Respiratory muscle weakness in facioscapulohumeral muscular dystrophy
Funding information: Sanofi-Genzyme, Neu-Isenburg, Germany
Abstract
Introduction
The purpose of this study was to comprehensively evaluate respiratory muscle function in adults with facioscapulohumeral muscular dystrophy (FSHD).
Methods
Fourteen patients with FSHD (9 men, 53 ± 16 years of age) and 14 matched controls underwent spirometry, diaphragm ultrasound, and measurement of twitch gastric and transdiaphragmatic pressures (twPgas and twPdi; n = 10) after magnetic stimulation of the lower thoracic nerve roots and the phrenic nerves. The latter was combined with recording of diaphragm compound muscle action potentials (CMAPs; n = 14).
Results
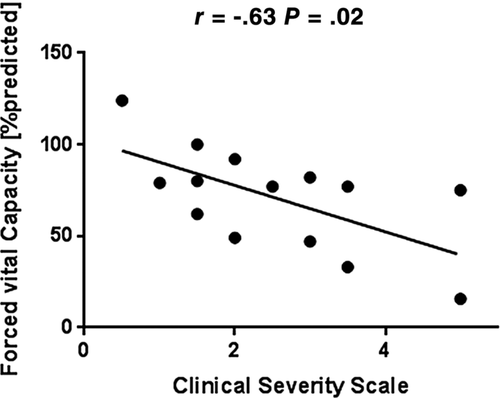
The following parameters were significantly lower in patients vs controls: forced vital capacity (FVC); maximum inspiratory and expiratory pressure; peak cough flow; diaphragm excursion amplitude; and thickening ratio on ultrasound, twPdi (11 ± 5 vs 20 ± 6 cmH2O) and twPgas (7 ± 3 vs 25 ± 20 cmH2O). Diaphragm CMAP showed no group differences. FVC correlated inversely with the clinical severity scale score (r = −0.63, P = .02).
Discussion
In FSHD, respiratory muscle weakness involves both the diaphragm and the expiratory abdominal muscles.
Abbreviations
-
- BMI
-
- body mass index
-
- CMAP
-
- compound muscle action potential
-
- CMS
-
- cervical magnetic stimulation
-
- coughPgas
-
- gastric pressure during maximum cough
-
- CSS
-
- Clinical Severity Scale
-
- DTR
-
- diaphragm thickening ratio
-
- FEV1
-
- forced expiratory volume in the first second
-
- FRC
-
- functional residual capacity
-
- FSHD
-
- facioscapulohumeral muscular dystrophy
-
- FVC
-
- forced vital capacity
-
- MEP
-
- maximum expiratory pressure
-
- MIP
-
- maximum inspiratory pressure
-
- NIV
-
- non-invasive ventilation
-
- Pdi
-
- transdiaphragmatic pressure
-
- sniffPdi
-
- transdiaphragmatic pressure during maximum sniff
-
- TLC
-
- total lung capacity
-
- twPdi
-
- twitch transdiaphragmatic pressure
-
- twPes
-
- twitch esophageal pressure
-
- twPgas
-
- twitch gastric pressure
1 INTRODUCTION
Facioscapulohumeral muscular dystrophy (FSHD) is characterized by progressive atrophy and muscle weakness. Symptom onset is usually in the second decade of life, and facial muscles usually are involved first. As the disease progresses, weakness may also affect shoulder and upper arm muscles, and the tibialis anterior and abdominal muscles. FSHD is one of the most common muscular dystrophies, with a prevalence of 1 in 20.000.1-3 Respiratory muscle involvement has been considered rare and restricted to patients with severe disease manifestation, spine deformities, and loss of ambulation.2, 3
To date, respiratory muscle function in FSHD has only been indirectly assessed by means of spirometry.4-8 Reduction of forced vital capacity (FVC) has been used as a surrogate marker for inspiratory muscle weakness, and has been reported as ranging from 10% to 38% of predicted in FSHD patients.4-8 Little is known about respiratory muscle involvement in patients with mild-to-moderate disease manifestation and, to date, detailed characterization of respiratory muscle weakness applying direct and non-volitional tests has not yet been performed in FSHD.
One of these methods is diaphragm ultrasound, which has been proposed as an objective bedside test for assessment of overall diaphragm function and morphology.9 Specifically, the diaphragm thickening ratio (DTR) may correlate with measures of inspiratory muscle strength.9 Another technique is invasive recording of twitch transdiaphragmatic pressure (twPdi) after stimulation of the phrenic nerve roots, which has been acknowledged as the diagnostic gold standard for objective measurement of inspiratory muscle strength.10 Both electrical and magnetic stimulation can be used for twPdi measurements and also for electrophysiological assessment of phrenic nerve function. Cervical magnetic stimulation (CMS) of the phrenic nerves is preferable to electrostimulation because it is less uncomfortable for patients.10
Because bedside tests such as FVC and maximum inspiratory pressure may be limited by insufficient co-operation or leakage of air due to incomplete lip seal, measurement of twPdi may provide more accurate evidence of the true burden of inspiratory muscle weakness in FSHD.10 Likewise, magnetic stimulation of the abdominal muscles along with recording of twitch gastric pressure (twPgas) has been introduced for non-volitional assessment of expiratory muscle function.11, 12 Abdominal muscles are known to be involved in the typical pattern of affected muscles in FSHD,13, 14 but, to date, selective functional assessment of these muscles has not been performed.
This case–control study comprehensively investigated the extent and pathophysiological characteristics of inspiratory and expiratory muscle function in adult patients with FSHD using measurement of mouth occlusion pressures, diaphragm ultrasound, phrenic nerve conduction studies, and invasive pressure recordings after magnetic stimulation of the diaphragm and the expiratory abdominal muscles.
2 METHODS
2.1 Experimental study design
This cross-sectional case–control study was conducted from November 2017 to October 2018. Ethical approval was obtained from the local ethics committee (Ethikkommission der Ärztekammer Westfalen-Lippe und der WWU Münster, AZ 2016-072-f-S). It was part of a wider project investigating the nature of respiratory muscle strength and function in neuromuscular disorders and chronic obstructive pulmonary disease (ClinicalTrials.gov Identifier: NCT03032562).
2.2 Study population
Twenty consecutive patients with genetically proven FSHD from the neuromuscular outpatient clinic at Münster University Hospital were consecutively invited to participate in the study. Consent was given by 14 individuals. All patients carried a chromosomal rearrangement within the subtelomeric region of chromosome 4q35 referred to as the D4Z4 locus.15 Neurological impairment was assessed using the Clinical Severity Scale (CSS), which reflects weakness in various muscle groups, including the spread of symptoms to pelvic and proximal leg muscles, which unequivocally indicates disease progression.16 The CSS score ranges from 0.5 (“facial weakness”) to 5 (“wheelchair-bound”) (see Table S1 online). Clinical symptoms indicative of respiratory muscle weakness or sleep-disordered breathing were assessed using a validated German language questionnaire (Boentert et al, manuscript submitted for publication). Healthy control subjects were consecutively recruited and matched 1:1 for age (±5 years), gender, and body mass index (BMI; ±3 kg/m2). All participants gave written informed consent to participate in the study.
2.3 Spirometry, maximum inspiratory, and expiratory pressures
Lung function tests were performed according to standard recommendations using an electronic spirometer (Vitalograph 3000, Vitalograph, Hamburg, Germany).10 Participants were encouraged to provide a maximum effort during assessment of their FVC and forced expiratory volume in the first second (FEV1) in the upright position. At least five consecutive tests were performed until the best result was achieved and showed less than 10% variation from the preceding test. FVC was expressed as percentage of the predicted value based on gender, height, and age.17 Maximum inspiratory and expiratory pressures (MIP and MEP, respectively) were obtained using a handheld electronic manometer equipped with a large-diameter flanged mouthpiece (MicroRPM, Care Fusion, Baesweiler, Germany). All participants performed at least three tests (with a 1-second plateau in 3- to 4-second efforts and a 1-minute pause between efforts). Subjects who failed to achieve less than 10% variability from the preceding test within the first 3 runs repeated the test until they were able to achieve more consistent results. MIP was measured first (from residual volume), and MEP was measured second (from total lung capacity), both with the subject sitting upright. All measurements were performed using a nasal clip to prevent air leakage.18
2.4 Diaphragm ultrasound
A portable ultrasound machine (Logiq S8-XD Clear, GE Healthcare, London, UK) with a 3.5-MHz convex transducer was used for assessment of diaphragm excursions in the subcostal view, and a 10-MHz linear transducer was used for evaluation of diaphragm thickness in the zone of apposition. Measurements were performed on the right hemidiaphragm in the supine position. All sonographic recordings were saved for later analysis. All measurements were performed three times and the average value for each parameter was calculated. For real-time evaluation of diaphragm excursions, the probe was positioned in the subcostal area between the midclavicular and anterior axillary line and directed cranially (Figure S1A online). Diaphragm excursion amplitude was assessed during tidal breathing (Figure S1A online), at total lung capacity (TLC), and after a voluntary sniff. Assessment of diaphragm excursion velocity was performed during tidal breathing and after a voluntary sniff maneuver only (Figure S1B online). Excursion amplitude was defined as the upright perpendicular distance from the minimum to the maximum point of diaphragm displacement, and excursion velocity was defined as the upright diagonal distance from the minimum to the maximum point.
Diaphragm thickness was measured as the vertical distance between the pleural and peritoneal layer at both TLC and functional residual capacity (FRC) (Figure S1C,D online). The 10-MHz probe was positioned in the posterior axillary line between the eighth and tenth intercostal space (Figure S1C online). Diaphragm thickness was defined as the distance from the inner part of the pleural layer to the inner part of the peritoneal layer, measured at its thickest portion adjacent to the lung. Diaphragm thickening ratio (DTR) was calculated as thickness at TLC divided by thickness at FRC.
2.5 Phrenic nerve conduction studies after posterior cervical magnetic stimulation
To exclude phrenic nerve dysfunction, diaphragm electrical activity was bilaterally recorded after posterior cervical magnetic stimulation. Surface electromyogram was recorded using a electromyography device (Dantec 2000, Dantec Medical, Skovlunde, Denmark) and surface electrodes were filled with electrode paste. Electrodes were placed in the seventh intercostal space approximately on the anterior axillary line with the reference electrode positioned cranially to the xiphoid process (16-cm interelectrode distance). The ground electrode was placed around the right wrist. Magnetic stimulation was performed with the subject in the seated position. After a 20-minute resting period with quiet breathing and no speaking, stimuli were delivered using a magnetic stimulator equipped with a 12-cm circular coil (C-100 MagPro Compact, MagVenture, Willich, Germany). Square-pulse stimulus duration was 0.1 milliseconds. Equal stimulus intensity was achieved by using maximum magnetic flux density (2 Tesla) for all stimulations. The coil was first placed at C7 and then moved up toward C6 until the highest reproducible diaphragm compound muscle action potential (CMAP) was obtained. For each run of tests, at least five stimuli were delivered to achieve the highest possible diaphragm CMAP amplitude showing less than 10% variation from the preceding two stimulations. To avoid twitch potentiation, stimuli were separated by at least 40 seconds. Stimulation at FRC was determined by visual observation of abdominal movements after instructing subjects to breathe out and hold their breath until the magnetic stimulus was delivered (the stimulus was then deployed within 1 second after FRC had been reached). Figure S2 (online) displays representative diaphragm CMAP values after CMS at FRC.
2.6 Invasive inspiratory muscle strength measurements
Twitch esophageal pressure (twPes) and twPgas were simultaneously obtained using balloon catheters (Cooper Surgical, Trumbull, Connecticut) transnasally inserted into the stomach and the distal esophagus.19 The esophageal and gastric balloons were filled with 1.0 mL and 2.5 mL of air, respectively. To ensure constant filling, both balloons were deflated and refilled after every run of tests. Balloon catheters were connected to a differential pressure transducer (DPT-100, Utah Medical Products, Athlone, Ireland) and a carrier amplifier (ADInstruments, Oxford, UK). Pressure data for twPgas, twPes, and twPdi (defined as twPes minus twPgas) were continuously displayed using LabChart software (ADInstruments) on a personal computer. Figure S3A (online) shows representative tracings of twPdi after CMS at FRC.
In addition to nonvolitional tests, subjects were instructed to perform consecutive maximum sniff maneuvers with a resting period of 5 minutes in between. Subjects were encouraged to achieve maximum deflection of the Pdi curve. After participants had learned and practiced the maneuver several times five measurements were recorded for each, and the highest value was taken for analysis. Figure S3B (online) shows representative pressure tracings after maximum voluntary sniff.
2.7 Invasive expiratory muscle strength measurements
As proposed by Polkey and coworkers,11 the abdominal nerve roots were magnetically stimulated at the 10th vertebrae with a clear instruction to go up and down the vertebral column (by no more than 2 vertebrae) to determine the optimal position where the highest reproducible diaphragm CMAP could be achieved. Abdominal muscle CMAPs were recorded bilaterally using surface electrodes placed in the anterior axillary line close to the lower costal margin. Stimulus duration was 0.1 millisecond. Stimulation intensity was 100% of the maximum magnetic output, as described above. Again, stimulation at FRC was determined by visual observation of abdominal movements. In addition, subjects were instructed to perform a voluntary maximum cough with a resting period of 5 minutes between different maneuvers. Gastric pressure during the cough maneuver and twPgas in response to magnetic stimulation of the abdominal muscles were recorded using a gastric balloon catheter and the same technical setup as described earlier. Representative pressure tracings are shown in Figure S3C and S3D (online).
2.8 Statistical analysis
All analyses were performed using SigmaPlot software version 13.0 (Systat Software, Erkrath, Germany). The data distribution was tested using the Kolmogorov–Smirnov test. Results are expressed as mean and standard deviation for continuous variables, and the t test for independent samples was used for between-group comparisons. For nonparametric data, the Mann–Whitney U test was used for comparison between groups as appropriate. For all analyses, P < .05 was considered statistically significant. GraphPad Prism 7 (GraphPad Software, San Diego, California) was used for graphical illustrations.
3 RESULTS
3.1 Subjects
Fourteen patients were enrolled in the study. Diagnosis of FSHD was genetically confirmed in all subjects, and exact contraction length of the D4Z4 repeat was known in 10 individuals. Fourteen matched control subjects (were also recruited (Table 1, and Table S2 online). Patients had a moderate to severe disease manifestation based on the CSS score (range, 1.0–5.0; median, 2.75; interquartile range [IQR], 1.50–3.38). Two patients required a wheelchair for ambulation. These two patients also had kyphoscoliosis. Four patients were receiving long-term non-invasive ventilation (NIV) during the night for known sleep-related hypoventilation, as reflected by nocturnal hypercapnia (transcutaneous carbon dioxide tension >50 mmHg or increase from awake baseline >10 mm Hg). Weakness of the lateral abdominal wall was observable in five individuals. According to the respiratory symptom questionnaire, exertional dyspnea was the most common complaint among FSHD patients (n = 9/14), followed by speech dyspnea (n = 6/14), non-restorative sleep (n = 5/14), sleep disturbances (n = 4/14), and daytime sleepiness (n = 3/14). The sum score derived from this questionnaire did not correlate with FVC and MIP, respectively (data not shown).
| FSHD patients (n = 14) | Controls (n = 14) | P value | |
|---|---|---|---|
| Male [n (%)] | 9 (64) | 9 (64) | NS |
| Age (years) | 53.4 ± 16.3 | 51.2 ± 12.7 | NS |
| Body mass index (kg/m2) | 24.3 ± 3.7 | 24.2 ± 1.9 | NS |
| CSS score | 2.75 (1.50–3.38) | – | – |
| Lung function data | |||
| FVC (L) | 3.1 ± 1.5 | 4.7 ± 1.1 | <.01 |
| FVC (% predicted) | 70.9 ± 27.9 | 112.0 ± 18.5 | <.01 |
| FEV1/VC (%) | 72.2 ± 22.4 | 75.9 ± 5.7 | NS |
| PEF (L/s) | 5.3 ± 1.5 | 8.8 ± 2.0 | <.01 |
| PEF (% predicted) | 65.1 ± 12.7 | 107.6 ± 22.5 | <.01 |
| PCF (L/min) | 322.3 ± 108.6 | 576.9 ± 109.6 | <.01 |
| MIP (cmH2O) | 61.8 ± 22.5 | 89.4 ± 15.7 | <.01 |
| MEP (cmH2O) | 68.9 ± 39.3 | 129.0 ± 15.8 | <.01 |
- Abbreviations: CSS, Clinical Severity Scale; FEV1, forced expiratory volume in the first second; FSHD, facioscapulohumeral muscular dystrophy; FVC, forced vital capacity; MEP, maximum expiratory pressure; MIP, maximum inspiratory pressure; NS, not statistically significant; PCF, peak cough flow; PEF, peak expiratory flow; VC, vital capacity.
- * Data are presented as mean ± standard deviation, number of patients, or percent, as indicated.
3.2 Spirometry and maximum inspiratory and expiratory pressures
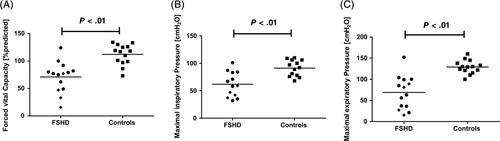
Compared with controls, FSHD patients showed significantly lower FVC, MIP, MEP, and PCF (Table 1 and Figure 1). FVC correlated inversely with clinical disease severity (as reflected by the CSS score) (Figure 2). When patients receiving nocturnal NIV were compared with patients without home ventilatory support, no differences were found between the two groups. Among FSHD patients, bedside tests of respiratory muscle function (FVC, MIP, MEP and PCF) did not correlate with the length of the D4Z4 contraction (data not shown).
3.3 Diaphragm ultrasound
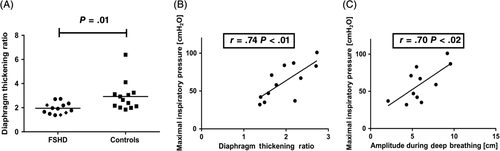
Diaphragm excursion amplitude during deep breathing and DTR were reduced in FSHD patients compared with controls (Table 2). Reduction of either DTR or diaphragm excursion amplitude during deep breathing was found in FSHD patients with and without NIV (Figure 3). MIP showed significant negative correlation with DTR and diaphragm excursion amplitude derived from ultrasound (Figure 3B,C). No correlation was found between clinical disease severity (CSS score) and diaphragm excursion amplitude or DTR, respectively.
| FSHD patients (n = 14) | Controls (n = 14) | P value | |
|---|---|---|---|
| Diaphragm excursion | |||
| Amplitude during tidal breathing (cm) | 1.5 ± 0.8 | 1.5 ± 0.6 | NS |
| Velocity during tidal breathing (cm/s) | 1.1 ± 0.3 | 1.2 ± 0.5 | NS |
| Amplitude during voluntary sniff (cm) | 2.8 ± 1.2 | 3.0 ± 1.3 | NS |
| Velocity during voluntary sniff (cm/s) | 6.4 ± 2.6 | 6.9 ± 2.3 | NS |
| Amplitude during maximum inspiration (cm) | 5.9 ± 2.1 | 7.9 ± 2.1 | .03 |
| Diaphragm thickness | |||
| At FRC (cm) | 0.18 ± 0.06 | 0.24 ± 0.10 | NS |
| At TLC (cm) | 0.34 ± 0.14 | 0.63 ± 0.23 | <.01 |
| DTR | 2.0 ± 0.5 | 2.9 ± 1.2 | .01 |
- Abbreviations: DTR, diaphragm thickening ratio; FRC, functional residual capacity; FSHD, facioscapulohumeral muscular dystrophy; NS, not statistically significant; TLC, total lung capacity.
3.4 Phrenic nerve conduction studies
Reproducible diaphragm CMAP could be obtained in all study participants, and no side-to-side difference was found with regard to latency or amplitude in those with FSHD or healthy controls (Table 3). Analysis of CMAP latencies and amplitudes revealed no abnormalities (Table 3).
| Cervical magnetic stimulation at FRC | FSHD patients (n = 14) | Controls (n = 14) | P value |
|---|---|---|---|
| Right-sided latency (ms) | 5.7 ± 1.2 | 4.8 ± 1.4 | NS |
| Right-sided amplitude (mV) | 0.10 (0.10–0.20) | 0.30 (0.10–0.48) | NS |
| Left-sided latency (ms) | 5.6 ± 1.2 | 5.1 ± 1.2 | NS |
| Left-sided amplitude (mV) | 0.20 (0.10–0.20) | 0.28 (0.10–0.40) | NS |
- Abbreviations: FRC, functional residual capacity; FSHD, facioscapulohumeral muscular dystrophy; NS, not statistically significant.
- * Data are presented as mean ± standard deviation or as median (interquartile range).
3.5 Invasive inspiratory muscle strength measurements
Transnasal insertion of balloon catheters was declined by 4 patients with FSHD, leaving 10 patients and 10 control subjects for group comparison of invasive pressure recordings. The twPdi was lower in the FSHD patients compared with controls (Table S3 online, and Figure 4A). Both twPdi and sniffPdi did not differ between patients with (n = 4) and without (n = 6) NIV (Table S2 online). No correlation was found between clinical disease severity (CSS score) and invasively obtained inspiratory muscle strength measures, and neither MIP nor FVC were significantly correlated with twPdi (data not shown). SniffPdi and twPdi showed a statistical correlation in control subjects (r = 0.77; P < .05), but not in FSHD patients. CoughPgas and twPgas after stimulation at Th10 were not associated in both groups (data not shown). FSHD patients were dichotomized for the median MIP and sniffPdi value, and individual patients with more severe inspiratory muscle weakness reported sleep disturbances, exertional dyspnea, and weakness of cough more frequently than patients with better inspiratory muscle function (data not shown).
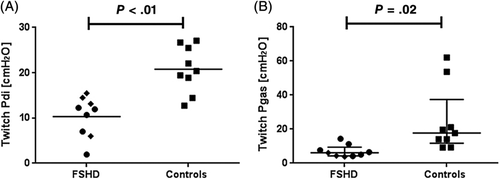
3.6 Invasive expiratory muscle strength measurements
After magnetic stimulation of the abdominal muscles, mean twPgas was lower in FSHD patients compared with healthy controls (Table S2 online, and Figure 4B). Pgas values after maximum voluntary cough (coughPgas) showed no differences between groups. Again, twitch Pgas did not differ between patients with and without nocturnal NIV. CoughPgas, twPgas, and MEP were not related to the CSS score (data not shown).
4 DISCUSSION
This study has confirmed that, in adult patients with FSHD, respiratory muscle weakness involves both the diaphragm and the expiratory abdominal muscles.
Respiratory muscle involvement has been described previously in FSHD, but was only indirectly diagnosed by means of spirometry or FVC, in particular.4–8,20 Indeed, the largest study investigating respiratory function in FSHD included 100 unselected patients and 38% of those patients showed a restrictive pattern (as defined by FVC <50% of predicted).4 In a population-based study, 1% of patients with FSHD required NIV,7 although more recent reports suggested that nocturnal ventilatory support may be indicated in a higher proportion of patients.4, 5 Early detection of respiratory muscle weakness could be useful to determine the possible need for further diagnostic work-up (including sleep studies and capnometry), which may reveal the need for nocturnal ventilatory support in more FSHD patients than previously thought. Interestingly, one study postulated that respiratory compromise in FSHD is mainly related to expiratory muscle weakness.20 In that study, relative preservation of diaphragm function was deduced from the fact that positional drop of FVC was normal in FSHD patients. However, electrophysiological measures and non-volitional pressure recordings were not performed. In contrast, in the present study, we comprehensively assessed respiratory muscle function. Significant diaphragm weakness was present in the majority of FSHD patients, including those who were still ambulatory or not receiving home ventilatory support. Inspiratory muscle dysfunction was reflected by significant reduction of MIP, twPdi, and sniffPdi in the present study. Diaphragm ultrasound revealed decreased DTR and diaphragm excursion amplitude in patients with FSHD.
Pathophysiologically, diaphragm involvement in FSHD may mainly be characterized by reduced contractility, as probably best reflected by DTR and twPdi. As the DUX4 protein, which is crucial for the molecular pathogenesis of FSHD,2–5,15 has been reported to exert toxic effects mainly on upper and lower limb muscles, it may be hypothesized that a similar pathology may also affect diaphragm.
FVC was inversely correlated with clinical disease severity, as reflected by the CSS score. Interestingly, non-volitional measures of respiratory muscle strength and diaphragm indices were not associated with the CSS score, which possibly reflects that respiratory muscle weakness may also be present in patients with milder disease manifestation. However, subjective symptoms of inspiratory muscle strength impairment were more frequent in patients with objective impairment of inspiratory muscle strength measures such as MIP and sniffPdi.
Magnetic phrenic nerve conduction studies revealed normal results in FSHD patients compared with healthy controls, excluding a neural contribution to diaphragm weakness.
Weakness of the abdominal muscles is known to be prevalent in FSHD, potentially resulting in abdominal wall prolapse or Beevor's sign (cranial dislocation of the umbilicus when the head is lifted in the flat supine position). However, the impact of abdominal muscle dysfunction on expiratory muscle strength has not yet been studied using magnetic stimulation and invasive recording of twPgas in this condition. Our study has revealed expiratory muscle weakness in FSHD patients, as shown by reduction of PCF, MEP, twPgas, and coughPgas. The clinical significance of expiratory muscle strength testing in lung and neuromuscular diseases is still being debated.12 On the one hand, there is a consensus that MEP may be regarded as an easy bedside marker to rule-out expiratory muscle weakness if values exceed the lower limit of normal.10 For patients with borderline MEP values, twPgas or coughPgas may then be useful to distinguish weakness from normality.12 Conversely, the clinical significance of expiratory muscle weakness, namely the inability to clear secretions from the lung, has not been proven in FSHD. However, our study findings imply that affected patients may be at risk of weakness of forced expiration caused by both diaphragm and abdominal wall involvement. Thus, weakness of cough and mucus retention should be routinely assessed by asking for corresponding symptoms and measuring the PCF, especially as the disease progresses.
Despite its comprehensive approach, our study has several limitations. First, the sample size is rather small, which clearly limits our findings. Second, inter- and intraobserver variability may have affected magnetic stimulation. We tried to minimize this by extensive training and by performing up to five stimulations per patient until variability was under 10%. Third, threshold testing for magnetic output was not performed. Instead, the maximum magnetic flux density of the coil (100% of the power output by default) was used to warrant comparability of CMAP and pressure amplitudes in both patients and control subjects. Fourth, impaired lip seal may have influenced FVC, MIP, and MEP measurements. Air leakage was minimized by the use of flanged and large-diameter mouthpieces, but sniff nasal inspiratory pressure (which also reflects global inspiratory muscle strength) would have been desirable to obtain. In addition, FVC was not measured in the supine position, and calculating the positional drop of FVC would have added valuable clinical information on diaphragm function. Because our study aimed to investigate diaphragm involvement in FSHD from a basic respiratory physiology rather than a clinical perspective, the focus was placed on diagnostic methods that more directly reflect diaphragm performance. Furthermore, subgroup analysis showed that inspiratory muscle weakness was related to the presence of home ventilatory support (as expected) but could also be found in nonventilated patients. To further evaluate this finding, it would have improved our study if we obtained sleep studies and overnight transcutaneous capnometry in the latter group. Future studies should specifically address the association between inspiratory muscle dysfunction and nocturnal hypoventilation in patients with FSHD. Finally, selection bias must be taken into account, as patients were recruited from a specialty outpatient clinic. It is likely that patients with more severe disease manifestation were overrepresented in this study population. However, patients were not preselected for the presence of respiratory complaints, and we did not intend to estimate the prevalence of respiratory muscle dysfunction in FSHD but rather aimed to provide general pathophysiological insights.
In conclusion, in this work we have revealed respiratory muscle weakness as an intrinsic feature of FSHD, which may involve both the diaphragm and the expiratory abdominal muscles.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical support provided by Judith Kemper and Dr Gerold Kierstein (ADInstruments, Oxford, UK). English language editing assistance was provided by Nicola Ryan, independent medical writer.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to this work.
ETHICAL PUBLICATION STATEMENT
The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with these guidelines.