Vertical open-bore MRI scanners generate significantly less radiofrequency heating around implanted leads: A study of deep brain stimulation implants in 1.2T OASIS scanners versus 1.5T horizontal systems
Ehsan Kazemivalipour
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Department of Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey
National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey
Search for more papers by this authorBhumi Bhusal
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorJasmine Vu
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Department of Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, Illinois, USA
Search for more papers by this authorStella Lin
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorBach Thanh Nguyen
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorJohn Kirsch
A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, Massachusetts, USA
Search for more papers by this authorElizabeth Nowac
Department of Neurosurgery, Albany Medical Center, Albany, New York, USA
Search for more papers by this authorJulie Pilitsis
Illinois Bone and Joint Institute (IBJI), Wilmette, Illinois, USA
Search for more papers by this authorJoshua Rosenow
Department of Neurological Surgery, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorErgin Atalar
Department of Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey
National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey
Search for more papers by this authorCorresponding Author
Laleh Golestanirad
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Department of Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, Illinois, USA
Correspondence
Laleh Golestanirad, Department of Radiology, Feinberg School of Medicine, Northwestern University, 737 N Michigan Ave, Suite 1600, Chicago, IL 60611, USA.
Email: [email protected]
Search for more papers by this authorEhsan Kazemivalipour
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Department of Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey
National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey
Search for more papers by this authorBhumi Bhusal
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorJasmine Vu
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Department of Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, Illinois, USA
Search for more papers by this authorStella Lin
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorBach Thanh Nguyen
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorJohn Kirsch
A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, Massachusetts, USA
Search for more papers by this authorElizabeth Nowac
Department of Neurosurgery, Albany Medical Center, Albany, New York, USA
Search for more papers by this authorJulie Pilitsis
Illinois Bone and Joint Institute (IBJI), Wilmette, Illinois, USA
Search for more papers by this authorJoshua Rosenow
Department of Neurological Surgery, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Search for more papers by this authorErgin Atalar
Department of Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey
National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey
Search for more papers by this authorCorresponding Author
Laleh Golestanirad
Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Department of Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, Illinois, USA
Correspondence
Laleh Golestanirad, Department of Radiology, Feinberg School of Medicine, Northwestern University, 737 N Michigan Ave, Suite 1600, Chicago, IL 60611, USA.
Email: [email protected]
Search for more papers by this authorBhumi Bhusal and Ehsan Kazemivalipour contributed equally to this work.
Abstract
Purpose
Patients with active implants such as deep brain stimulation (DBS) devices are often denied access to MRI due to safety concerns associated with the radiofrequency (RF) heating of their electrodes. The majority of studies on RF heating of conductive implants have been performed in horizontal close-bore MRI scanners. Vertical MRI scanners which have a 90° rotated transmit coil generate fundamentally different electric and magnetic field distributions, yet very little is known about RF heating of implants in this class of scanners. We performed numerical simulations as well as phantom experiments to compare RF heating of DBS implants in a 1.2T vertical scanner (OASIS, Hitachi) compared to a 1.5T horizontal scanner (Aera, Siemens).
Methods
Simulations were performed on 90 lead models created from post-operative CT images of patients with DBS implants. Experiments were performed with wires and commercial DBS devices implanted in an anthropomorphic phantom.
Results
We found significant reduction of 0.1 g-averaged specific absorption rate (30-fold, P < 1 × 10−5) and RF heating (9-fold, P < .026) in the 1.2T vertical scanner compared to the 1.5T conventional scanner.
Conclusion
Vertical MRI scanners appear to generate lower RF heating around DBS leads, providing potentially heightened safety or the flexibility to use sequences with higher power levels than on conventional systems.
Supporting Information
| Filename | Description |
|---|---|
| mrm28818-sup-0001-TableS1-3.docxWord document, 30.3 KB |
TABLE S1 Maximum of 0.1g SAR values for patient 47 (ID47) with different convergence thresholds TABLE S2 Maximum of 0.1g SAR TABLE S3 Measured temperature increase at the lead tip for each implant model |
| mrm28818-sup-0002-VideoS1.mp4MPEG-4 video, 8.2 MB | VIDEO S1 Incident E field (arrows) and Etan (color field overlaid on leads) for varying intrinsic phases along lead trajectories in patient #47 for the 1.2 T vertical OASIS coil and 1.2 T and 1.5 T horizontal birdcage coils. Simulations were done for the head imaging landmark and the coil's input power was adjusted to generate a mean 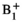 = 4 μT over a circular plane placed on an axial plane passing through the coil's iso-center = 4 μT over a circular plane placed on an axial plane passing through the coil's iso-center |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Rezai AR, Finelli D, Nyenhuis JA, et al. Neurostimulation systems for deep brain stimulation: in vitro evaluation of magnetic resonance imaging–related heating at 1.5 Tesla. J Magn Reson Imaging. 2002; 15: 241-250.
- 2McElcheran CE, Yang B, Anderson KJT, et al. Parallel radiofrequency transmission at 3 Tesla to improve safety in bilateral implanted wires in a heterogeneous model. Magn Reson Med. 2017; 78: 2406-2415.
- 3Medical SJ. MRI Procedure Information; 2018. https://manuals.sjm.com/~/media/manuals/product-manual-pdfs/d/6/d6db9679-aaa7-4e22-b1e3-1d8a2ac43b98.pdf. Accessed April 20, 2021.
- 4Schäfer A, Forstmann BU, Neumann J, et al. Direct visualization of the subthalamic nucleus and its iron distribution using high-resolution susceptibility mapping. Hum Brain Mapp. 2012; 33: 2831-2842.
- 5Slavin K, Thulborn K, Wess C, et al. Direct visualization of the human subthalamic nucleus with 3T MR imaging. Am J Neuroradiol. 2006; 27: 80-84.
- 6Sudhyadhom A, Haq IU, Foote KD, et al. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the fast gray matter acquisition T1 inversion recovery (FGATIR). Neuroimage. 2009; 47: T44-T52.
- 7Yeung CJ, Susil RC, Atalar E. RF heating due to conductive wires during MRI depends on the phase distribution of the transmit field. Magn Reson Med. 2002; 48: 1096-1098.
- 8Golestanirad L, Angelone LM, Iacono MI, et al. Local SAR near deep brain stimulation (DBS) electrodes at 64 and 127 MH z: a simulation study of the effect of extracranial loops. Magn Reson Med. 2017; 78: 1558-1565.
- 9Golestanirad L, Keil B, Angelone LM, et al. Feasibility of using linearly polarized rotating birdcage transmitters and close-fitting receive arrays in MRI to reduce SAR in the vicinity of deep brain simulation implants. Magn Reson Med. 2017; 77: 1701-1712.
- 10Golestanirad L, Kirsch J, Bonmassar G, et al. RF-induced heating in tissue near bilateral DBS implants during MRI at 1.5 T and 3T: The role of surgical lead management. Neuroimage. 2019; 184: 566-576.
- 11Golestanirad L, Angelone LM, Kirsch J, et al. Reducing RF-induced heating near implanted leads through high-dielectric capacitive bleeding of current (CBLOC). IEEE Trans Microw Theory Tech. 2019; 67: 1265-1273.
- 12Kazemivalipour E, Vu J, Lin S, et al. RF heating of deep brain stimulation implants during MRI in 1.2 T vertical scanners versus 1.5 T horizontal systems: a simulation study with realistic lead configurations. 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC): IEEE. 2020, pp. 6143-6146.
- 13Ibrahim T, Lee R, Baertlein B, et al. B1 field homogeneity and SAR calculations for the birdcage coil. Phys Med Biol. 2001; 46: 609.
- 14Ibrahim TS, Lee R, Baertlein BA, et al. Computational analysis of the high pass birdcage resonator: finite difference time domain simulations for high-field MRI. Magn Reson Imaging. 2000; 18: 835-843.
- 15Collins CM, Liu W, Wang J, et al. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J Magn Reson Imaging. 2004; 19: 650-656.
- 16Collins CM, Li S, Smith MB. SAR and B1 field distributions in a heterogeneous human head model within a birdcage coil. Magn Reson Med. 1998; 40: 847-856.
- 17Lucano E, Liberti M, Mendoza GG, et al. Assessing the electromagnetic fields generated by a radiofrequency MRI body coil at 64 mhz: defeaturing versus accuracy. IEEE Trans Biomed Eng. 2015; 63: 1591-1601.
- 18Wolf S, Diehl D, Gebhardt M, et al. SAR simulations for high-field MRI: how much detail, effort, and accuracy is needed? Magn Reson Med. 2013; 69: 1157-1168.
- 19Fujimoto K, Zaidi TA, Lampman D, et al. Specific absorption rate (SAR) comparison in the conventional and open MRI systems utilizing an anatomical human computational model. Proc Intl Soc Mag Reson Med. 2021; 28: 1616.
- 20Ochi H, Soutome Y, Bito Y, Suzuki S, Shimoda T, Taniguchi T. High frequency coil for magnetic resonance imaging device. Patent Number WO/2008/108048. December 9, 2008.
- 21Yeo DT, Wang Z, Loew W, et al. Local SAR in high pass birdcage and TEM body coils for multiple human body models in clinical landmark positions at 3T. J Magn Reson Imaging. 2011; 33: 1209.
- 22Yeung CJ, Susil RC, Atalar E. RF safety of wires in interventional MRI: using a safety index. Magn Reson Med. 2001; 47: 187-193.
- 23Nguyen BT, Pilitsis J, Golestanirad L. The effect of simulation strategies on prediction of power deposition in the tissue around electronic implants during magnetic resonance imaging. Phys Med Biol. 2020; 65: 185007.
- 24Golestanirad L, Rahsepar AA, Kirsch JE, et al. Changes in the specific absorption rate (SAR) of radiofrequency energy in patients with retained cardiac leads during MRI at 1.5 T and 3T. Magn Reson Med. 2019; 81: 653-669.
- 25Nordbeck P, Weiss I, Ehses P, et al. Measuring RF-induced currents inside implants: impact of device configuration on MRI safety of cardiac pacemaker leads. Magn Reson Med. 2009; 61: 570-578.
- 26Golestanirad L, Kazemivalipour E, Keil B, et al. Reconfigurable MRI coil technology can substantially reduce RF heating of deep brain stimulation implants: first in-vitro study of RF heating reduction in bilateral DBS leads at 1.5 T. PLoS One. 2019; 14:e0220043.
- 27Vu J, Bhusal B, Nguyen BT, et al. Evaluating accuracy of numerical simulations in predicting heating of wire implants during MRI at 1.5 T. 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC): IEEE. 2020, pp. 6107-6110.
- 28Nordbeck P, Ritter O, Weiss I, et al. Impact of imaging landmark on the risk of MRI-related heating near implanted medical devices like cardiac pacemaker leads. Magn Reson Med. 2011; 65: 44-50.
- 29Bhusal B, Nguyen BT, Sanpitak P, et al. Effect of device configuration and patient’s body composition on the RF heating and non-susceptibility artifact of deep brain stimulation implants during MRI at 1.5 T and 3 T. J Magn Reson Imaging. 2020; 53: 599-610.
- 30Bhusal B, Keil B, Rosenow J, et al. Patient’s body composition can significantly affect RF power deposition in the tissue around DBS implants: ramifications for lead management strategies and MRI field-shaping techniques. Phys Med Biol. 2021; 66:015008.
- 31Bhusal B, Nguyen BT, Vu J, et al. Device configuration and patient’s body composition significantly affect RF heating of deep brain stimulation implants during MRI: an experimental study at 1.5 T and 3T. 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC): IEEE. 2020, pp. 5192-5197.
- 32Zhang M, Che Z, Chen J, et al. Experimental determination of thermal conductivity of water− agar gel at different concentrations and temperatures. J Chem Eng Data. 2011; 56: 859-864.
- 33 IT’IS Foundation. Thermal Conductivity. https://itis.swiss/virtual-population/tissue-properties/database/thermal-conductivity/. Accessed April 20, 2021.
- 34Davidson B, Tam F, Yang B, et al. Three-Tesla magnetic resonance imaging of patients with deep brain stimulators: results from a phantom study and a pilot study in patients. Neurosurgery. 2021; 88: 349-355.
- 35Boutet A, Rashid T, Hancu I, et al. Functional MRI safety and artifacts during deep brain stimulation: experience in 102 patients. Radiology. 2019; 293: 174-183.
- 36Park SM, Kamondetdacha R, Nyenhuis JA. Calculation of MRI-induced heating of an implanted medical lead wire with an electric field transfer function. J Magn Reson Imaging. 2007; 26: 1278-1285.
- 37 Neurostimulatin devices: Market estimation & trend analysis from 2016 to 2024. San Francisco, CA: Grand View Research, Inc; 2016.
- 38Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005; 28: 326-328.
- 39Naehle CP, Zeijlemaker V, Thomas D, et al. Evaluation of cumulative effects of MR imaging on pacemaker systems at 1.5 Tesla. Pacing Clin Electrophysiol. 2009; 32: 1526-1535.
- 40Falowski S, Safriel Y, Ryan MP, et al. The rate of magnetic resonance imaging in patients with deep brain stimulation. Stereotact Funct Neurosurg. 2016; 94: 147-153.
- 41Cui Z, Pan L, Song H, et al. Intraoperative MRI for optimizing electrode placement for deep brain stimulation of the subthalamic nucleus in Parkinson disease. J Neurosurg. 2016; 124: 62-69.
- 42Ramani S, Schulte R, Mckinnon G, et al. Accurate localization of individual DBS contacts by MRI using zero-TE phase images. Proc Intl Soc Mag Reson Med. 2018; 26: 2687.
- 43DiMarzio M, Madhavan R, Joel S, et al. Use of functional magnetic resonance imaging to assess how motor phenotypes of Parkinson's disease respond to deep brain stimulation. Neuromodulation: Technology at the Neural Interface. 2020; 23: 515-524.
- 44DiMarzio M, Rashid T, Hancu I, et al. Functional MRI signature of chronic pain relief from deep brain stimulation in Parkinson disease patients. Neurosurgery. 2019; 85: E1043-E1049.
- 45Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019; 15: 148-160.
- 46Horn A. The impact of modern-day neuroimaging on the field of deep brain stimulation. Curr Opin Neurol. 2019; 32: 511-520.
- 47Golestanirad L, Iacono MI, Keil B, et al. Construction and modeling of a reconfigurable MRI coil for lowering SAR in patients with deep brain stimulation implants. Neuroimage. 2017; 147: 577-588.
- 48Kazemivalipour E, Keil B, Vali A, et al. Reconfigurable MRI technology for low-SAR imaging of deep brain stimulation at 3T: application in bilateral leads, fully-implanted systems, and surgically modified lead trajectories. Neuroimage. 2019; 199: 18-29.
- 49McElcheran CE, Golestanirad L, Iacono MI, et al. Numerical simulations of realistic lead trajectories and an experimental verification support the efficacy of parallel radiofrequency transmission to reduce heating of deep brain stimulation implants during MRI. Sci Rep. 2019; 9: 1-14.
- 50McElcheran CE, Yang B, Anderson KJT, et al. Investigation of parallel radiofrequency transmission for the reduction of heating in long conductive leads in 3 Tesla magnetic resonance imaging. PLoS One. 2015; 10:e0134379.
- 51Eryaman Y, Guerin B, Akgun C, et al. Parallel transmit pulse design for patients with deep brain stimulation implants. Magn Reson Med. 2014; 73: 1896-1903.
- 52Eryaman Y, Kobayashi N, Moen S, et al. A simple geometric analysis method for measuring and mitigating RF induced currents on deep brain stimulation leads by multichannel transmission/reception. Neuroimage. 2019; 184: 658-668.
- 53Olsen JM, Hrdlicka GA, Wahlstrand CD, et al. Lead Electrode for Use in an MRI-Safe Implantable Medical Device. US Patent US7877150B2. 2010.
- 54McCabe S, Scott J. A novel implant electrode design safe in the RF field of MRI scanners. IEEE Trans Microw Theory Tech. 2017; 65: 3541-3547.
- 55Stevenson RA, Halperin HR, Lardo AC, et al. Implantable Lead Bandstop Filter Employing an Inductive Coil with Parasitic Capacitance to Enhance MRI Compatibility of Active Medical Devices. US Patent US8903505B2. 2012.
- 56Atalar E, Allen J, Bottomley P et al. MRI-Safe High Impedance Lead Systems. US Patent US8055351B2. 2011.
- 57Vase A, Sethna DN. Implantable Medical Lead Configured for Improved MRI Safety. US Patent US20090270956A1. 2008.
- 58Serano P, Angelone LM, Katnani H, et al. A novel brain stimulation technology provides compatibility with MRI. Sci Rep. 2015; 5: 1-10.
- 59Kakugawa S, Kitamura S, Hara N, et al. Open MRI System with a Vertical Static Field and an Imaging Volume Closer to the Lower Than to the Upper Magnet Assembly. US Patent US6600318B1. 2003. https://patentimages.storage.googleapis.com/cc/39/e1/ab1cf9f453ca41/US6600318.pdf. Accessed April 20, 2021.
- 60Yeung CJ, Susil RC, Atalar E. RF heating due to conductive wires during MRI depends on the phase distribution of the transmit field. Magn Reson Med. 2002; 48: 1096-1098.
- 61Golestanirad L, Pilitsis J, Martin A, et al. Variation of RF heating around deep brain stimulation leads during 3.0 T MRI in fourteen patient-derived realistic lead models: the role of extracranial lead management. Proc Intl Soc Mag Reson Med. 2017; 25: 0484.
- 62Brühl R, Ihlenfeld A, Ittermann B. Gradient heating of bulk metallic implants can be a safety concern in MRI. Magn Reson Med. 2017; 77: 1739-1740.
- 63 ISO/TS 10974:2018. Assessment of the Safety of Magnetic Resonance Imaging for Patients with an Active Implantable Medical Device. Geneva, Switzerland: International Organization for Standardization; 2018.




