospectively recorded at baseline and after 6 and 12 months of treatment: body mass index (BMI), percent predicted forced vital capacity (FVC%), the Revised Upper Limb Module (RULM),5 the Egen Klassification (EK2),6 and the Amyotrophic Lateral Sclerosis Functional Rating Scale—Revised (ALSFRS-R).7 Moreover, those measurements at 12 months before treatment were retrospectively recorded. Clinically meaningful changes for each scale were defined as suggested in an earlier work8 (≥2 points for RULM, EK2, and ALSFRS-R) and, based on our experience and previous literature, changes of greater than 5% in BMI and FVC were also considered meaningful. Three personalized functional goals were established at the baseline visit (Table 1) after discussion with each patient, and re-evaluated after 12 months with the Global Attainment Scale (GAS).9 The use of noninvasive ventilation (NIV), cough assist or percutaneous endoscopic gastrostomy (PEG), the patient's global impression of change (P-GIC), and the clinical impression of global change (C-GIC)10 were also recorded at the 12-month visit. Safety was evaluated through the monitoring and recording of adverse events and laboratory assessments at 1, 6, and 12 months.
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age of loss of sitting ability (years) | 9 | 4 | 12 | 12 | 3 | 3 |
| Sex | F | M | M | F | M | F |
| Age (years) | 44 | 20 | 17 | 38 | 33 | 46 |
| SMN2 copynumber | 3 | 3 | 3 | 1 | 3 | 4 |
| FVC (%) | NA | NA | NA | 16 | 18 | 34.4 |
| Ventilatory support | NIV | NIV | NIV | NIV | No | No |
| BMI (kg/m2) | 8 | 6.6 | 16.6 | 17.9 | 17.4 | 26.3 |
| Nutritional sSupport | Oral | Oral | No | Oral | No | No |
| Previous SMA treatment | No | Nusinersen (9 doses) | No | Nusinersen (3 doses) | No | No |
| RULM EI | 0 | 3 | 3 | 1 | 1 | 1 |
| RULM score | 0 | 10 | 8 | 0 | 1 | 0 |
| EK2 | 45 | 28 | 22 | 37 | 30 | 29 |
| ALSFRS – R | 11 | 17 | 23 | 13 | 24 | 21 |
| Goals (GAS) | 1. Improve swallowing 2. Talk without shortness of breath 3. Use cutlery alone |
1. Improve head control 2. Eating and drinking in sitting position 3. Less shortness of breath |
1. Eating alone 2. Improve typing 3. Improve head control |
1. Drink independently (with straw) 2. Reduce fatigue 3. Improve typing |
1. Eating alone 2. Improve speech 3. Improve nutrition |
1. Avoid NIV initiation 2. Reduce fatigue 3. Improve “pincer grip” |
- Abbreviations: ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale---Revised; BMI, body mass index; EI, entry item; EK2, Egen Klassifikation 2; F, female; FVC%, percent predicted forced vital capacity; GAS, Goal Attainment Scale; M, male; NA, not available; NIV, noninvasive ventilation; RULM, Revised Upper Limb Module; SMA, spinal muscular atrophy; SMN2, survival motor neuron 2 gene.
Excel 365 (Microsoft Corp, Redmond, WA) was used to calculate the mean and standard deviation for both the EK2 and ALSFRS-R.
All patients provided informed consent before the start of risdiplam. Data collection and processing were approved by the ethics committee for biomedical research of the Hospital la Fe.
3 RESULTS
Six non-sitter SMA patients over 16 years of age started treatment with risdiplam. Their baseline demographics and clinical characteristics are summarized in Table 1 and Table S1. Briefly, five patients showed a low BMI (<18.5), four patients used NIV routinely, four patients showed only minimal residual mobility in the upper limbs (RULM entry item ≤1), and two patients had been treated previously with nusinersen (one was discontinued due to the lack of lumbar access and the other due to the lack of efficacy).
Regarding respiratory function, there were no changes in use of ventilatory support, nor were there clinically meaningful changes in FVC%, albeit a valid FVC could not be obtained in half of patients (Table 2). Nevertheless, three patients reported an improvement in cough ability on the EK2, resulting in a reduced need for cough assist. At the nutritional level, three patients reported improved swallowing in functional scale scores, with two of whom showing clinically meaningful improvements in BMI (Table 2). Of note, in one of these patients, PEG had been proposed due to sustained weight loss in previous visits but was no longer recommended after the improvement with risdiplam.
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| ΔBMI (%) | −2.5 | 12.1 | 0 | −0.6 | 9.8 | −1.1 |
| ΔFVC% | NA | NA | NA | 2 | −2 | 1.5 |
| ΔRULM score | 0 | 1.5 | 3.5 | 3 | 0 | 0 |
| ΔEK2 | 6 | 3 | 1 | 7 | 4 | 3 |
| ΔALSFRS-R | 7 | 4 | 1 | 9 | 1 | 1 |
| C-GIC | 1 | 1 | 1 | 1 | 1 | 1 |
| P-GIC | 1 | 1 | 0 | 1 | 2 | 1 |
| GAS | 0 | 5.9 | 0 | 22.8 | 18.3 | 20.4 |
- Note: Bold indicates clinically meaningful result.
- Abbreviations: ALSFRS-R, revised version of the amyotrophic lateral sclerosis functional rating scale; BMI, body mass index; C-GIC, clinical global impression of change; EK2, Egen Klassifikation 2; FVC%, percent predicted forced vital capacity; GAS, Goal Attainment Scale; NA, not available; P-GIC, patient global impression of change; RULM, Revised Upper Limb Module.
Three patients scored 0 for their baseline RULM assessment. Nonetheless, two patients showed clinically meaningful improvements, while the others remained stable or improved mildly (Table 2). Conversely, no floor effect was found on the EK2 or ALSFRS-R. Moreover, five patients showed clinically meaningful improvements in either their motor (head control and distal upper limbs), bulbar (speech and swallowing), or respiratory (coughing) domains (Table 1 and Figure 1).
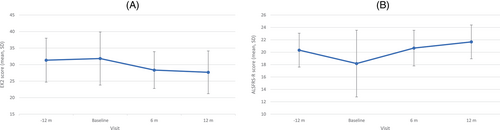
Overall, all patients showed clinically meaningful improvements on at least one outcome measure, but none showed clinically meaningful deterioration in any measure. Table S2 details the results of the scales evaluated pre- and posttreatment.
Accordingly, the C-GIC showed mild improvements for all patients. One the P-GIC, four patients reported mild improvements, with one showing a moderate improvement and another having no change in global condition. Finally, on the GAS, two patients reported some improvement but did not achieve any of their goals set at the baseline visit, whereas the other four achieved at least one goal (Table 2). Table S3 details the goals established by each patient for the GAS.
One patient reported mild AEs (gastrointestinal symptoms and headache) and discontinued the medication after 10.5 months of treatment. However, she had a functional deterioration after withdrawal and restarted treatment at 12 months without any new AEs, yet with recurrence of her previous mild AEs. No clinically relevant laboratory changes were found in any of the patients.
4 DISCUSSION
In this study, risdiplam was well tolerated and there were clinically meaningful improvements for all patients in at least one domain, also suggesting its efficacy.
The safety of risdiplam has been recently reported in two EAP-based studies, which included some patients not considered in the clinical trials.11, 12 However, data on risdiplam efficacy were not reported.
SMA is a progressive disease in type 2 non-sitter patients, as shown in natural history studies.13-15 Therefore, any real improvement (or even long-term stability) in such patients could be considered clinically meaningful, because improvement is unexpected in the natural history of the disease. To make sure that these changes were not due to reliability problems,16 we used conservative thresholds of improvement for each measure based on previous studies. Moreover, most patients showed clinically meaningful changes in at least two of the outcome measures, including some fine motor (head control, distal upper limbs, fatigability) and bulbar (speech and swallowing) or respiratory (coughing) domains, which are the most relevant issues for non-sitter patients. As suggested in an earlier study,17 these changes were better captured with functional scales as compared with RULM or FVC%. Indeed, these domains are either difficult to measure or not measurable at all with most motor scales, which are currently used as the “gold standard” outcome measures in both clinical trials and clinical practice4, 17 because they usually focus on gross motor function. Specifically, in our series, half of the patients could not perform or showed floor effects with both RULM and FVC, which are probably the most widely used measures in non-sitters.4, 17 This explains the disparities found in this study between functional scale scores and RULM and FVC%. Conversely, better agreement was found between changes in functional scales, GAS, and BMI. For example, body-weight gains in patients with low BMI were accompanied by improvements in the swallowing and eating domain scores of the EK2 and ALSFRS-R. Moreover, in line with previous studies,17 our results show that functional scales (specifically EK2) and the GAS are helpful for setting personalized goals and measuring individual responses to treatment in non-sitter patients over 16 years of age because they can detect subtle but meaningful changes in relevant aspects of the daily life. Hence, regulatory agencies increasingly recommend the use of scales assessing function in activities of daily living in both clinical trials and practice. The goals set here before treatment show that finger movements, head control, swallowing, breathing, and fatigue are the most important issues for non-sitter patients. The scarce use of scales measuring these items explains the reduced access of non-sitter adults to clinical trials and the lack of efficacy data from clinical practice, resulting in limited access to disease-modifying treatments.18 Despite all patients reporting some improvement, no patient reached all goals set at baseline. This could reflect overambitious expectations.19 Nevertheless, 1 year is probably insufficient time to attain full response to treatment, as seen with nusinersen,20 and thus it may still be possible for these patients to reach their previously established goals.
This study has limitations related to the small size of the patient sample and the lack of a control group. Despite the limitations, our results suggest that risdiplam is both safe and well tolerated and may be effective in non-sitter SMA patients over 16 years of age. Moreover, the data support the usefulness of functional scales and GAS for measuring changes in these patients. Larger studies are warranted to confirm our findings.
AUTHOR CONTRIBUTIONS
Nancy Carolina Ñungo Garzón: Data curation; formal analysis; writing – original draft; writing – review and editing. Inmaculada Pitarch Castellano: Writing – review and editing. Teresa Sevilla: Writing – review and editing. Juan Francisco Vázquez-Costa: Conceptualization; data curation; formal analysis; methodology; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
The authors thank Fernando Mora and M. Carmen Baviera for their role in assessment of the patients. We also thank the SMA patients for their participation. The Centro de Investigación Biomédica en Red de Enfermedades Raras is an initiative from the Instituto de Salud Carlos III (ISCIII). T.S. and J.F.V.-C. are members of the European Reference Network for Rare Neuromuscular Diseases. The sponsors of this work did not participate in the study design, data acquisition, analysis, data interpretation, or writing of the article.
FUNDING INFORMATION
CUIDAME (PIC188-18); Instituto de Salud Carlos III (JR19/00030, principal investigator: J.F.V.-C); Generalitat Valenciana (EMERGENTES/ CIGE 2021/055).
CONFLICT OF INTEREST STATEMENT
J.F.V.-C. served on advisory boards and received travel and speaker honoraria from Biogen and Roche. I.P.C. served on advisory boards for Avexis and Biogen, received travel and speaker honoraria from Biogen and Roche, and is principal investigator for an ongoing Biogen clinical trial. The remaining authors have no disclosures.
ETHICAL PUBLICATION STATEMENT
All authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
JFVC and NCÑG had full access to the database population used to create the study population. All data supporting our findings are available on reasonable request.