Oxidant-induced atrogin-1 and transforming growth factor-β1 precede alcohol-related myopathy in rats
Corresponding Author
Jeffrey S. Otis PhD
Pulmonary, Allergy and Critical Care Medicine, Atlanta VA Medical Center and Emory University School of Medicine, 1670 Clairmont Road, 12C-191, Decatur, Georgia 30033, USA
Pulmonary, Allergy and Critical Care Medicine, Atlanta VA Medical Center and Emory University School of Medicine, 1670 Clairmont Road, 12C-191, Decatur, Georgia 30033, USASearch for more papers by this authorLou Ann S. Brown PhD
Department of Pediatrics, Emory University School of Medicine, 2015 Uppergate Drive, Atlanta, Georgia, USA
Search for more papers by this authorDavid M. Guidot MD
Pulmonary, Allergy and Critical Care Medicine, Atlanta VA Medical Center and Emory University School of Medicine, 1670 Clairmont Road, 12C-191, Decatur, Georgia 30033, USA
Search for more papers by this authorCorresponding Author
Jeffrey S. Otis PhD
Pulmonary, Allergy and Critical Care Medicine, Atlanta VA Medical Center and Emory University School of Medicine, 1670 Clairmont Road, 12C-191, Decatur, Georgia 30033, USA
Pulmonary, Allergy and Critical Care Medicine, Atlanta VA Medical Center and Emory University School of Medicine, 1670 Clairmont Road, 12C-191, Decatur, Georgia 30033, USASearch for more papers by this authorLou Ann S. Brown PhD
Department of Pediatrics, Emory University School of Medicine, 2015 Uppergate Drive, Atlanta, Georgia, USA
Search for more papers by this authorDavid M. Guidot MD
Pulmonary, Allergy and Critical Care Medicine, Atlanta VA Medical Center and Emory University School of Medicine, 1670 Clairmont Road, 12C-191, Decatur, Georgia 30033, USA
Search for more papers by this authorAbstract
Alcohol-related chronic myopathy is characterized by severe biochemical and structural changes to skeletal muscle. Our goals were to: (1) identify early regulatory elements that precede the overt manifestation of plantaris atrophy; and (2) circumvent these derangements by supplementing alcohol-fed rats with the glutathione precursor, procysteine. After 6 weeks of daily ingestion, before the development of overt atrophy of the plantaris muscle, alcohol increased several markers of oxidative stress and increased gene expressions of atrogin-1 and transforming growth factor-β1 (TGF-β1) by ∼60- and ∼65-fold, respectively, which were attenuated by procysteine supplementation. Interestingly, after 28 weeks of alcohol ingestion, when overt plantaris atrophy had developed, atrogin-1 and TGF-β1 gene expression had returned to baseline levels. Together, these findings suggest that alcohol-induced, redox-sensitive alterations drive pro-atrophy signaling pathways that precede muscle atrophy. Therefore, targeted anti-oxidant treatments such as procysteine supplementation may benefit individuals with chronic alcohol abuse, particularly if given prior to the development of clinically significant myopathy. Muscle Nerve, 2007
REFERENCES
- 1 Adachi J, Asano M, Ueno Y, Reilly M, Mantle P, Peters TJ, et al. 7-α and 7 β-hydrocycholesterol-5-en 3β-ol in muscle as indices of oxidative stress: response to ethanol dosage in rats. Alcohol Clin Exp Res 2000; 24: 675–681.
- 2 Aoyama Y, Urushiyama S, Yamada M, Kato C, Ide H, Higuchi S, et al. MFB-1, an F-box-type ubiquitin ligase, regulates TGF-beta signaling. Genes Cells 2004; 9: 1093–1101.
- 3 Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, et al. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol 2005; 289: L363–L370.
- 4 Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001; 294: 1704–1708.
- 5 Cederbaum AI, Rubin E. Molecular injury to mitochondria produced by ethanol and acetaldehyde. Fed Proc 1975; 34: 2045–2051.
- 6 Cederbaum AI. Introduction—serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med 2001; 313: 1524–1526.
- 7 Chan KM, Decker EA. Endogenous skeletal muscle anti-oxidants. Crit Rev Food Sci Nutr 1994; 34: 403–426.
- 8 Clark DA, Coker R. Transforming growth factor-beta (TGF-β). Int J Biochem Cell Biol 1998; 30: 293–298.
- 9 Durán Castellón MC, González-Reimers E, López-Lirola A, MartÍn Olivera R, Santolaria-Fernández F, Galindo-Martín L, et al. Alcoholic myopathy: lack of effect of zinc supplementation. Food Chem Toxicol 2005; 43: 1333–1343.
- 10 Fernandez-Sola J, Estruch R, Grau JM, Pare JC, Urbano-Marquez A, Rubin E. The relation of alcoholic myopathy to cardiomyopathy. Curr Opin Neurol 1994; 9: 400–405.
- 11 Fernandez-Sola J, Villegas E, Nicolas JM, Deulofeu R, Antunez E, Sacanella E, et al. Serum and muscle levels of alpha-tocopherol, ascorbic acid, and retinol are normal in chronic alcoholic myopathy. Alcohol Clin Exp Res 1998; 22: 422–427.
- 12 Fernandez-Sola J, Garcia G, Elena M, Tobias E, Sacanella E, Estruch R, et al. Muscle anti-oxidant status in chronic alcoholism. Alcohol Clin Exp Res 2002; 26: 1858–1862.
- 13 Fernandez-Sola J, Nicolas JM, Fatjo F, Garcia G, Sacanella E, Estruch R, et al. Evidence of apoptosis in chronic alcoholic skeletal myopathy. Hum Pathol 2003; 34: 1247–1252.
- 14 Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 2001; 98: 14440–14445.
- 15 Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res 2000; 24: 1070–1076.
- 16 Hanid A, Slavin G, Mair W, Sowter C, Ward P, Webb J, et al. Fibre type changes in striated muscle of alcoholics. J Clin Pathol 1981; 34: 991–995.
- 17 Kiessling KH, Pilstrom L, Bylund AC, Piehl K, Saltin B. Effects of chronic ethanol abuse on structure and enzyme activities of skeletal muscle in man. Scand J Clin Lab Invest 1975; 35: 601–607.
- 18 Koll M, Beeso JA, Kelly FJ, Simanowski UA, Seitz HK, Peters TJ, et al. Chronic alpha-tocopherol supplementation in rats does not ameliorate either chronic or acute alcohol-induced changes in muscle protein metabolism. Clin Sci (Colch) 2003; 104: 287–294.
- 19 Koo-Ng R, Falkous G, Reilly M, Peters TJ, Mantle D, Preedy VR. Carbonyl levels in type I and II fiber-rich muscles and their response to chronic ethanol feeding in vivo and hydroxyl and superoxide radicals in vitro. Alcohol Clin Exp Res 2000; 24: 1862–1868.
- 20 Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-1 stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol 2002; 283: E917–E928.
- 21 Lang CH, Frost RA, Svanberg E, Vary TC. IGF-1/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol 2004; 286: E916–E926.
- 22 Lieber CS, Casini A, Decarli LM, Kim C, Lowe N, Sasaki R, et al. S-Adenosyl-L-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology 1990; 11: 165–172.
- 23 Liu J, Yeo HC, Övervik-Douki E, Hagen T, Doniger SJ, Chu DW, et al. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous anti-oxidants. J Appl Physiol 2000; 89: 21–28.
- 24
Livak KJ,
Schmittgen TD.
Analysis of relative gene expression data using real-time quantitative PCR and the 2
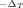 method.
Methods
2001;
25:
402–408.
method.
Methods
2001;
25:
402–408.
- 25 Lundberg IE. The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 2000; 2: 216–224.
- 26 Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart and skeletal muscles: protective effects of anti-oxidants. J Pharmacol Exp Ther 2001; 298: 737–743.
- 27 Martin CJ, Goeddeke-Merickel CM. Oxidative stress in chronic kidney disease. Nephrol Nurs J 2005; 32: 683–685.
- 28 Martin J, White JM. Fluorometric determination of oxidized and reduced glutathione in cells and tissues by high-performance liquid chromatography following derivatization with dansyl chloride. J Chromatogr 1991; 568: 221–225.
- 29 Preedy VR, Peters TJ. The effect of chronic ethanol ingestion on protein metabolism in type-I- and type-II-fibre-rich skeletal muscles of the rat. Biochem J 1988; 254: 631–639.
- 30 Preedy VR, Adachi J, Asano M, Koll M, Mantle D, Niemela O, et al. Free radicals in alcoholic myopathy: indices and preventive study. Free Rad Biol Med 2001; 32: 683–687.
- 31 Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, et al. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil 2003; 24: 55–63.
- 32 Reilly ME, Patel VB, Peters TJ, Preedy VR. In vivo rates of skeletal muscle protein synthesis in rats are decreased by acute ethanol treatment but are not ameliorated by supplemental alpha-tocopherol. J Nutr 2000; 130: 3045–3049.
- 33 Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr 2000; 72(suppl): 653S–669S.
- 34 Trounce I, Byrne E, Dennett X. Biochemical and morphological studies of skeletal muscle in experimental chronic alcoholic myopathy. Acta Neurol Scand 1990; 82: 386–391.
- 35 Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and anti-oxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84.
- 36 Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, et al. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res 2002; 26: 1245–1251.
- 37 Vingren JL, Koziris LP, Gordon SE, Kraemer WJ, Turner RT, Westerlind KC. Chronic alcohol intake, resistance training, and muscle androgen receptor content. Med Sci Sports Exerc 2005; 37: 1842–1848.
- 38 Worrall S, Niemela O, Parkkila S, Peters TJ, Preedy VR. Protein adducts in type I and type II fibre predominant muscles of the ethanol-fed rat: preferential localization in the sarcolemmal and subsarcolemmal region. Eur J Clin Invest 2001; 31: 723–730.




