A split-label design for simultaneous measurements of perfusion in distant slices by pulsed arterial spin labeling
Celine Baligand and Lydiane Hirschler contributed equally to this work.
Funding information
The Netherlands Organization for Scientific Research, under research program VIDI (project number 917.164.90) and under research program VICI (project number 016.160.351)
Abstract
Purpose
Multislice arterial spin labeling (ASL) MRI acquisitions are currently challenging in skeletal muscle because of long transit times, translating into low-perfusion SNR in distal slices when large spatial coverage is required. However, fiber type and oxidative capacity vary along the length of healthy muscles, calling for multislice acquisitions in clinical studies. We propose a new variant of flow alternating inversion recovery (FAIR) that generates sufficient ASL signal to monitor exercise-induced perfusion changes in muscle in two distant slices.
Methods
Label around and between two 7-cm distant slices was created by applying the presaturation/postsaturation and selective inversion modules selectively to each slice (split-label multislice FAIR). Images were acquired using simultaneous multislice EPI. We validated our approach in the brain to take advantage of the high resting-state perfusion, and applied it in the lower leg muscle during and after exercise, interleaved with a single-slice FAIR as a reference.
Results
We show that standard multislice FAIR leads to an underestimation of perfusion, while the proposed split-label multislice approach shows good agreement with separate single-slice FAIR acquisitions in brain, as well as in muscle following exercise.
Conclusion
Split-label FAIR allows measuring muscle perfusion in two distant slices simultaneously without losing sensitivity in the distal slice.
1 INTRODUCTION
Arterial spin labeling (ASL) MRI has become a popular technique for assessing perfusion in muscle. The method is strictly noninvasive and does not require any contrast agent, making it ideal to monitor transient muscle perfusion changes in response to exercise. Such measurements have been used to explore physiology in healthy muscle, as well as in aging.1 Although the lack of sensitivity of the technique to low perfusion values and the need for an exercise challenge have limited its widespread use in muscle so far, ASL remains nonetheless particularly relevant to the study of microvascular reactivity in disorders such as peripheral arterial disease or diabetes.2-4 In these disorders, the muscle’s ability to timely match the oxygen and energy demand during physical activity is compromised, leading to severe complications. Circulatory abnormalities have also been hypothesized to play a role in certain inherited neuromuscular disorders like Duchenne and Becker muscular dystrophies and polymyositis.5-7 However, the exact role of perfusion in these disorders is still unknown.
Several ASL variants exist for muscle perfusion measurements that differ primarily in the approach that is used to magnetically label arterial blood.2-4 Continuous and pseudo-continuous methods rely on flow-driven inversion of blood spins as they travel through an inversion plane, proximal to the region of interest. Although these (pseudo)-continuous approaches yield high SNR due to the long labeling duration, temporal resolution is inherently limited. This becomes critical for postexercise perfusion assessment, as muscles exhibit a large dynamic range of perfusion values and can show very rapid changes. In pulsed ASL approaches, label is created virtually instantaneously over a large volume.8-11 Combined with its ease of implementation, this is probably the reason why pulsed ASL and more specifically flow alternating inversion recovery (FAIR), has become the preferred ASL technique in muscle exercise studies.
Most muscle studies published so far have used FAIR with a single-slice readout, thereby relying on the assumption that the response in this single slice is representative of the complete muscle. However, a number of observations challenge this assumption. First, the mechanic load distribution during exercise may not be uniform.12-14 Moreover, fiber-type distribution and capillary density are known to vary regionally in healthy muscles,15-17 which can affect metabolic and vascular responses to exercise. Recent work using 31P-MRS and T2* imaging also brought evidence that postexercise phosphocreatine kinetics and BOLD signal responses were heterogeneous along the length of the tibialis anterior muscle, suggesting a higher oxidative capacity in the proximal than in the distal part.18 These results prompted a few subsequent studies on healthy-muscle postexercise responses to explore the hemodynamic contribution to this heterogeneity, such as using intravoxel incoherent motion imaging19 or ASL,20 although over a limited distance (3.5 cm). Importantly, proximo-distal heterogeneity of skeletal muscle properties should not be overlooked when monitoring progression of muscle wasting disorders. This has been highlighted for other biomarkers over a large volume, such as fat replacement in muscular dystrophies.21-25 Therefore, there is a need for large-coverage, multislice perfusion acquisitions in functional muscle studies.
Covering larger imaging volumes is challenging with ASL techniques, especially because slow flow can lead to long transit times. In the classic multislice implementation of FAIR, label is only created proximally of the complete imaging stack (Figure 1). This implies long transit times to the most distal slice when the functional response has to be measured over a distance of about 10-15 cm, which may considerably affect SNR, perfusion quantification, and sensitivity.
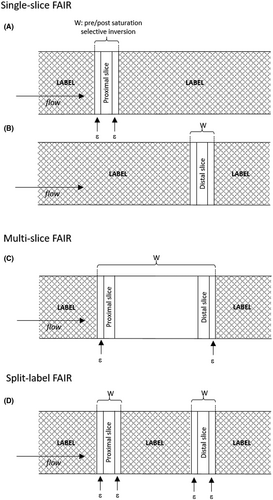
In this study, we propose a modified label scheme for FAIR, allowing perfusion measurements in two slices located 7 cm apart, while also preserving perfusion sensitivity and accuracy in the most distal slice (Figure 1). In this “split label” multislice (MS) approach, the presaturation/postsaturation and selective inversion modules are applied sequentially in the specific slices of interest instead of covering the entire imaging slab. To test our approach, we first validated our sequence in the brain to take advantage of the higher resting-state perfusion levels compared with muscle. In a second study, we applied the multislice split-label FAIR in lower leg muscle and validated it against single-slice FAIR in an interleaved protocol.
2 METHODS
2.1 Arterial spin labeling schemes
- Single slice: For single-slice FAIR, a water suppression enhanced through T1 effects saturation module27 was applied for presaturation, followed by a nonselective or selective 15-ms frequency offset–corrected inversion pulse28 for inversion, and immediately followed by a 5-ms, 90° postsaturation hard pulse. To minimize the effect of possible slice-profile imperfections, the selective inversion and the presaturation and postsaturation thicknesses were systematically 3 mm wider than the imaging slice thickness (Figure 1A,B). As a result, label was created surrounding the individual slice of interest.
- Standard MS: The standard MS scheme used the same elements as the single-slice scheme, with presaturation and postsaturation and inversion thicknesses encompassing the entire imaging stack with an additional 3 mm on both the proximal and distal sides (Figure 1C). Label was therefore created around the entire stack of images, but not in between slices.
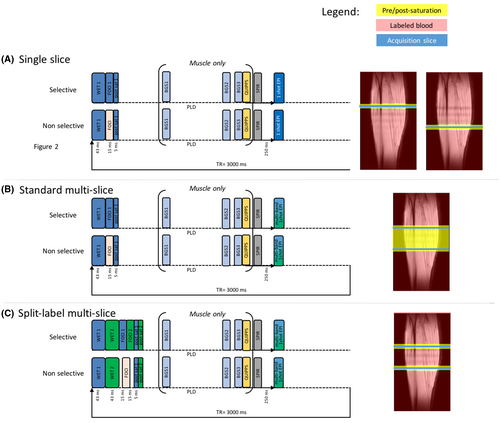
- Split-label MS: This newly proposed split label approach, as in the case of a two-slice acquisition, can be described as two interlaced single-slice schemes (Figure 1D). The first presaturation pulse was selective of the proximal slice and immediately followed by a presaturation pulse selective of the distal slice. The same was true for the selective inversion and the postsaturation pulse with selectivity widths identical to those used in the single-slice sequence. Therefore, label was created not only around the slices of interest, but also in between them, as depicted in Figure 1D. To ensure that possible magnetization transfer effects were identical for the selective and nonselective inversions, the power of the two selective frequency offset–corrected inversion pulses was half of that of the nonselective pulse (Figure 2).
2.2 Subjects
All subjects provided written informed consent, and all experiments were performed in accordance with the Leiden University Medical Center Institutional Review Board (The Netherlands) under authorization numbers NL16198.058 (brain study) and P18.034 (muscle study). A total of 9 subjects were included in this study. We first validated the split-label MS FAIR in the brain, where perfusion values are stable at rest and approximately an order of magnitude higher than in resting muscles.29-31 For this first study, 5 healthy volunteers were included (3 men, 2 women, age range: 25-33 years). In the second part of the study, we applied split-label MS FAIR to measure postexercise reactive hyperemia in the tibialis anterior muscle. Four healthy volunteers participated (2 men, 3 women, age range: 24-33 years) in this part of the study. Subjects were instructed to perform an isometric dorsiflexion against a resistance band for 10 minutes or until self-reported exhaustion to activate the tibialis anterior muscle of their right lower leg while lying in the magnet in feet-first supine position. The average exercise duration was 454 ± 130 seconds.
2.3 Experimental setup
All data were acquired on a Philips 3T MRI scanner (Ingenia; Philips, Best, the Netherlands) using a two-channel body transmit coil array, and either a 32-channel head coil (brain study) or a small extremity flex coil with eight elements placed around the lower leg (muscle study).
2.4 Brain study
2.4.1 Data acquisition
Single-slice, standard MS and split-label MS FAIR sequences were first compared in a resting-state brain study. A three-plane gradient-echo localizer was acquired for image planning. Preparation scans included B0 shimming, B1 calibration, and coil-sensitivity map acquisitions. For each subject, two single-slice FAIR images were acquired separately in two slices positioned parallel to the corpus callosum in sagittal view, with a 5-cm interslice distance as depicted in Figure 2, and referred to as proximal slice and distal slice. The smaller 5-cm gap (versus the 7-cm gap used later in muscle) between the proximal and distal slices was the maximum possible gap that still allowed acquisition of images in the brain with reasonable image quality. These images were used as a reference measurement for the evaluation of the multislice and split-label FAIR performances. Standard MS FAIR and split-label MS FAIR were acquired with a package of two slices positioned at the same location as the single-slice distal and proximal slices (Figure 2). All ASL images were acquired using a single-shot EPI readout (TR/TE = 3000/9.7 ms; acquisition matrix size = 76 × 76; SENSE factor = 2.8; reconstruction matrix size = 80 × 80; FOV = 240 × 240 cm; slice thickness = 5 mm; flip angle = 25°) and a Look-Locker scheme was used to acquire a train of 10 images at different postlabeling delays (PLDs). The PLDs were equally spaced from 250 ms to 2500 ms, to follow the inflow of the labeled blood. The two slices of the standard MS and split-label MS acquisitions were read out at the same time by using a multiband approach (multiband factor 2, multiband shift of 2). In addition, fat suppression was applied before imaging using a spectral presaturation with inversion recovery module. Fifty pairs of ASL images (selective and nonselective labeling) were acquired for signal averaging, yielding an acquisition time of 10 minutes per ASL acquisition. The protocol also included multi-TI scans with the same settings as the FAIR scans, but without presaturation and postsaturation and a single average, to calculate a T1 map for the two slices. Fifteen repetitions were performed, each with a different TI starting at 50 ms and incremented by 200 ms for subsequent acquisitions. The TR was set to 10 seconds to allow for complete relaxation between acquisitions. All other readout or labeling parameters remained unchanged. An M0 map was acquired using the same acquisition parameters as for the FAIR, with the labeling module turned off and using four averages.
2.4.2 Data processing
Data were processed in MATLAB (The MathWorks, Natick, MA). For each PLD, voxel-wise perfusion sensitivity maps were generated as the difference between the selective and nonselective condition, normalized to the M0: ΔMFAIR/M0 = (Mselective − Mnon-selective)/M0. This allowed us to compare the time course of ASL signal between the three different FAIR sequences. Next, T1 maps were calculated by fitting the following equation to the signal from each voxel of the inversion-recovery scans: M0 × (1-2 exp(−PLD/T1)), with M0 and T1 being the fitted parameters. These T1 maps were used to generate a gray-matter mask, including voxels with 750 ms < T1 < 1350 ms. In addition, the first two ΔMFAIR/M0 images (PLDs of 250 ms and 500 ms) were averaged and used to identify large vessels, which were manually outlined and discarded from the gray-matter mask. The final mask was applied to the PLD series of ΔMFAIR/M0 for each sequence. To allow a comparison of the ASL time curve between the three acquisition techniques, for each subject, ΔMFAIR/M0 values were normalized to the maximum value reached across all PLDs and acquisition techniques for that subject. This maximum value was always found in the single-slice FAIR data set. Normalized results are reported as mean ± SEM over the 5 subjects. Finally, cerebral blood flow (CBF) maps were fitted for each subject using the Bayesian Inference for Arterial Spin Labeling (BASIL) tool32 incorporated in the Oxford Center for Functional MRI of the Brain’s software library (FSL). Using the default priors set in BASIL, a macrovascular arterial component was fitted to remove vascular contamination in the CBF map.33 Additionally, the flip angle used in the Look-Locker readout was accounted for in the fitting, and the bolus duration was inferred from the data. Using the previously described gray-matter mask, a mean gray-matter CBF value was extracted for each subject in units of mL/min/100 g.
2.4.3 Statistical analysis
All statistical analyses were performed using GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla, CA). To compare the time courses of ASL signal among the three different methods, a two-way repeated-measures analysis of variance was performed with Holm-Sidak correction for multiple comparison (factor 1: PLD; factor 2: method). To compare mean gray-matter CBF between methods and slices, we performed an analysis of variance using a Friedman’s test and Dunn’s correction for multiple comparisons. A p-value < .05 was considered statistically significant.
2.5 Muscle study
Once validated in the brain, the performance of the split-label approach was tested for postexercise ASL measurements in two distant slices of the tibialis anterior muscle. To allow direct comparison, split-label MS FAIR acquisitions were interleaved with two consecutive single-slice FAIR acquisitions of the corresponding slices.
2.5.1 Data acquisition
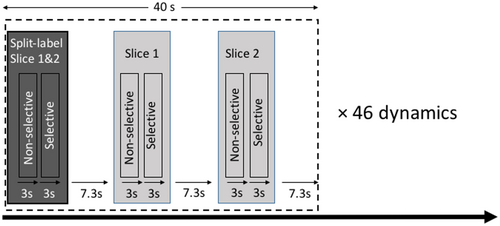
A three-plane gradient-echo localizer was acquired for image planning. Preparation scans included B0 shimming, B1 calibration, and coil sensitivity map acquisitions. For each subject, the hemodynamic response to exercise was monitored dynamically and at the same time by three interleaved FAIR scans acquired with the same PLD: a split-label MS scan and two single-slice scans. The slices were positioned identically for split-label MS and single-slice FAIR with a 7-cm distance and centered at the maximum girth of the calf. Two background suppression pulses, optimized to reduce fat and background signal (TI = 650 ms and 1300 ms, frequency offset–corrected inversion pulses), were applied during the PLD. Due to the large amount of subcutaneous fat and bone marrow present in the lower leg, a spectral presaturation with inversion recovery module was applied before the readout to suppress the remaining fat signal. A quantitative imaging of perfusion using a single subtraction (QUIPSS) module34 was applied 100 ms before imaging to obtain a sharp labeling bolus and allow better muscle perfusion quantification.29 It consisted of three 38-mm saturation slabs, evenly spaced in time, and applied 10 mm proximal to the imaging slice. For the split-label MS FAIR, the QUIPSS module of the proximal slice was duplicated and interleaved with a QUIPSS module for the distal slice, applied in between the two slices (Figure 2). All ASL images were acquired using a single-shot EPI readout (TR/TE = 3000/9.7 ms; acquisition matrix size = 68 × 68; SENSE factor = 2.5; reconstruction matrix 80 × 80; FOV = 180 × 200 mm; slice thickness = 8 mm) after a PLD of 1600 ms. For each method, the pair of ASL images (selective and nonselective) was acquired in 6 seconds. An incompressible interscan delay of 7.3 seconds separated two consecutive scans (as per scanner limitation to switch sequences). Therefore, a set of three different FAIR acquisitions (one split-label MS and two single-slice scans) was acquired within 40 seconds. This set of three scans was repeated 46 times over a total duration of 30 minutes (Figure 3). An M0 map was acquired using the same acquisition parameters as for the FAIR with the labeling module turned off and using four averages.
2.5.2 Data processing
Voxel-wise muscle perfusion (f) maps were calculated as 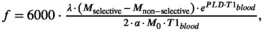 where
where  is the tissue-blood partition coefficient, α is the labeling efficiency assumed to be 0.98 for pulsed ASL, and 6000 is a unit conversion factor yielding f in units of mL/min/100 g. The value of T1blood was assumed to be 1.65 seconds at 3 T.29 Voxels within the muscle that contained vascular signal were excluded for all dynamics and were defined as voxels exceeding a threshold in one of the dynamics. This threshold was calculated from a region of interest drawn in one of the largest arteries and was set to be half a SD lower than the mean arterial signal.
is the tissue-blood partition coefficient, α is the labeling efficiency assumed to be 0.98 for pulsed ASL, and 6000 is a unit conversion factor yielding f in units of mL/min/100 g. The value of T1blood was assumed to be 1.65 seconds at 3 T.29 Voxels within the muscle that contained vascular signal were excluded for all dynamics and were defined as voxels exceeding a threshold in one of the dynamics. This threshold was calculated from a region of interest drawn in one of the largest arteries and was set to be half a SD lower than the mean arterial signal.
2.5.3 Statistical analysis
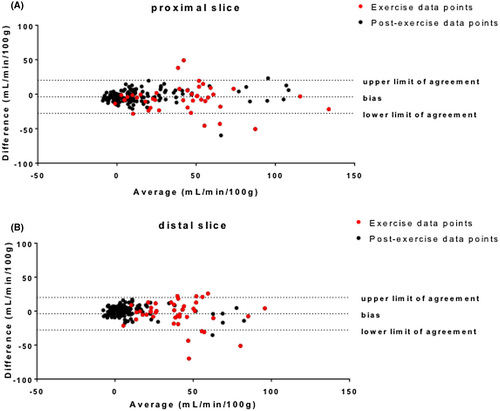
All statistical analyses were performed using GraphPad Prism version 6.01 for Windows. Bland-Altman analyses were used to compare the results obtained from the single-slice acquisition and the split-label MS acquisition for each slice.
3 RESULTS
3.1 Brain experiments
Our data showed a large difference between the perfusion contrast obtained with the single-slice FAIR (used as a reference) and the standard MS FAIR (Figure 4A,B). Statistical analysis showed that this difference was significant at all PLDs and for both slices (Figure 4C,D; P < .001).
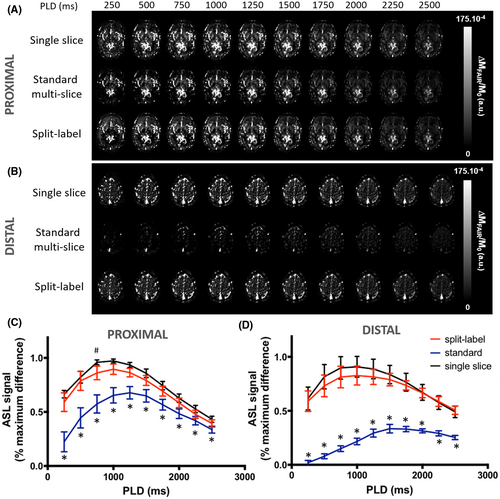
In the distal slice, the largest difference between the MS FAIR contrast and the single-slice contrast was seen at the first PLD (PLD = 250 ms). The MS FAIR contrast was 95 ± 10% lower than the single-slice FAIR contrast. This difference was the lowest at the last measured PLD (PLD = 2500 ms), with a difference of 47 ± 8%. There was also a delay in the time to reach maximum perfusion contrast. The peak ASL signal was reached earlier with the single-slice FAIR acquisition (PLD = 950 ± 275 ms) than with the standard MS FAIR (PLD = 1650 ± 137 ms).
In the proximal slice, the difference in perfusion contrast between single-slice FAIR and standard MS FAIR was also maximal at PLD = 250 ms with the standard MS-FAIR value 67 ± 31% lower that the single slice, on average. The standard MS-FAIR maximum perfusion contrast was reached, on average, at PLD = 1250 ± 177 ms, with a delay of 250 ms compared with the single-slice FAIR in the same slice for 4 of 5 subjects, and no delay in 1 of the subjects.
When using the split-label MS design in the brain, we found that the perfusion contrast was not significantly different from that acquired with the single-slice FAIR sequence in both the proximal and the distal slices (Figure 4C,D). The time courses of the perfusion contrast as a function of PLD did not show any statistical difference either. Notably, the time to reach maximum perfusion contrast was not different between the single-slice acquisitions and split-label MS acquisitions for both slices (PLD = 1000 ms).
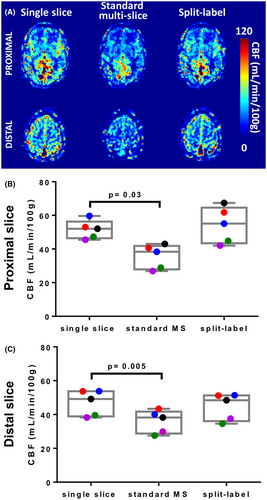
Quantified CBF maps from a representative subject are provided in Figure 5A. Data confirmed that standard MS FAIR led to an underestimation of perfusion compared with the single-slice FAIR acquisitions, which was corrected using the split-label FAIR. Using the same gray-matter masks as for the ASL signal time curves, we found that the group-average gray-matter perfusion value in the proximal slice was of 47 ± 7 mL/min/100 g with the single-slice FAIR, 36 ± 7 mL/min/100 g with the standard MS FAIR, and 45 ± 8 mL/min/100 g with the split-label FAIR. An analysis of variance using Friedman’s test and Dunn’s multiple-comparison correction showed that CBF results acquired with single-slice FAIR and split-label FAIR were not significantly different (P = .34), although CBF results acquired with standard MS were significantly lower than single-slice FAIR (P = .005; Figure 5B). The same results were found for the distal slice, with CBF of 51 ± 6 mL/min/100 g in the single-slice FAIR, 36 ± 7 mL/min/100 g in the standard MS FAIR, and 54 ± 11 mL/min/100 g in the split-label FAIR. The standard MS FAIR values were significantly lower that the single-slice values (P = .03), and no difference between single-slice and split-label FAIR (P > .99; Figure 5C).
3.2 Muscle experiments
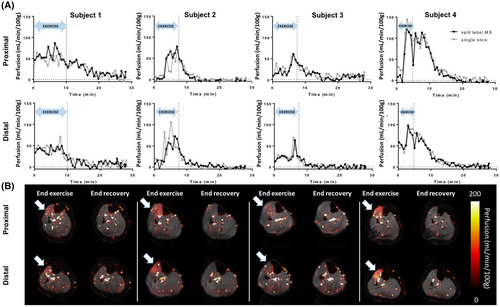
After isometric dorsiflexion exercise, an increase in perfusion was observed in the tibialis anterior, both using the split-label MS and single-slice FAIR. Postexercise responses from the proximal and distal slices were variable across subjects (Figure 6), both in terms of the amplitude and duration of the hyperemia. This can also be appreciated on end-exercise perfusion maps (Figure 6B), showing a specific response of the tibialis anterior as targeted by the type of exercise, but with variable intensity across subjects. Figure 6 also shows end-recovery maps established as the average over the last 5 minutes of acquisition. Mean end-recovery values in the tibialis anterior were low (0.2 ± 6.5 mL/min/100 g in the proximal slice, and 0.7 ± 3.9 mL/min/100 g in the distal slice), suggesting that the muscle had returned to resting state by that time. Using a Bland-Altman analysis, we showed a good level of agreement between the split-label MS and single-slice FAIR acquisitions for both slices (Figure 7), as 95.6% (proximal slice) and 93.5% (distal slice) of the data points lay within the limits of agreement between methods (data pooled over all subjects), and the bias was close to 0 in both slices. Interestingly, most of the points falling outside of the agreement limits were acquired during the exercise (red dots on Figure 7) (ie, were more likely to be affected by movement artifacts).
4 DISCUSSION
The ability to measure perfusion along a muscle is particularly important when studying skeletal muscle pathophysiology. In this study, we showed that the implementation of a split-label MS FAIR enabled the assessment of perfusion in two 7-cm distant slices simultaneously while preserving perfusion sensitivity and accuracy in the most distal slice.
We first validated our sequence in the brain to take advantage of the higher resting-state perfusion levels compared with muscle. We compared the proposed split-label MS approach to both a standard MS FAIR and to two separate single-slice FAIR acquisitions. We showed that by preserving the ability to create label in the gap between the two slices, split-label FAIR enables better perfusion sensitivity in the distal slice than the standard FAIR implementation. Moreover, using a standard MS FAIR in distant slices led to suboptimal perfusion sensitivity both in proximal and distal slices. This can be explained by the absence of label in the large gap between the two slices in the standard MS-FAIR case. Because of this large labeling slab, the labeled signal that eventually reaches the distal slice originates from a remote (proximal) location and has already decayed significantly due to longitudinal relaxation before arriving at this distal location. This effect could be even more prominent in muscle, where blood flow is about 6 times lower at rest,29-31 yielding longer transit times.
Suboptimal ASL signal was also found in the proximal slice when using the standard MS approach. This can be explained by a substantial contribution from blood, either venous or arterial, flowing from the distal side into the imaging slice. Venous enhancement is a well-known feature of FAIR. Indeed, with the FAIR sequence, and as opposed to other variations like PICORE35 or EPISTAR,36 label is created on both sides of the slice of interest, generating a positive signal from venous flow,37 whereas some arterial signal might also be labeled above the imaging slice for single-slice and split-label FAIR. By creating label in between the two slices, it is likely that our acquisitions proved more sensitive to this effect.
Using the split-label MS approach, where label is created in the gap between the two imaging slices, proper perfusion signal in both slices was restored. In the brain, we still noticed a (nonsignificant) trend to lower ASL values with split-label MS compared with the single-slice approach, likely due to the fact that blood within the proximal (resp. distal) slice is not labeled but saturated for both the label and control scan, and therefore does not contribute to signal in the distal (resp. proximal) slice. This effect is reduced when increasing the gap between slices, as can be seen in our muscle results.
In a second step, we applied the split-label MS FAIR, interleaved with two single-slice acquisitions, in the lower leg muscle during and after exercise. In our setup, the perfusion response to the exercise was variable among the 4 subjects. This is not surprising, as we did not standardize the exercise across participants in this technical proof-of-principle study, nor did we select a group of participants with consistent levels of training. In the current data set, it cannot be excluded that the amplitude and duration of the hyperemia also reflects differences in muscle types due to different levels of training. It is well documented that endurance athletes, compared with sedentary subjects38 or even sprinter athletes,11 have differential perfusion and BOLD response to exercise, and that age is also an important factor.1 Although future developments will include a standardization and calibration of the exercise protocol, and while future studies will factor in the self-reported level activity of the subjects, this variability in the exercise intensity gave us an opportunity to compare the split-label MS results with the single-slice results in a large range of perfusion values. We found that our proposed sequence performed in the same way as the two separate single-slice experiments (Figure 6), both in the presence of a strong increase after exercise (subjects 2 and 4) as well as for only modest increases (subjects 1 and 3). Altogether, we have shown that split-label MS FAIR is the technique of choice to measure perfusion in the muscle in two distant slices simultaneously: Split-label MS provides better contrast than standard MS, and higher time resolution than interleaved single-slice measurements. Moreover, the time resolution can be increased by subtracting each control image to both the preceding and the following labeled image, generating two ASL images (so-called surround subtraction). Surround subtraction is not possible when interleaving two single-slice FAIR sequences. In our acquisitions, slices were separated by 7 cm; the largest achievable distance between slices is eventually only limited by the RF coverage of the transmit and receive coil and by the gradient linearity.
Other techniques such as pseudo-continuous ASL (pCASL) have been used in muscle. The drawback of using pCASL in our particular context is that the label has to travel from the labeling plane located proximally from the imaging volume up to the slice of interest. Although this works well for a single slice when the travel distance is relatively small,31 or in multislice acquisitions with a narrow stack of slices,3 it becomes more problematic for larger slice gaps, as was the objective in our study. The large travel distance combined with the slow blood flow in muscle at rest makes pCASL suboptimal for studying distant slices separated by a large slice gap. Additionally, pCASL is sensitive to B1 and B0 inhomogeneities,1, 2 which could be enhanced in the case of a larger slice gap. Finally, it is much more challenging for pCASL to measure perfusion during a dynamic exercise, because movement of the target region will make it hard to label for a longer period (typically 1 to 2 seconds) by pCASL, as compared with the instantaneous labeling over the entire leg as is done with FAIR.
A direct and interesting application of the technique is the study of the differences in perfusion along the length of the muscle. Previous work already explored the variation in oxidative metabolism along the length of the tibialis anterior using 31P-NMR spectroscopy, showing higher oxidative capacity proximally.18 If the lower oxidative capacities simply reflect a different fiber type (fast twitch muscle), it is possible that functional hyperemia would be lower in distal parts of the muscle. However, the available data looking at the vascular component of the response to exercise do not allow full characterization of the role of blood supply in these proximal-distal differences, as they were performed either over a small stack of slices20 or with non-quantitative BOLD acquisitions.18 Moreover, it is possible that these proximal-distal differences are enhanced in pathological conditions. Future work will be aimed at using split-label MS FAIR to study proximo-distal heterogeneity of skeletal muscle properties in healthy subjects and neuromuscular disorders with a standardized exercise protocol. This will provide valuable insight in muscle physiology and possibly shed new light on the progression of muscle-wasting disorders.