Long-term safety and efficacy of eculizumab in generalized myasthenia gravis
ABSTRACT
Introduction: Eculizumab is effective and well tolerated in patients with antiacetylcholine receptor antibody-positive refractory generalized myasthenia gravis (gMG; REGAIN; NCT01997229). We report an interim analysis of an open-label extension of REGAIN, evaluating eculizumab's long-term safety and efficacy. Methods: Eculizumab (1,200 mg every 2 weeks for 22.7 months [median]) was administered to 117 patients. Results: The safety profile of eculizumab was consistent with REGAIN; no cases of meningococcal infection were reported during the interim analysis period. Myasthenia gravis exacerbation rate was reduced by 75% from the year before REGAIN (P < 0.0001). Improvements with eculizumab in activities of daily living, muscle strength, functional ability, and quality of life in REGAIN were maintained through 3 years; 56% of patients achieved minimal manifestations or pharmacological remission. Patients who had received placebo during REGAIN experienced rapid and sustained improvements during open-label eculizumab (P < 0.0001). Discussion: These findings provide evidence for the long-term safety and sustained efficacy of eculizumab for refractory gMG. Muscle Nerve 2019
Abstract
Abbreviations
-
- AChR+
-
- antiacetylcholine receptor antibody-positive
-
- aHUS
-
- atypical hemolytic uremic syndrome
-
- CI
-
- confidence interval
-
- gMG
-
- generalized myasthenia gravis
-
- IST
-
- immunosuppressive therapy
-
- IVIg
-
- intravenous immunoglobulin
-
- MG
-
- myasthenia gravis
-
- MG-ADL
-
- Myasthenia Gravis Activities of Daily Living
-
- MGC
-
- Myasthenia Gravis Composite scale
-
- MGFA
-
- Myasthenia Gravis Foundation of America
-
- MG-QOL15
-
- Myasthenia Gravis Quality of Life 15
-
- PNH
-
- paroxysmal nocturnal hemoglobinuria
-
- QMG
-
- Quantitative Myasthenia Gravis scale
Generalized myasthenia gravis (gMG) is a chronic, rare autoimmune disorder that is characterized by severe muscle weakness.1 Autoantibodies to the acetylcholine receptor are present in 73%–88% of patients with gMG.2-7 These autoantibodies initiate accelerated endocytosis and degradation of acetylcholine receptors and complement-mediated destruction of the neuromuscular junction, resulting in further reductions in acetylcholine and sodium channel receptors.1, 8-13 The complement-mediated pathological membrane changes reduce the efficiency of neurotransmission at the neuromuscular junction, resulting in the characteristic muscle weakness and fatigability that are observed in patients with MG.1, 14
Some patients (10%–15%) with MG do not respond adequately to long-term treatment with corticosteroids or multiple steroid-sparing immunosuppressive therapies (IST), experience intolerable side effects, or require ongoing treatment with intravenous immunoglobulin (IVIg) or plasma exchange.1, 15-18 Persistent myasthenic symptoms14-16, 18, 19 can adversely affect activities of daily living, including breathing, talking, swallowing, walking, and functional muscle strength as well as quality of life.15 Patients also have increased risks of myasthenic exacerbations and crises; an increased requirement for rescue therapies; and more frequent inpatient hospitalizations, intensive care unit admissions, and emergency room visits.20, 21
Eculizumab is a humanized monoclonal antibody that specifically binds with high affinity to human terminal complement protein C5. This inhibits enzymatic cleavage of C5 to C5a and C5b, thereby preventing C5a-induced chemotaxis of proinflammatory cells and formation of the C5b-induced membrane attack complex.22 Eculizumab was shown to have efficacy and was well tolerated in the 6-month randomized, double-blind, placebo-controlled REGAIN study (NCT01997229), producing clinically meaningful improvements in activities of daily living, muscle strength, functional ability and quality of life in patients with antiacetylcholine receptor antibody-positive (AChR+) refractory gMG.14 Eculizumab is approved for the treatment of adults with AChR+ gMG, in addition to paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS).
The open-label extension study of the phase 3 REGAIN (NCT02301624) was designed to evaluate the long-term safety and efficacy of eculizumab in patients with AChR+ refractory gMG. Here we report data from a preplanned interim analysis that was based on a median duration of almost 2 years of eculizumab treatment.
MATERIALS AND METHODS
Study Design and Participants
Participants who completed the 6-month, double-blind REGAIN study could enter this extension study. Full inclusion and exclusion criteria for the REGAIN study have been published previously.14 In brief, adults (aged ≥18 years) with the following were eligible for REGAIN: confirmed gMG with positive serology for acetylcholine receptor antibodies, an MG Activities of Daily Living (MG-ADL) total score of 6 or higher, and failed treatment with 2 or more ISTs or at least 1 IST with requirement for chronic IVIg or plasma exchange therapy over the preceding 12 months. Patients were excluded if they had a history of thymoma or other thymic neoplasm, had undergone thymectomy in the 12 months before screening, experienced ocular-only MG symptoms (Myasthenia Gravis Foundation of America [MGFA] class I) or myasthenic crisis (MGFA class V) at screening, or required treatment with IVIg or plasma exchange within the 4 weeks before randomization.14
Eligible patients who elected to continue into the open-label extension were required to enter it within 2 weeks of completing REGAIN. The first patient was enrolled on November 12, 2014, and the last patient was enrolled on March 4, 2016. Participants received open-label eculizumab until it was otherwise available to them, up to a maximum of 4 years in the extension study. The study was completed in January 2019.
All patients provided written, informed consent. Written approval for the study protocol and all study amendments was obtained from independent ethics committees or institutional review boards at all participating sites. All human studies were approved by the appropriate ethics committee and have been performed in accordance with the ethical standard laid down in the 1964 Declaration of Helsinki.
Dosing and Administration
Patients who received eculizumab in REGAIN continued to receive eculizumab during the open-label study (eculizumab/eculizumab group). Those who received placebo in REGAIN started eculizumab treatment when they entered the open-label study (placebo/eculizumab group).
To preserve the blinded nature of REGAIN, patients who entered the open-label study first underwent a 4-week blinded induction phase during which investigators, patients, and study personnel remained blinded to all treatment assignments (Fig. 1). During this phase, patients who had been assigned to eculizumab in REGAIN received eculizumab 1,200 mg (4 vials) on day 1 and week 2 and placebo (4 vials) at weeks 1 and 3. Patients who had been assigned to placebo in REGAIN received eculizumab (900 mg, 3 vials) and placebo (one vial) on day 1 and at weeks 1, 2, and 3 (Fig. 1). In the open-label maintenance phase, which started at week 4, all patients received open-label eculizumab (1,200 mg) every 2 weeks.
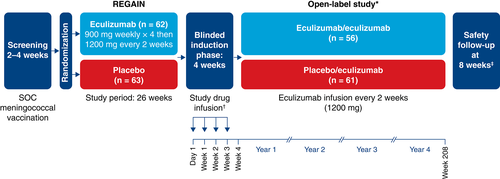
Procedures
To mitigate the increased risk of meningococcal infection associated with terminal inhibition of the complement system,23 all patients in REGAIN were required to receive Neisseria meningitidis vaccination according to local guidelines at least 2 weeks before starting blinded study treatment; if they were not vaccinated at the appropriate time, patients received prophylactic antibiotics until 2 weeks after vaccination. In the open-label study, patients were revaccinated according to local guidelines, which recommend revaccinating patients after 2–5 years to maintain active coverage.
Patients who entered the open-label study were on a stable regimen of concomitant MG therapies, including ISTs that could include but were not limited to corticosteroids, azathioprine, mycophenolate mofetil, methotrexate, cyclosporine, tacrolimus, and cyclophosphamide. Adjustment of concomitant MG therapies, including ISTs, was permitted at the discretion of the study investigator but was not required by the study protocol. Rescue therapy (e.g., high-dose intravenous corticosteroids, IVIg, or plasma exchange) was available at the discretion of the study investigator for patients who experienced disease exacerbation.
Validated MG assessments of activities of daily living, muscle strength, functional ability, and quality of life were used to evaluate the long-term efficacy of eculizumab. These assessments consisted of the MG-ADL scale,24 the Quantitative MG scale (QMG),25 the MG Composite scale (MGC),26 and the 15-item MG Quality of Life questionnaire (MG-QOL15).27 MG-ADL, QMG, MGC, and MG-QOL15 assessments were performed on day 1. MG-ADL, QMG, and MGC assessments were performed weekly from week 1 through week 3. MG-ADL, QMG, MGC, and MG-QOL15 assessments were then performed at weeks 4, 8, 12, 16, 20, 26, 40, and 52 in year 1, every 6 months thereafter, and at each patient's end of study visit. These same 4 efficacy measures were used in the REGAIN study.14
Outcomes
We report an interim analysis of safety and efficacy data, including the occurrence of adverse events, and changes in activities of daily living, muscle strength, functional ability, and quality of life over time using 4 MG-specific disease measures (data cutoff December 31, 2017).
The primary objective of this open-label study was to evaluate the long-term safety of eculizumab. Safety was assessed by incidences of adverse events, serious adverse events, study discontinuations due to adverse events, exacerbations, hospital admissions, and rescue therapy administrations. Adverse events were coded by preferred term by using the Medical Dictionary for Regulatory Activities Version 20.1. The number of patients who experienced an adverse event of special interest (meningococcal infections, aspergillus infections, sepsis, any serious infections, infusion-related reactions, serious cutaneous reactions, cardiac disorders, or angioedema) during each 3-month study period was determined. For this study, a clinical deterioration/exacerbation was defined as 1 of the following: MG crisis, substantial symptomatic worsening (to a score of 3 or a 2-point worsening on any 1 of the individual MG-ADL items, excluding ocular items), or health in jeopardy if rescue therapy was not given, as determined by the treating physician. The exacerbation and hospitalization event rates were compared with rates during the year before entering REGAIN (collected at REGAIN baseline assessment); the rescue therapy event rate was compared with the rate in the placebo group during REGAIN.
The primary efficacy endpoint was change in mean MG-ADL total score from baseline over time. Changes from REGAIN and open-label baselines were evaluated. Secondary efficacy endpoints included changes from baseline in mean QMG, MGC, and MG-QOL15 total scores over time and the proportions of patients achieving clinically meaningful responses to eculizumab, prospectively defined as improvements from baseline of at least 3 points in MG-ADL total score or at least 5 points in QMG total score.28-30 Other efficacy endpoints included MGFA postinterventional status, a disease-specific outcome measure that captures the physician's global assessment of the patient's clinical status after initiation of MG treatment.31 The same neurologist skilled in evaluating patients with MG assessed the MGFA postinterventional status throughout the study (at weeks 26 and 40 and then every 26 weeks until end of study/early termination) according to the following categories relative to baseline: improved (a substantial decrease in clinical manifestations or in MG medications), unchanged (no substantial change in clinical manifestations or reduction in MG medications), or worse (a substantial increase in clinical manifestations or MG medications).31 Patients who had improved were also evaluated for minimal manifestation and pharmacological remission status.31
Statistical Analysis
Safety analyses were performed for all patients who received at least 1 dose of eculizumab in the open-label study (safety set). Efficacy analyses were conducted by using the full analysis set, which comprised all patients who received at least 1 dose of eculizumab in the open-label study and had at least 1 postdose efficacy assessment.
For exacerbations, hospitalizations, and rescue therapy use, model-based event rates per 100 patient-years were calculated. This was based on a generalized estimating equation Poisson regression repeated-measures model with the number of events as the dependent variable, the logarithm of patient-years as the offset variable, and the study phase indicator (prestudy, placebo, or eculizumab) as the factor assuming a compound symmetry correlation structure.
Two baselines were used for the efficacy analyses, REGAIN baseline, defined as assessment at day 1 in the REGAIN study; and open-label baseline, defined as the last available assessment before first eculizumab infusion (this was typically the day-1 assessment in the open-label study; when the day-1 data were missing, the most recent assessment from REGAIN was used as the open-label baseline). Changes from baseline in MG-ADL, QMG, MGC, and MG-QOL15 total scores at a particular visit were based on repeated-measures models. Separate repeated-measures models were used for the eculizumab/eculizumab and placebo/eculizumab groups because patients in the eculizumab/eculizumab group had already received 6 months of eculizumab in REGAIN. Assessing the change from the open-label baseline allowed for evaluation of the eculizumab treatment effect in the placebo/eculizumab arm and the effect of continued, long-term treatment in the eculizumab/eculizumab arm. The change from REGAIN baseline allowed for an assessment of all changes from time of entry to REGAIN. Missing efficacy endpoint assessments were not imputed.
The responder analyses measured the proportion of patients with clinically meaningful improvements from REGAIN baseline. The proportions of patients experiencing this improvement with and without prior on-study rescue therapy were determined at each visit for both groups. Exact (Clopper–Pearson) 95% confidence intervals (CIs) for the true proportions are presented.
Data are presented as least-squares means (changes from open-label baseline) and 95% CIs. All statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina). This study did not have a data monitoring committee.
RESULTS
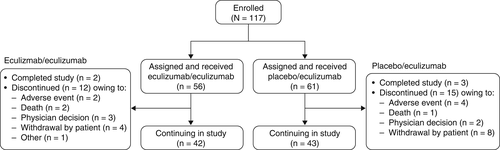
In this interim analysis, 117 of the 118 patients who completed REGAIN enrolled in the open-label study (eculizumab/eculizumab, 56; placebo/eculizumab, 61) and were included in the safety analysis, and 116 patients were included in the efficacy analysis (eculizumab/eculizumab, 56; placebo/eculizumab, 60); approval for the inclusion of 1 patient in the interim efficacy analyses was not given by their national health authority.
At the time of data cutoff, study participation was ongoing for 73% of patients. Five patients (eculizumab/eculizumab, 2; placebo/eculizumab, 3) had completed the study. Among the 27 patients who discontinued the study, 6 patients discontinued because of 1 or more adverse events, and 3 patients died. In addition, study investigators withdrew 5 patients, 12 patients withdrew consent, and 1 patient discontinued for a reason described as “other” (Fig. 2) after a median period of eculizumab therapy of 379 days because of various factors including ongoing comorbidities, perceived lack of clinical benefit, and logistical problems with clinic attendance (Supp. Info. Table 1).
Patient demographics for the study population were reported for REGAIN.14 Patient characteristics were similar between the eculizumab/eculizumab and placebo/eculizumab groups (Table 1). In total, 38 patients required revaccination against N meningitidis (eculizumab/eculizumab, 20; placebo/eculizumab, 18).
| Variable | Eculizumab/ eculizumab, n = 56 | Placebo/ eculizumab, n = 61 |
All patients, N = 117 |
|---|---|---|---|
| Mean age (SD), y* | 47.2 (15.5) | 47.5 (17.9) | 47.4 (16.7) |
| Sex, n (%) | |||
| Men | 18 (32.1) | 20 (32.8) | 38 (32.5) |
| Women | 38 (67.9) | 41 (67.2) | 79 (67.5) |
| Race, n (%) | |||
| Asian | 3 (5.4) | 16 (26.2) | 19 (16.2) |
| Black | 0 (0.0) | 2 (3.3) | 2 (1.7) |
| White | 47 (83.9) | 41 (67.2) | 88 (75.2) |
| Multiple | 1 (1.8) | 0 (0.0) | 1 (0.9) |
| Unknown | 1 (1.8) | 0 (0.0) | 1 (0.9) |
| Other | 4 (7.1) | 2 (3.3) | 6 (5.1) |
| MG duration, mean (SD), y† | 10.7 (7.9) | 9.8 (8.5) | 10.2 (8.2) |
- MG, myasthenia gravis; SD, standard deviation.
- * On day 1 of the open-label extension study.
- † Time from MG diagnosis to first dose date in the open-label extension study.
The median duration of eculizumab treatment for all 117 patients during the open-label study was 22.7 months (range, 1 day to 37.3 months). Reported data are based on 227 patient-years of open-label eculizumab exposure at the time of data cutoff.
The most common adverse events were headache and nasopharyngitis, which were experienced by 37.6% and 31.6% of patients, respectively (Table 2). The most common serious adverse event was MG worsening, which occurred in 12.8% of patients (Table 2). Three patients experienced a serious adverse event of MG crisis, which resulted in study discontinuation for 2 of them. Six patients withdrew from the study because of a serious adverse event. All serious adverse events are listed in Supporting Information Table 2.
| Event | Events, n | Patients experiencing an event, n (%) | Events per 100 PY* |
|---|---|---|---|
| All adverse events | 1,816 | 113 (96.6) | 800.0 |
| Most common adverse events† ,‡, >10% of all patients, N = 117 | |||
| Headache | 71 | 44 (37.6) | 31.3 |
| Nasopharyngitis | 76 | 37 (31.6) | 33.5 |
| Diarrhea | 40 | 27 (23.1) | 17.6 |
| Upper respiratory tract infection | 55 | 27 (23.1) | 24.2 |
| Myasthenia gravis§ | 40 | 23 (19.7) | 17.6 |
| Arthralgia | 29 | 22 (18.8) | 12.8 |
| Nausea | 26 | 21 (17.9) | 11.5 |
| Pain in extremity | 21 | 18 (15.4) | 9.3 |
| Cough | 21 | 17 (14.5) | 9.3 |
| Fatigue | 21 | 17 (14.5) | 9.3 |
| Urinary tract infection | 32 | 17 (14.5) | 14.1 |
| Influenza | 24 | 16 (13.7) | 10.6 |
| Gastroenteritis | 15 | 14 (12.0) | 6.6 |
| Bronchitis | 22 | 13 (11.1) | 9.7 |
| Pyrexia | 17 | 13 (11.1) | 7.5 |
| Fall | 24 | 12 (10.3) | 10.6 |
| All serious adverse events | 147 | 52 (44.4) | 64.8 |
| Most common (≥2 patients) MG- and infection-related serious adverse events† ,‡ ,‖ | |||
| Myasthenia gravis§ | 28 | 15 (12.8) | 12.3 |
| Death | 3 | 3 (2.6) | 1.3 |
| Myasthenia gravis crisis | 3 | 3 (2.6) | 1.3 |
| Pyrexia | 3 | 3 (2.6) | 1.3 |
| Gastroenteritis | 3 | 3 (2.6) | 1.3 |
| Pneumonia | 3 | 3 (2.6) | 1.3 |
| Sepsis | 3 | 3 (2.6) | 1.3 |
| Bronchitis | 3 | 2 (1.7) | 1.3 |
| Influenza | 2 | 2 (1.7) | 0.9 |
| Upper respiratory tract infection | 2 | 2 (1.7) | 0.9 |
| Urinary tract infection | 3 | 2 (1.7) | 1.3 |
| Aspiration pneumonia | 2 | 2 (1.7) | 0.9 |
- MedDRA, Medical Dictionary for Regulatory Activities; MG, myasthenia gravis; PY, patient year.
- * Patient-years is the sum of all years for all patients and observed event rate is the number of events per PY multiplied by 100.
- † When a patient had more than 1 adverse event for a particular preferred term, that patient was counted only once for that preferred term.
- ‡ MedDRA preferred term.
- § Worsening (increased frequency and/or intensity) of a preexisting condition, including myasthenia gravis, is considered to be an adverse event.
- ‖ Serious adverse events are adverse events that are life-threatening or result in death, hospitalization, or persistent or significant disability or incapacity, are congenital anomalies or birth defects, or are important medical events. All serious adverse events in the open-label study are listed in Supporting Information Table 1.
At the time of this interim analysis, 3 deaths had occurred. The death of 1 patient who was concomitantly receiving azathioprine was attributed to hemophagocytic lymphohistiocytosis associated with cytomegalovirus infection of the liver resulting in multiple organ failure. The second death was attributed to end-stage liver disease in a patient with cryptogenic liver cirrhosis and a medical history of fatty liver, and the third death was due to pulmonary embolism that occurred in a patient who was in the hospital recovering from cardiogenic shock secondary to sepsis complicated by deep vein thrombosis.
During the open-label study, 22 (18.8%) patients experienced an infectious event of special interest, including 5 cases of sepsis, septic shock, or pseudomonas sepsis and 1 case each of aspergillus, cytomegalovirus, and pseudomonas infection (Supp. Info. Table 3). The proportion of patients experiencing these infections or other adverse events of special interest (infusion-related reactions, serious cutaneous reactions [urticaria], and cardiac disorders) was in line with that observed during REGAIN. Similar proportions of patients in the eculizumab/eculizumab and placebo/eculizumab groups experienced these adverse events, and rates did not change over time in the open-label study (Supp. Info. Table 4). There were no cases of meningococcal infection at the interim data cutoff date; 1 case, which was resolved with antibiotic treatment, occurred after this date.
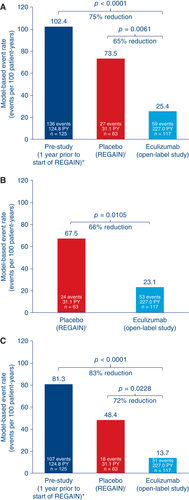
In total, 59 MG exacerbations (including MG crises, substantial symptomatic worsening, and health in jeopardy if rescue therapy was not given) were experienced by 29 patients during the open-label study before data cutoff. Compared with the year before REGAIN start, the exacerbation rate was reduced by 75.2% (prestudy, 102.4 exacerbations per 100 patient-years; open-label study, 25.4 exacerbations per 100 patient-years; P < 0.0001; Fig. 3). The exacerbation rate was also significantly lower than in the REGAIN placebo group (73.5 exacerbations per 100 patient-years; P = 0.0061). The rate of rescue therapy use was 23.1 events per 100 patient-years in the open-label study, compared with 67.5 events per 100 patient-years in patients receiving placebo during REGAIN (P = 0.0105; Fig. 3). The rate of MG-related hospitalizations was reduced by over 80% compared with the year before REGAIN start (prestudy, 81.3 hospitalizations per 100 patient-years; open-label study, 13.7 hospitalizations per 100 patient-years; P < 0.0001; Fig. 3). It was also lower than in the REGAIN placebo group (48.4 hospitalizations per 100 patient-years; P = 0.0228; Fig. 3).
Improvements that had been demonstrated for patients during 6 months of blinded eculizumab in REGAIN were sustained during the open-label study for a total maximum eculizumab treatment duration of 3 years (eculizumab/eculizumab group; Fig. 4). The mean MG-ADL total score from open-label baseline did not change significantly in this group at each assessment (−0.8 change from baseline to week 130 [n = 13]; P = 0.0990; Fig. 5A2). This sustained treatment effect was also supported by similar findings with mean total scores for QMG (0.1 change from baseline to week 130 [n = 13]; P = 0.8949; Fig. 5B2), MGC (−1.3 change from baseline to week 130 [n = 13]; P = 0.1531; Fig. 5C2), and MG-QOL15 (−1.2 change from baseline to week 130 [n = 13]; P = 0.4756; Fig. 5D2).
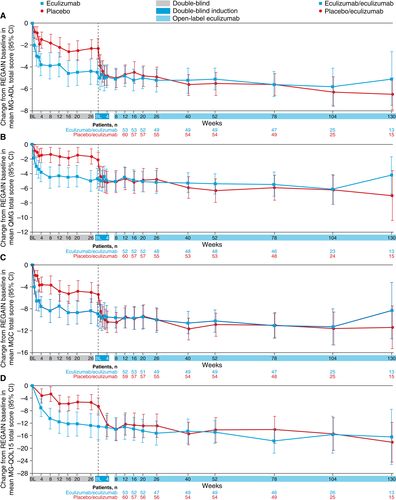
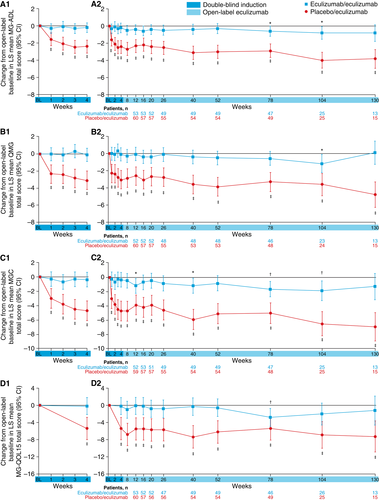
Patients who had received placebo during REGAIN experienced a rapid and significant improvement when they commenced eculizumab at the start of the open-label extension (Fig. 5A1, B1, C1, D1). Improvements from open-label baseline in mean MG-ADL total score as well as QMG, MGC, and MG-QOL15 total scores were of similar magnitude to those experienced by eculizumab-treated patients in REGAIN. The mean change from open-label baseline was statistically significant as early as the first visit after eculizumab administration (assessed at week 1 for MG-ADL [−1.6 from baseline], QMG [−2.3 from baseline], and MGC [−3.0 from baseline] and at week 4 for MG-QOL15 [−5.4 from baseline]; all P < 0.0001; Fig. 5A1, B1, C1, D1), with over half of the improvement occurring in the first 3 months. These improvements were sustained over 30 months (mean change in MG-ADL total score from open-label baseline to month 30, −3.8; P < 0.0001; n = 15).
At the last assessment for this interim analysis, 55.2% of patients in the open-label study demonstrated a clinically meaningful response in activities of daily living (at least a 3-point improvement from REGAIN baseline in MG-ADL total score without use of rescue therapy). When analysis was performed regardless of rescue therapy, 71.6% of patients experienced this improvement. Over one-third (39.7%) of patients experienced a clinically meaningful response in muscle strength (at least a 5-point improvement from REGAIN baseline in QMG total score without rescue therapy use), and 48.3% experienced this improvement regardless of rescue therapy use.
At the final MGFA postinterventional status evaluation before this data cutoff, 74.1% (86/116) of patients were reported by their investigator to have clinically improved compared with REGAIN baseline. In addition, most (65/116 [56.0%]) patients were deemed to have achieved minimal manifestations of MG or pharmacological remission.
DISCUSSION
Inhibition of terminal complement activity with eculizumab is a biologically rational approach to prevent damage at the neuromuscular junction in patients with AChR+ refractory gMG.22 Eculizumab has been reported to produce rapid and clinically meaningful improvements in activities of daily living, muscle strength, functional ability, and quality of life and is well tolerated in these patients.14 This interim analysis of the open-label extension study provides evidence that the safety and efficacy of eculizumab in this patient population are sustained with long-term treatment.
The safety data for eculizumab in this study are in line with the current safety profile in patients with refractory gMG and with its well-characterized safety profile (on the basis of >10 years of postmarketing experience) in the other approved indications, PNH and aHUS.14, 32-34 Consistent with the mode of action of eculizumab, infections were the most commonly reported events among the adverse events of special interest. The prevalence of infections and other events of special interest did not change with continued eculizumab exposure. To mitigate the risk of meningococcal infection, patients were required to receive meningococcal vaccination as described in Materials and Methods. There were no occurrences of meningococcal infection by data cutoff; 1 nonfatal case after this date has been reported. Three fatal events were reported in patients with important comorbidities that likely contributed to the clinical outcome.
The rapid response to eculizumab across multiple disease-specific measures in patients who had previously received placebo confirms the effect of eculizumab recorded in REGAIN and exceeded the response to placebo that was observed during REGAIN. The maintenance of this effect in patients who received eculizumab for up to 3 years provides evidence of its durability. The open-label study not only supports and extends the body of evidence for the treatment effect of eculizumab but also provides new data regarding its positive impact on clinical burden in patients who experience persistent MG symptoms despite having a history of using multiple ISTs. Among such patients, rates of potentially life-threatening exacerbations,35 with consequent hospitalizations and rescue therapy use, are typically high.20 We have demonstrated that eculizumab treatment significantly reduces the rates of MG exacerbations and MG-related hospitalizations in comparison with the last complete year before entering the study and the rate of rescue therapy requirement compared with placebo-treated patients in REGAIN. In addition, most patients were reported by their physicians to have shown global clinical improvements during the open-label study, and over half achieved minimal manifestations status or pharmacological remission according to MGFA postinterventional status evaluation. These interim results add to the findings of REGAIN in providing evidence of the range of clinical benefits provided by eculizumab and the impact it has on alleviating the burden of disease in patients with previously refractory gMG.
These results add to the evidence that complement protein C5 inhibition is a relevant therapeutic target in AChR+ refractory gMG and provide evidence of the long-term benefits of complement inhibition by eculizumab for patients with this disease. Additional investigations into the structural and functional changes at the neuromuscular junction in response to eculizumab that correlate with clinical improvement in patients with MG may help to build on the current preclinical evidence,36-38 fully characterize the pathophysiology of MG including the role of complement inhibition, and elucidate the mechanism underlying the rapid response to eculizumab.
The main limitation of this study is the open-label design, which could yield unconscious bias in reporting, particularly of adverse events. The blinded induction phase was designed to preserve the blinded nature of REGAIN during the period of potential initial response. Although the lack of a control group may also be considered a limitation, comparisons can be made with the placebo group in REGAIN and with pre-REGAIN data for the overall study population. Because over 90% of REGAIN participants enrolled in the open-label extension study, selection bias based on inclusion in the open-label study population is unlikely.
Future analyses of long-term eculizumab data, including changes in IST and corticosteroid use during eculizumab therapy; the time course of clinical improvements; and achievement of minimal manifestations will be important for understanding the role of eculizumab in the treatment of individuals with refractory gMG.
In conclusion, results of this interim analysis confirm the rapid and robust response to eculizumab that was observed during REGAIN and support the long-term clinical effectiveness and safety of eculizumab in patients with AChR+ refractory gMG who previously experienced persistent symptoms and significant morbidities despite concomitant IST. Improvements in activities of daily living, muscle strength, functional ability, and quality of life were maintained through 3 years in patients receiving eculizumab. This study also provides new evidence for other clinical benefits of eculizumab in these patients, including reducing the frequency of disease exacerbations. The completion of this open-label extension study will provide additional opportunities to continue studying and understanding the long-term benefits of eculizumab in patients with gMG.
The authors thank the patients who took part in REGAIN and the open-label extension study as well as their families; all of the investigators and collaborators for their contributions to the completion of the study; Angela Kaya, PhD, and Gus Khursigara, PhD, (formerly of Alexion Pharmaceuticals) and Róisín Armstrong, PhD, Diaa Diab, MBBS, and Kelley Capocelli, MD, (Alexion Pharmaceuticals) for critical review of the manuscript, and Cindy Lane, MS, (Alexion Pharmaceuticals) for clinical study oversight. We acknowledge Vicky Sanders, PhD, and Ruth Gandolfo, PhD (Oxford PharmaGenesis, Oxford, UK), who provided medical writing support in the production of this manuscript (funded by Alexion Pharmaceuticals). All members of the REGAIN study group are listed in the Supporting Information materials.
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.