Characterization of patients with Becker muscular dystrophy by histology, magnetic resonance imaging, function, and strength assessments
Funding information: Italfarmaco; Regione Lombardia
Abstract
Introduction/Aims
Becker muscular dystrophy (BMD) is characterized by variable disease severity and progression, prompting the identification of biomarkers for clinical trials. We used data from an ongoing phase II study to provide a comprehensive characterization of a cohort of patients with BMD, and to assess correlations between histological and magnetic resonance imaging (MRI) markers with muscle function and strength.
Methods
Eligible patients were ambulatory males with BMD, aged 18 to 65 years (200 to 450 meters on 6-minute walk test). The following data were obtained: function test results, strength, fat-fraction quantification using chemical shift-encoded MRI (whole thigh and quadriceps), and fibrosis and muscle fiber area (MFA) of the brachial biceps.
Results
Of 70 patients screened, 51 entered the study. There was substantial heterogeneity between patients in muscle morphology (histology and MRI), with high fat replacement. Total fibrosis correlated significantly and mostly moderately with all functional endpoints, including both upper arm strength assessments (left and right elbow flexion rho −.574 and −.588, respectively [both P < .0001]), as did MRI fat fraction (whole thigh and quadriceps), for example, with four-stair-climb velocity −.554 and −.550, respectively (both P < .0001). Total fibrosis correlated significantly and moderately with both MRI fat fraction assessments (.500 [P = .0003] and .423 [.0024], respectively).
Discussion
In this BMD cohort, micro- and macroscopic morphological muscle parameters correlated moderately with each other and with functional parameters, potentially supporting the use of MRI fat fraction and histology as surrogate outcome measures in patients with BMD, although additional research is required to validate this.
Abbreviations
-
- 6MWT
-
- 6-minute walk test
-
- BMD
-
- Becker muscular dystrophy
-
- CINRG
-
- Cooperative International Neuromuscular Research Group
-
- DMD
-
- Duchenne muscular dystrophy
-
- LOESS
-
- locally estimated scatterplot smoothing
-
- MFA
-
- muscle fiber area
-
- MFM
-
- Motor Function Measure
-
- MRI
-
- magnetic resonance imaging
-
- SSRI
-
- selective serotonin reuptake inhibitor
1 INTRODUCTION
Becker muscular dystrophy (BMD) is a heterogeneous condition, with substantial clinical variability in age at onset and rate of progression.1 Histologically, BMD involves active myonecrosis and regeneration in the early stages; later in the disease course chronic myopathic changes are more likely, including skeletal muscle fiber size variability and fibrosis.2 BMD and Duchenne muscular dystrophy (DMD) are caused by mutations in the dystrophin gene, with full-length dystrophin absent in DMD, whereas reduced levels of truncated protein are present in BMD. BMD is much rarer than DMD,3 and less is known about the micro- and macroscopic involvement in various muscles in patients with BMD, with information particularly limited on histological markers.
Assessments, including the 6-minute walk test (6MWT), are often used to evaluate functional impairment in BMD and DMD.2 Because these assessments are effort-dependent, they can be challenging to incorporate in clinical trials without introducing excessive variability in study outcomes. Magnetic resonance imaging (MRI) allows objective assessment of fat replacement in skeletal muscle, and is therefore being explored as a potential surrogate endpoint in clinical trials in BMD and DMD.2 In DMD, muscle fat fraction has been shown to predict future function and clinical milestones, which is an important consideration in qualification of the technique as a surrogate endpoint.4-6
There are no treatments specifically approved for BMD, with current therapies aimed at control of symptoms. The efficacy and safety of givinostat, a histone deacetylase pan-inhibitor, is being investigated in patients with BMD in an ongoing phase II study. We used baseline data from this study to describe the clinical, MRI, and histopathological features of a cohort of patients with BMD.
2 METHODS
This phase II, randomized, double-blind, placebo-controlled study aims to evaluate the effects of givinostat on micro- and macroscopic muscle morphology and function. The primary endpoint is the mean change from baseline in total percent of fibrosis after 12 months, using data from muscle biopsies. Baseline data from this study were used for the analyses reported herein.
Eligible patients were men aged 18 to 65 years, inclusive, with a diagnosis of BMD confirmed by genetic testing (based on patient records), who were able to walk between 200 and 450 meters on a 6MWT, and who, if receiving a systemic corticosteroid, angiotensin- converting enzyme inhibitor, and/or α- or β-adrenergic receptor blocker, were expecting no significant change in dose or dosing regimen immediately before the start of study treatment. Among the reasons for exclusion were: use of any pharmacologic treatment in the 3 months before study entry, other than corticosteroids, or surgery that may have an effect on muscle strength or function; symptomatic cardiomyopathy or heart failure (New York Heart Association class III or IV) or left ventricular ejection fraction <50% at screening or with heart transplant; and contraindications to muscle biopsy or MRI. All patients provided written informed consent before any study-related procedure. Full inclusion and exclusion criteria are listed in the Supporting Information online. This study was approved by an independent ethics committee at each institution, and was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization notes for guidance on Good Clinical Practice (ICH/CPMP/135/95). The study is registered at ClinicalTrials.gov (NCT03238235).
At the screening visit, patients undertook a series of timed function tests (rise from floor, run/walk 10 meters, and climb four standard steps) and a 6MWT.7 They then completed the Motor Function Measure (MFM) test,8-10 followed by bilateral strength measures (knee extension and elbow flexion) using hand-held myometry (microFET Dynamometer; Hoggan Scientific LLC, Salt Lake City, Utah). All functional and strength assessments were evaluated by qualified functional evaluators (all of whom were physiotherapists), with the timed function tests and 6MWT standardized by a study-specific manual.
At one or more subsequent visits during the screening period, open muscle biopsies of the brachial biceps and MRI of the lower limbs were performed. Muscle biopsy samples (three per patient, each approximately 0.5 cm3) were immediately frozen in liquid nitrogen–cooled 2-methylbutane and then stored in liquid nitrogen. Serial transverse 8-μm-thick cryosections were processed for routine staining with hematoxylin-eosin. On each section, four randomly selected, nonoverlapping fields were photographed at 20× magnification, using an optical microscope (Leica DC200) equipped with a camera and IM50 image analysis software (Leica Microsystems, Wetzlar, Germany). Each muscle biopsy was evaluated in a blinded manner by two operators. All morphometric analyses were performed using ImageJ 1.51j8 (https://imagej.nih.gov/ij/download.html) and LAS 4.9.0 (Leica Application Suite) software, consistent with the methods of Peverelli and colleagues.11
MRI scans were acquired in a 1.5T (at the center in Milan, Italy; Philips Achieva, Phillips Healthcare, Amsterdam, Netherlands) or 3T (at the center in Leiden, The Netherlands; Ingenia Philips, Philips Healthcare) whole body magnet. Chemical-shift encoded MRI can be accurately measured across field strengths,12 and fat and copper sulfate phantoms scanned regularly in the current study confirmed that measured fat fractions were not field-strength-dependent. To generate fat fraction maps, multi-echo axial gradient echo images were acquired in the thigh (TR = 430 milliseconds or greater; TE = 4.61, 6.91, 9.21 milliseconds [1.5T], 4.61, 5.76, and 6.91 milliseconds [3T]). Slices were chosen to include the origin of the short head of the biceps femoris, which served as the landmark. All MRI data were processed at a central reading laboratory (University of Florida, Gainesville, Florida). Fat fraction maps were generated, and the borders of the quadriceps group (vastus lateralis, vastus medialis, vastus intermedius, rectus femoris), hamstrings group (biceps femoris, semitendinosus, semimembranosus), and medial compartment of the thigh (adductor magnus, adductor longus, gracilis, sartorius) were manually traced on three contiguous slices (landmark and two distal slices) using custom-written software with mean values reported for each muscle.13
2.1 Outcomes
Outcomes from the timed function tests included the time to climb four standard steps and the velocity achieved, the time to rise from the floor and the velocity achieved, and the time to walk/run 10 meters and the velocity achieved (see Appendix S1 for more details). In addition to the 6MWT at the screening visit, patients completed a 6MWT at the randomization visit, with the mean of the results from these two visits used for the current analyses. For the MFM, data from both elbows were used, but we only included data from the right knee, as the MRI evaluation was performed on the right limb (see Appendix S1 for more details).
Chemical shift–encoded MRI was used to measure fat fraction in the whole thigh and in the quadriceps. Fat fraction for each compartment was calculated as the average of all pixels on the three slices, and whole-thigh fat fraction was calculated as the average fat fraction of the quadriceps, hamstrings, and medial thigh compartments. The percentages of muscle fiber area (MFA), fibrosis, adipose tissue, other histological structures (necrotic cells, vessels, and any other nonconnective tissue present in the microscopic field) and fiber with nuclear centralization were determined from biopsy of the brachial biceps, using the mean of available fields.11 Patients were randomized on entry to the study, such that half had their right arm biopsied at baseline with the left arm to be biopsied at the end of the study, and the other half to have their left arm biopsied on baseline and their right arm at the study end.
2.2 Sample size and statistical methods
All patients recruited into the study were included in the current analyses, which are not formally powered. Correlations were assessed using Spearman correlation coefficients, with P < .05 considered statistically significant. Strong or very strong correlations were considered to be those with coefficients of .70 to 1.00, moderate correlations .40 to .69, and weak .10 to .39.14 To control for the false discovery rate, P values considered statistically significant were adjusted for multiple comparisons by applying the correction method of Benjamini-Hochberg, assuming a false discovery rate of .05. Locally estimated scatterplot smoothing (LOESS) best-fit lines were also derived for the correlation figures to illustrate the direction of the correlations.
3 RESULTS
3.1 Participants
The participants were recruited and are being studied in two centers: Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (Italy), and Leiden University Medical Center (The Netherlands). Of the 70 patients screened, 51 entered the study, with 17 not meeting the inclusion/exclusion criteria, and 2 withdrawing consent.
Table 1 summarizes the patients' baseline characteristics. Deletion was the most prevalent mutation type, with two thirds of patients having an exon 45-x deletion. Only three (5.9%) patients were receiving concomitant steroids, all on an “every-other-day” regimen. There was substantial heterogeneity in the muscle morphology parameters, both when assessed using histology and using MRI (Table 1). Examples of brachial muscle biopsy images from three participants are provided in Appendix S1. Figure S1 clearly illustrates the marked variability between patients. Similarly, MRI images of the thigh from three individuals illustrate the variability in fat replacement both within a muscle and between individuals (Figure S2 in Appendix S1).
| Parameter | Overall (n = 51) |
|---|---|
| Age (years), mean (SD), range | 37.4 (10.99), 19-61 |
| Time since genetic diagnosis (years), mean (SD), range | 13.0 (8.46), 0.1-27.7 |
| Mutation type, n (%) | |
| Deletion | 48 (94.1) |
| Duplication | 1 (2.0) |
| Point mutation | 2 (3.9) |
| Mutated exon category, n (%) | |
| Del 45-x | 34 (66.7) |
| Other | 17 (33.3) |
| Total fibrosis (%), mean (SD), rangea | 25.4 (11.86), 9.72-56.63 |
| MFA (%), mean (SD), rangea | 72.0 (14.82), 27.81-90.28 |
| Total fibers,b mean (SD), rangea | 98.9 (29.74), 43-178 |
| MRI fat fraction (%), mean (SD), range | |
| Whole thigh | 59.0 (13.32), 23-81 |
| Quadriceps | 57.0 (15.31), 16-79 |
| MFM, mean (SD), range | |
| D1 dimension | 48.9 (12.66), 25.6-87.2 |
| D2 dimension | 98.0 (3.43), 83.3-100.0 |
| D3 dimension | 98.5 (2.78), 90.5-100.0 |
| Total score | 78.1 (6.17), 66.7-94.8 |
| 6-minute walk test (m), mean (SD), rangec | 355.0 (70.00), 229-469 |
| Time to walk 10 m (s), mean (SD), range | 9.1 (4.90), 5.2-38.3 |
| 10 m walk/run velocity (m/s), mean (SD), range | 1.2 (0.35), 0.26-1.91 |
| Concomitant medication, n (%) | |
| Agents acting on the renin-angiotensin system | 19 (37.3) |
| Beta-blocking agents | 7 (13.7) |
| Steroids | 3 (5.9) |
| Deflazacortd | 2 (3.9) |
| Prednisoned | 1 (2.0) |
| Creatine | 2 (3.9) |
| Carnitine, levocarnitine, or ubidecarenone | 6 (11.8) |
| SSRIs | 3 (5.9) |
| Etizolam or olanzapine | 2 (3.9) |
- Abbreviations: MFA, muscle fiber area; MRI, magnetic resonance imaging; SSRI, selective serotonin reuptake inhibitor.
- a Data available from 49 patients (disease too advanced in 2 patients for evaluation).
- b Data are the mean of the total fibers evaluated for each subject.
- c Data are the mean of the tests at the screening and baseline tests.
- d All three patients receiving steroids at baseline were on an “every-other-day” steroid regimen.
3.2 Correlation analyses
The correlation analyses are shown in Table 2. Total fibrosis in the biceps brachialis assessed by muscle biopsy showed statistically significant, moderate negative correlations with the two upper arm strength assessments (left and right elbow flexion), with MFA showing significant, moderate positive correlations with these two endpoints (individual patient data for total fibrosis vs right elbow flexion are shown in Figure 1A).
| Age (years) vs | Total fibrosis (%) vs | MFA (%) vs | MRI fat fraction in whole thigh (%) vs | MRI fat fraction in quadriceps (%) vs | ||
|---|---|---|---|---|---|---|
| Histology parameters (from arm biopsy) | Total fibrosis (%) | 0.094;* 0.5187 | --- | −.979a; <.0001* | .500a; .0003* | .423a; .0024* |
| MFA (%) | −.135a; .3552 | −.979a; <.0001* | --- | −.496a; .0003* | −.444a; .0014* | |
| Adipose tissue (%) | .045 a; .7574 | .542 a; <.0001* |
−.627a; <.0001* | .424a; .0024* | .449 a; .0012* | |
| Other histological structures (%) | .148a; .3103 | .219a; .1302 | −.316a; .0270* | .033a; .8214 | .024a; .8710 | |
| Fiber with nuclear centralizations (%) | .143a; .3285 | −.123a; .4001 | .171a; .2409 | .116a; .4265 | .150a; .3024 | |
| Functional and strength endpoints | Distance walked after 6 min (m) | −.159; .2656 | −.490a; .0003* | .503a; .0002* | −.451; .0009* | −.440; .0012* |
| Time to climb four steps (s) | .032b; .8285 | .493c; .0005* | −.481c; .0007* | .535b; <.0001* |
.598b; <.0001* | |
| Four-stair-climb velocity (steps/s) | −.060d; .6782 | −.547a; <.0001* | .530a; .0001* | −.554d; <.0001* | −.550d; <.0001* | |
| Time to rise from floor (s) | −.160e; .3174 | .380e; .0156* | −.384e; .0143* | .378e; .0147* | .420e; .0062* | |
| Rise from floor velocity (1/s) | −.018; .9026 | −.499a; .0003* | .521a; .0001* | −.586; <.0001* | −.559; <.0001* | |
| Time to walk 10 m (s) | .260; .0653 | .548a; <.0001* | −.566a; <.0001* | .571; <.0001* | .543; <.0001* |
|
| 10-m walk/run velocity (steps/s) | −.260; .0653 | −.548a; <.0001* | .566a; <.0001* | −.571; <.0001* | −.543; <.0001* | |
| MFM D1 | −.161; .2584 | −.594a; <.0001* | .608a; <.0001* | −.664; <.0001* | −.595; <.0001* | |
| MFM D2 | −.201; .1580 | −.311a; .0297* | .349a; .0139* | −.369; .0078* | −.283; .0445 | |
| MFM D3 | .143; .3169 | −.445a; .0014* | .477a; .0005* | −.318; .0229* | −.410; .0028* | |
| MFM total score | −.139; .3290 | −.597a; <.0001* | .623a; <.0001* | −.647; <.0001* | −.593; <.0001* | |
| Right knee extension | .091a; .5342 | −.596f; <.0001* | .584f; <.0001* | −.438a; .0016* | −.419;a .0027* | |
| Left elbow flexion | .316b; .0285* | −.574b; <.0001* | .542b; <.0001* | −.311b; .0317* | −.240b; .1001 | |
| Right elbow flexion | .225a; .1195 | −.588a; <.0001* | .566a; <.0001* | −.340a; .0167* | −.400a; .0044* |
- Notes: Data expressed as rho; P value determined from Spearman correlation. Data available from 51 patients, except for an=49, bn=48, cn=46, dn=50, en=41, and fn=47.
- Abbreviations: MFA, muscle fiber area; MFM, Motor Function Measurement (D1, standing and transfers; D2, axial and proximal motor function; D3, distal motor function); MRI, magnetic resonance imaging.
- Note: *P value considered statistically significant with application of the correction method of Benjamini-Hochberg, assuming a false discovery rate of .05.
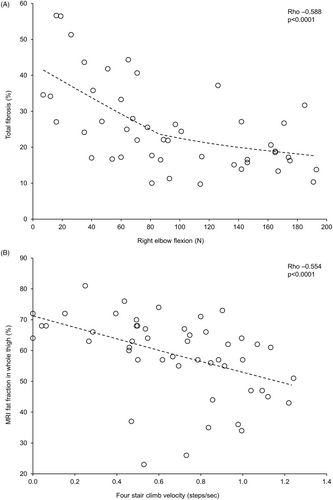
Both MRI fat fraction endpoints had significant, moderate positive correlations with all time-based functional endpoints, and significant, mostly moderate negative correlations with velocity-based functional endpoints (the MRI whole thigh fat fraction vs four-stair-climb velocity patient data are shown in Figure 1B). There were also significant, moderate negative correlations between the two MRI endpoints and right knee extension, and between the quadriceps endpoint and right elbow flexion.
There were significant, moderate positive correlations between total fibrosis (from arm biopsy) and the two leg MRI fat fraction assessments (the total fibrosis vs MRI whole thigh fat fraction data are shown in Appendix S1, Figure S3). Similarly, there were significant, moderate negative correlations between MFA and the MRI fat fraction assessments. Total fibrosis and MFA (from arm biopsy) correlated significantly and mostly moderately with all of the leg-based functional and strength endpoints (individual patient data for total fibrosis vs four-stair-climb velocity are shown in Appendix S1, Figure S4). Age, in years, correlated significantly only with left elbow flexion, with the correlation being weak.
4 DISCUSSION
Overall, the fat fractions in the MRI whole thigh and quadriceps of the patients recruited into this clinical trial were high, with the mean values exceeding 50%. Despite this significant fat replacement, the population was relatively functional, not only in terms of the 6MWT (as required by the inclusion criteria), but also the full set of functional and strength tests.5 The Cooperative International Neuromuscular Research Group (CINRG) presented the baseline characteristics of 83 adults and children recruited into the BMD Natural History Study, which focuses on the demographics, dystrophin gene deletions, and function.15 Although the CINRG study obviously recruited a broader population than the current study, the two studies clearly confirm the heterogeneity of BMD.
We found a range of statistically significant correlations between the histology parameters and the arm strength, functional and strength endpoints. These correlations were generally moderate, perhaps reflecting the heterogeneity of BMD---with the example figures clearly illustrating the high between-patient variability of the various endpoints.
There were also significant moderate correlations between the two MRI fat fractions in the whole thigh and quadriceps and most leg-based functional and strength endpoints. Indeed, previous studies in patients with DMD have shown a relationship between MRI fat fraction and time to loss of ambulation,4 and functional performance,5 with MRI fat fraction increasing with age.5, 6 A number of studies used MRI imaging to evaluate muscle involvement in BMD.16-26 Two of those had findings consistent with those of the current study, in that skeletal muscle MRI fat fractions correlated with motor function.16, 26 Furthermore, in one of the studies, the MRI fat fraction changes helped to predict functional deterioration.16 Although only limited longitudinal data are available on MRI fat fraction values in patients with BMD, available cross-sectional data suggest that clinical function correlates with the extent of fatty degeneration of the muscle, in particular muscle fat fraction in the thigh muscles evaluated by the Dixon MRI technique.9, 26 Of course, such use as a marker in BMD needs to be validated in prospective clinical studies.
In addition to significant moderate correlations with most of the functional and strength endpoints, total fibrosis and MFA correlated significantly, although again moderately, with MRI fat fractions in the whole thigh and quadriceps. This was surprising because the histology samples were from arm (brachial biceps) biopsies and the MRI measures were performed in the leg. However, this could indicate that the thigh and biceps muscles follow a similar progression, in line with the proximal-distal order of muscle involvement in BMD, suggesting that both the arms and legs capture overall disease progression in individuals with BMD. A previous MRI study in patients with DMD showed that fat replacement in the arm muscles seems to coexist with fat replacement in the leg muscles, with the result that the deltoid, biceps brachii, and triceps brachii were affected to the same extent as the soleus and medial gastrocnemius.27 Our results suggest that this is also the case in BMD.
Our analyses have some limitations. These data are from a single visit and are therefore cross-sectional. Second, as these patients have been recruited into an interventional clinical trial, we applied a series of specific inclusion and exclusion criteria. Overall, this may impact the generalizability of the results and the strength of the correlations between measures (due to the limited range of disease severity in the recruited participants), although, as discussed earlier, our overall results are consistent with previously published data.16 Finally, the histology data are from samples taken from patients' arms, whereas the MRI fat fraction evaluations were performed on the legs. This indicates we cannot directly compare the two sets of results within a specific muscle---although, as noted earlier, the results suggest that the evolution of muscle degeneration is similar in the upper and lower limbs.
Evaluations of histology using muscle biopsies and of fat fraction using MRI have advantages and disadvantages. Although recognized as useful in diagnosing BMD and potentially as an endpoint for evaluating efficacy in clinical trials,28 muscle biopsies are invasive procedures, and are susceptible to human error---both in terms of specimen processing and sampling technique. For example, when selecting the area to biopsy, the surgeon may avoid areas that are overtly fibrotic or fat replaced, and instead excise samples that appear to contain the most healthy muscle. This, together with not being able to take biopsy samples from two patients with advanced disease, could partly explain why there was a weaker correlation between the extent of fibrosis seen on histology and fat fractions on MRI. In addition, although such procedures can provide data that cannot be obtained through other means (and have been used as an outcome measure in muscular dystrophy trials, including exon-skipping trials in children with DMD), repeated biopsies can reduce the willingness of patients to participate in such trials. Furthermore, the high variability in fat replacement within the muscle of an individual with BMD means that four randomly selected fields (as was the case in our study) may not provide a representative evaluation of disease progression within that individual. In contrast, the MRI fat fraction evaluation is noninvasive, avoids the inadvertent surgeon bias, and can be used to monitor disease progression in BMD. The muscle groups we selected for study have been shown to be most suitable for assessing BMD disease progression, based on standardized mean response, correlation with function, and interrater variability26; further studies (especially longitudinal) are needed to validate the accuracy of MRI fat fraction assessments to monitor disease progression in BMD. Given the heterogeneity of BMD, as evidenced by the interpatient variability in both our analyses and another study,26 such studies should also identify the thresholds and minimum clinically important differences of the various histology and MRI endpoints when used as outcomes.26 Ideally, such studies should include patients with a range of BMD severities, and follow patients for a sufficient period to be able to observe disease progression. This will then help to determine the clinical relevance of the correlations that we observed.
In conclusion, in this cohort of adults with BMD who retained the ability to walk unassisted, micro- and macroscopic morphological results from analyses of the muscles correlated moderately not only with each other, but with many functional parameters, potentially supporting the use of MRI and histology as surrogate outcome measures in BMD, although additional research is required to validate this.
ACKNOWLEDGMENTS
Statistical analyses were performed by Gabriele Lanzi, Elena Pasquali, and Roberta Tritto of OPIS, and funded by Regione Lombardia. Writing support was provided by David Young of Young Medical Communications and Consulting, Ltd. This support was funded by Italfarmaco SpA. This study was funded by Italfarmaco SpA and Regione Lombardia, Grant 231836, as part of the European Regional Development Fund (ERDF) of the Regional Operational Program (ROP) 2014− 2020. This work was generated within the European Reference Network for Neuromuscular Diseases. Several of the authors are members of the European Reference Network for Rare Neuromuscular Diseases. The Dutch authors are members of the Netherlands Neuromuscular Center. The University of Florida authors are part of the ImagingDMD Consortium.
CONFLICT OF INTEREST
G.P.C. has served on scientific advisory boards of Avexis, PTC Therapeutics, Italfarmaco, Sarepta, Roche, and Biogen. E.H.N. has worked as an investigator on the Italfarmaco SpA clinical trial described in this article. He also reports ad hoc consultancies for BioMarin, Summit, WAVE, and Regenxbio, and worked as an investigator in clinical trials for BioMarin, GSK, Lilly, Santhera, WAVE, Roche, NS Pharma, Reveragen, and Sarepta, all outside the submitted work. All reimbursements were received by the LUMC. H.E.K. reports grants from Netherlands Organization for Health Research and Development, grant 91716490, research support from Philips Healthcare, trial support from ImagingDMD-UF, and has served as a consultant for Esperare and PTC Therapeutics (no personal fees collected, all payments to the LUMC). KV reports grants from the National Institutes of Health (R01 AR056973) and research service support from Sarepta Therapeutics, Catabasis Pharmaceuticals, PTC Therapeutics, Eli Lilly, Summit Therapeutics, ML Bio/VCU directed to the University of Florida. N.M.v.d.V. has worked as a subinvestigator in clinical trials of givinostat, WAVE, Roche, and NS Pharma, all outside the submitted work. All reimbursements were received by the LUMC (no personal fees collected). S.C. and P.U.B. are employees of Italfarmaco SpA, the sponsor of the study. The remaining authors have no conflicts of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
As this study is ongoing, individual participant data will not be shared.