Toward more reliable measurements of NOE effects in CEST spectra at around −1.6 ppm (NOE (−1.6)) in rat brain
Funding information: National Institutes of Health, Grant/Award number: EB017873
Abstract
Purpose
Recently, a new relayed nuclear Overhauser enhancement (NOE) saturation transfer effect at around −1.6 parts per million, termed NOE(−1.6), and its potential applications in tumor and stroke were reported by several institutes. However, there is a concern of the reproducibility of NOE(−1.6) measurements because it is not reported by many other publications. This paper aims to study the influence of typically overlooked experimental settings on the NOE(−1.6) signal and to build a framework for more reliable measurements of NOE(−1.6) at 9.4T.
Methods
Z-spectra were obtained in rat brains. A fitting approach was performed to quantify all known saturation transfer effects except NOE(−1.6). Residual signals were obtained by removing these confounding effects from Z-spectra and were then used to quantify NOE(−1.6). Multislice imaging was performed to study the NOE(−1.6) dependence on brain regions. The influences of euthanasia, anesthesia, breathing gases, and RF irradiation power were also evaluated.
Results
Results demonstrate that the NOE(−1.6) signal contributions are often not clearly observable in raw Z-spectra at relatively high irradiation powers due to, for example, the direct water saturation effect, but they can be visualized after removing other nonspecific effects. In addition, the NOE(−1.6) effect depends on brain region, decreases postmortem, shifts after long-duration anesthesia, and may be enhanced by increasing O2 and N2O breathing air concentrations.
Conclusion
Because the NOE(−1.6) effect is more susceptible to the direct water saturation effect and more sensitive to physiological conditions than are other CEST effects, incorporating known sensitivities into the experimental design and data analysis is necessary to ensure more reliable NOE(−1.6) results.



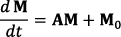 , where A is a 19 × 19 matrix. The water and solute pools each has three coupled equations representing their x, y, and z components. The semi-solid MT pool has a single coupled equation representing the z component, with a Lorentzian absorption lineshape. fs, ksw, Δ, and T2w were varied individually with all other parameters remaining at the value listed in the following table. T1 and T2 represent the longitudinal and transverse relaxation time of each pool.
, where A is a 19 × 19 matrix. The water and solute pools each has three coupled equations representing their x, y, and z components. The semi-solid MT pool has a single coupled equation representing the z component, with a Lorentzian absorption lineshape. fs, ksw, Δ, and T2w were varied individually with all other parameters remaining at the value listed in the following table. T1 and T2 represent the longitudinal and transverse relaxation time of each pool.
