Proteomic diversification of spermatostyles among six species of whirligig beetles
Abstract
Seminal fluid protein composition is complex and commonly assumed to be rapidly divergent due to functional interactions with both sperm and the female reproductive tract (FRT), both of which evolve rapidly. In addition to sperm, seminal fluid may contain structures, such as mating plugs and spermatophores. Here, we investigate the evolutionary diversification of a lesser-known ejaculate structure: the spermatostyle, which has independently arisen in several families of beetles and true bugs. We characterized the spermatostyle proteome, in addition to spermatostyle and FRT morphology, in six species of whirligig beetles (family Gyrinidae). Spermatostyles were enriched for proteolytic enzymes, and assays confirmed they possess proteolytic activity. Sperm-leucylaminopeptidases (S-LAPs) were particularly abundant, and their localization to spermatostyles was confirmed by immunohistochemistry. Although there was evidence for functional conservation of spermatostyle proteomes across species, phylogenetic regressions suggest evolutionary covariation between protein composition and the morphology of both spermatostyles and FRTs. We postulate that S-LAPs (and other proteases) have evolved a novel structural role in spermatostyles and discuss spermatostyles as adaptations for delivering male-derived materials to females.
1 INTRODUCTION
Ejaculates are comprised of sperm and seminal fluid, the latter being a complex mixture predominantly of proteins, sugars, and lipids (Gillott, 2003). For example, human seminal plasma is believed to contain thousands of proteins (Samanta et al., 2018), and seminal fluid of the fruit fly, Drosophila melanogaster, includes approximately 100 proteins that have been demonstrated to be transferred to females during mating (McCullough et al., 2022). Ejaculate proteins have diverse impacts on postcopulatory events shaping fertility, including inducing changes to female reproductive tract (FRT) immunity, stimulating ovulation, and influencing female metabolism, physiology, and receptivity to mating (e.g., Abu-Raya et al., 2020; Berland et al., 2016; Schwenke & Lazzaro, 2017; Tsukamoto et al., 2014; Walker et al., 2015). Ejaculates can also include distinct structures. These may be produced by males and transferred to females along with seminal fluid and sperm, such as the fine strands and vesicles observed in the semen of D. melanogaster (Perotti, 1971; Wainwright et al., 2021) and numerous other Drosophila species (S. P., personal observation), the nutrient-containing spermatophylax of some crickets and katydids (Alexander & Otte, 1967; Sakaluk, 1984), and the spermatophores (i.e., capsule, sheath, or mass surrounding sperm) of diverse animals lacking direct transfer of the ejaculate by the male into the FRT (e.g., scorpions, salamanders, and octopuses; Mann, 1984; Schaller, 1971). Alternatively, ejaculate structures may form within the FRT during and/or immediately following copulation from components transferred by males. Examples of such structures include the spermatophores of butterflies, beetles, flies, and other insects (Davey, 1960; Mann, 1984) and the mating plugs of diverse taxa (e.g., primates, rodents, garter snakes, insects; Avila et al., 2015; Dixson & Anderson, 2002; Mcdonough-Goldstein et al., 2022; Schneider et al., 2016; Voss, 1979). Here, we report a molecular and evolutionary investigation of another somewhat obscure class of ejaculate structure: spermatostyles.
Spermatostyles are slender, hyaline rods to which sperm attach to form conjugates (Figure 1). Conjugation refers to the phenomenon of two or more sperm physically uniting for motility or transport through the FRT (Higginson & Pitnick, 2011). The term “spermatostyle” was coined by Breland and Simmons (1970) when describing the sperm conjugates of Dineutus spp. whirligig beetles, although the structures were first described by Gilson (1884) in an investigation of ground beetles (where they were incorrectly referred to as “spermatophores”). Spermatostyles occur in several families of adephagan beetles (ground beetles, diving beetles, and kin; families Carabidae, Dytiscidae, Gyrinidae, and Haliplidae; Breland & Simmons, 1970; Dallai et al., 2019, 2020; Giglio et al., 2024; Gómez & Maddison, 2020; Higginson et al., 2015; Higginson & Pitnick, 2011; Mercati et al., 2023; Salazar et al., 2022, 2023) and auchenorrhynch true bugs (cicadas, planthoppers, and spittlebugs; families Aphrophoridae, Cercopidae, Cicadidae, and Cicadellidae; Chawanji et al., 2005, 2006; Chevaillier, 1963; Chevaillier & Maillet, 1965; Folliot & Maillet, 1970; Hayashi & Kamimura, 2002a, 2002b; Maillet, 1959; Roberston & Gibbs, 1937; Sodré et al., 2024). Spermatostyles vary in their length, from 17 µm to 4.1 cm, the location, density, and organization of attached sperm, and their ultrastructural appearance (e.g., Giglio et al., 2024; Gómez & Maddison, 2020; Higginson & Pitnick, 2011). Spermatostyles clearly have independent evolutionary origins in true bugs and beetles. However, ancestral state reconstructions suggest spermatostyles are ancestral to adephagan beetles, including ground, diving, and whirligig beetles (Gómez & Maddison, 2020; Gomez et al., 2023).
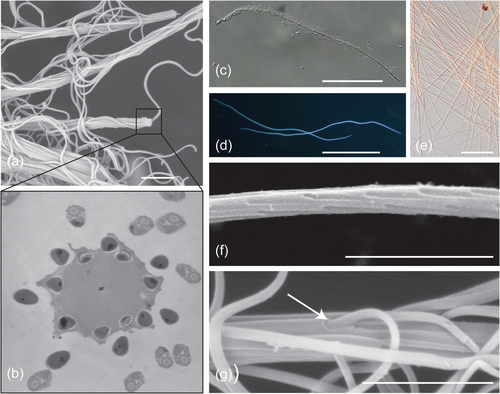
Although spermatostyles are often functionally associated with sperm conjugation, there is no direct evidence to support this as their primary adaptive function, and alternative hypotheses have been proposed (see Higginson & Pitnick, 2011). In fact, spermatostyles are neither required for conjugation nor are they the only mechanism by which sperm unite to form conjugates. Conjugation has independently evolved numerous times and includes a wide array of sperm binding mechanisms exclusive of spermatostyles (Higginson et al., 2012a; Higginson & Pitnick, 2011). In addition to serving as scaffolding in conjugation, spermatostyles might also perform adaptive functions within the FRT (Pitnick et al., 2020). Under this general scenario, it is intriguing to reconsider the interaction of sperm with spermatostyles as one in which sperm are responsible for the delivery of the spermatostyle (and its molecular cargo) to a precise location in the FRT. For internally fertilizing species, sperm, seminal fluid, and ejaculate structures all engage in complex interactions with the FRT that influence fertilization efficiency and competitive fertilization success (Lüpold et al., 2020; Pitnick et al., 2020; Pitnick, Wolfner, et al., 2009; Ravi Ram & Wolfner, 2007; Sirot & Wolfner, 2015; Sirot et al., 2014). In fact, female-derived proteins substantively contribute to sperm and ejaculate (i.e., spermatophores and copulatory plugs) form and function within the FRT (Dean et al., 2011; McCullough et al., 2022; McDonough-Goldstein et al., 2022; Meslin et al., 2017). Post-insemination functionality of spermatostyles would be consistent with the expansive role of seminal fluid proteins (SFP) in mediating postmating responses by females (Hopkins et al., 2017).
Discrimination among alternative hypotheses for the adaptive value of ejaculate structures requires a deeper understanding of the interactions underpinning their form-function relationships inside FRTs (Higginson et al., 2012b; Lüpold et al., 2020; Meslin et al., 2017; Pitnick, Wolfner, et al., 2009; Syed et al., in review). For example, the initial discovery that bumblebee copulatory plugs consist of fatty acids (Baer et al., 2000) led researchers to identify linolic acid as the major plug constituent responsible for female remating delays (Baer et al., 2001). Here, we combine phylogenetic, morphological, and molecular approaches to (i) characterize spermatostyle proteomes and structures, (ii) assay spermatostyle proteolytic activity, and (iii) test for covariation between spermatostyle proteome composition and the form of both spermatostyle and the FRT in whirligig beetles.
2 METHODS
2.1 Specimen collection and spermatostyle isolation
Our investigation included a representative of two of the three clades of Gyrinidae known to produce spermatostyles (Gustafson & Miller, 2017; Salazar et al., 2022). Six species were sampled: five species of North American Dineutus (tribe Dineutini; D. ciliatus, D. discolor, D. nigrior, D. assimilis, and D. hornii) and the Palearctic whirligig Orectochilus villosus (tribe Orectochilini). Beetles were collected using aquatic nets in ponds and creeks in the United States of America and France (see Supporting Information S1: Table 1 for study sites). All studied specimens are stored in the personal research collection of the lead author and are available upon request.
Spermatostyles were isolated for proteomics by microdissection from the male seminal vesicles (SV, Figure 1c; Supporting Information S1: Table 1). SV contents were evacuated into a clean drop of phosphate-buffered saline (PBS) supplemented with protease inhibitors (cOmplete Protease Inhibitors Cocktail; Roche) on a subbed slide. Following the removal of any extraneous tissue, spermatostyles were pooled and stored at −80°C.
2.2 Characterizing spermatostyle and FRT morphology
Using the methods described above, males were dissected (N = 5 per species) to isolate sperm and spermatostyles for morphological study. Total sperm length, sperm head length, and spermatostyle length were measured from 10 sperm or spermatostyles per male using routine DAPI staining (Gómez & Maddison, 2020). Female genitalia were dissected (N = 3−5 females per species) and prepared following the methods of Miller and Bergsten (2012) to quantify spermatheca maximal length, spermatheca maximal width, spermatheca area, spermathecal duct length, fertilization duct length, and the diameter of the apical orifice of the fertilization duct. All morphometric measurements (Supporting Information Material S1) were obtained from photomicrographs (differential interference contrast, darkfield, or fluorescent) acquired using an Olympus DP-71 camera mounted on an Olympus BX60 microscope using FIJI (Schindelin et al., 2012).
2.3 In vitro sperm removal and spermatostyle protein solubilization
Sperm were removed from isolated spermatostyles by sonication using a Misonix S-4000 sonicator (Misonix) followed by centrifugation and purification with a cell strainer filter (pore size 40−70 µm) to remove sperm (Figure 1d). Spermatostyles on the filter were washed with PBS and centrifuged before transfer to 40 µL of 1-2X Laemmli sample buffer with 25 mM Bond-Breaker TCEP solution (Thermo Fisher Scientific) and protease inhibitors. Vortexing and heating (95°C for 5 min) were used to ensure complete solubilization. To evaluate the effectiveness of our spermatostyle purification protocol, five samples of sonicated and unsonicated D. assimilis spermatostyles were prepared for electron microscopy (EM) following the methods of Dallai et al. (2020). EM of samples prepared for proteomic analysis confirmed that our protocol successfully removes bound sperm, although we note the persistence of a small amount of residual sperm fragments (Supporting Information S1: Figure 1).
2.4 RNA extraction, RNAseq, and de novo transcriptomics
De novo transcriptome assemblies and gene model annotations were completed for all six species to facilitate phylogenetic analyses and establish species-specific, predicted protein databases for tandem mass spectrometry (MS/MS) spermatostyle characterization (Supporting Information S1: Tables 1-2). Total RNA was extracted from whole males to prepare Illumina TruSeq Stranded mRNA libraries for sequencing on an Illumina NextSeq. 550 with 75 base pairs, paired-end reads. Between 6 and 8 library preps were multiplexed per sequencing lane to obtain approximately 50 million reads per transcriptome. The raw reads are available via the NCBI SRA (BioProject Accession PRJNA1063663).
The transcriptomes were assembled de novo using the Trinity pipeline (version 2.13.2; Grabherr et al., 2011). Quality control with BUSCO scores (≥89%) and transrate (≥0.25) (Supporting Information S1: Table 2) were used as filters. De novo annotation was performed with the Trinotate pipeline (Bryant et al., 2017), including protein prediction with TransDecoder (Grabherr et al., 2011) for transcripts with a minimum open reading frame length of 20 amino acids (-m 20) or significant homology.
2.5 MS/MS and protein identification
Two replicate samples were prepared for each species by pooling spermatostyles from either 20 males per species in Dineutus spp. or 50 males of O. villosus. The entire volume of each sample was separated on a 1.5 mm 12% SDS-PAGE gel stained with colloidal Coomassie dye and divided into four slices. Slices were analyzed with a Dionex UltiMate 300 rapid separation liquid chromatography nanoUPLC system (Thermo Fisher Scientific) coupled with a Lumos mass spectrometer (Thermo Fisher Scientific) (see McDonough-Goldstein et al., 2021 for details of MS/MS methods).
Mass spectra data were searched in a species-specific manner against their predicted proteome (see above) using the PEAKS Studio X software (Bioinformatics Solutions Inc.). We appended each reference proteome with the cRAP v. 1.0 contaminant database (thegpm.org) and used the following PEAKS parameters: semi-specific digestion with up to three missed tryptic cleavages, parent monoisotopic mass error of 15.0 ppm, and fragment ion mass tolerance of 0.5 Da. Three posttranslational modifications were included in our searches: carabamidomethylation (cysteine; fixed), oxidation (methionine; variable), and deamidation (glutamine and arginine; variable). For each species, we included those PSMs with −10logP scores that yielded a total false discovery rate of 1% (estimated with a decoy-fusion approach; Zhang et al., 2012), a PTMA score >100, or a de novo identified score ≥ to 50. We retained only those proteins with a −10log p ≥ to 20, identification by at least two unique peptides and at least six spectral hits. A single representative protein was included for protein groups resulting from isoforms. These criteria resulted in the retention of between 779 and 1086 spermatostyle proteins per species (Supporting Information Material S2). Protein abundances were established with label-free quantitation, relying on feature intensity from MS1 as the basis for estimating protein abundances (Cox & Mann, 2008). To facilitate comparisons among species, these values were normalized following Wisconsin double standardization. MS/MS data is available via the ProteomeXchange Consortium (Project accession PXD048928).
2.6 Confocal microscopy and immunohistostaining
To validate our proteomic discovery of sperm-leucylaminopeptidases (S-LAPs) at high abundance in spermatostyles, D. assimilis spermatostyles were stained using a mouse polyclonal antibody against D. melanogaster S-LAP-1 (Laurinyecz et al., 2019) and the methods of White-Cooper (2004) with the dilution factor originally used by Laurinyecz et al. (2019). Confocal images were acquired using a LSM980 confocal microscope with high-resolution airyscan (Blatt Bioimaging Center; Syracuse University).
2.7 Orthology and phylogeny characterization
To conduct evolutionary comparisons among spermatostyle proteomes, we reconstructed orthology relationships for our transcriptomic data using Orthofinder v. 2.5.4 (Emms & Kelly, 2015, 2019). Orthofinder recovered pairwise orthology relationships for 51.6%–73.1% (average = 63.0%) of all genes, and 7999/27,908 (28.6%) of orthogroups were present in all species. 2851/27,908 (10.2%) of orthogroups were estimated to be single-copy and present in all transcriptomes. The maximum-likelihood species tree was determined based on single-copy orthogroups with RAxML-NG v. 1.1, as implemented by Orthofinder (Kozlov et al., 2019).
2.8 Protease assay
We assayed the proteolytic activity of D. assimilis spermatostyles using the Pierce fluorescent protease assay (Thermo Fisher Scientific). Two samples of sonicated spermatostyles isolated from 20 males and pooled in PBS supplemented with 0.01% Triton-X 100 were incubated in parallel with six serial dilutions of 0.5 µg/mL trypsin standards at room temperature for an hour, and changes in fluorescence polarization were recorded using a SpectraMax Microplate Reader.
2.9 Gene ontology (GO)
GO terms were obtained from the Trinotate pipeline for the transcriptome of each species. Significantly enriched GO terms (using a Fisher's exact test) from each spermatostyle proteome were identified using the R package TopGO (Alexa & Rahnenfuhrer, 2023) after limiting the search to the highest abundance proteins that account for 75% of the cumulative protein abundance per proteome.
2.10 Phylogenetic analysis of proteome and reproductive trait co-diversification
To facilitate phylogenetic comparative analyses of all morphological traits and proteome composition, the species tree from Orthofinder was ultrametricized using established divergence time estimates (Gustafson, Prokin, Bukontaite, et al., 2017). Protein abundance values were summed by orthogroup. All phylogenetic comparative analyses and principle component analyses (PCAs) were conducted in R v. 4.1.2 (R Core Team, 2021). Phylomorphospaces were reconstructed using phytools v. 1.9.6 (Revell, 2012). Phylogenetic generalized least squares (PGLS) and phylogenetic ANOVA were conducted using the R packages caper v. 1.0.2 (Orme, 2018) and phytools, respectively, to examine covariation between proteome composition and morphological traits (spermatostyle length, fertilization duct length, the width of the apical orifice of the fertilization duct, and spermatheca area). Correlation coefficients were obtained using phylogenetic independent contrasts with ape v. 5.7.1 (Paradis & Schliep, 2019) for PGLS models that fit Brownian motion or Pearson's correlation for models that did not require phylogenetic correction (i.e., λ = 0).
3 RESULTS
3.1 Spermatostyle, sperm, and FRT evolution
A well-resolved ultrametric phylogeny was obtained from maximum-likelihood analysis of 2851 one-to-one orthologs present in our de novo transcriptome annotations for all six species (1,168,346 concatenated bps; Figure 2a). Dineutus and Orectochilus were each recovered as distinct lineages. Divergence time estimates for the previously unsampled assimilis-group species suggest that D. hornii diverged from its sister group 12.4 million years ago (mya), and D. assimilis diverged from D. nigrior 9.4 mya. The phylogenetic distribution of spermatostyles in whirligigs suggests that they have possibly been retained in these beetles across hundreds of millions of years (Gustafson & Miller, 2017; Gustafson, Prokin, Bukontaite, et al., 2017).

Spermatostyles from male SV vesicles appear as rope-like bundles (Figure 1a,c) due to the hundreds of sperm embedded via their heads in peripheral cavities found in the homogenous spermatostyle material (Figure 1b,f,g; Salazar et al., 2022). Electron micrographs of spermatostyles after the removal of sperm showed that these cavities are arranged with highly ordered positioning between cavities (Figure 1f). Spermatostyles vary morphologically among our sampled species principally in length (Figure 2a), and because of the regular pattern of sperm attachment, spermatostyle length closely covaries with the number of sperm with which they associate (R. A. G., personal observation). Linear morphometrics of sperm and FRTs also exhibit substantial interspecific variation (Figure 2a; Supporting Information Material S1).
Spermatostyles and the proteins of which they are composed may function in multiple sites in FRTs. Spermatostyles were observed in both the spermathecae and fertilization ducts of wild-caught D. assimilis females (n = 5 out of six females examined; Figure 1e). FRT morphology differed among species (Figure 2a), particularly the design of the fertilization duct (Figure 2a) and the size of the spermatheca (Figure 2c; Miller & Bergsten, 2012). Dineutus assimilis and D. nigrior are notable for possessing voluminous spermathecae (Figure 2a,c) capable of storing hundreds of spermatostyles (R. A. G., personal observation).
3.2 Composition of spermatostyle proteomes
MS/MS proteomic analysis of spermatostyles was conducted in a species-specific manner using predicted proteins from de novo transcriptome annotations in all six species. This resulted in a mean of 148,717 (range: 77,169–214,388) peptide-spectrum matches and the identification of 978 ± 135 (mean ± 1 SD, range: 779–1086) spermatostyle proteins per species (Supporting Information Material S2). Protein identification was highly reproducible between samples as nearly all spermatostyle proteins were identified in both biological replicates per species (range = 97.3%–99.5%). Mapping spermatostyle proteins to their corresponding orthology groups revealed that approximately one-quarter of all proteins (362/1472) were identified in the proteomes of all six species (Figure 3a). When excluding the outgroup species O. villosus, the remaining proteomes overlapped in at least one-half of their orthology groups (Figure 3a).
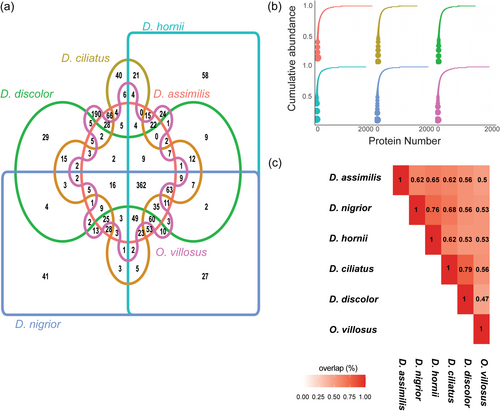
Spermatostyle proteomes possessed several notable features. First, the proteomes of all six species were largely comprised of a small number of highly abundant proteins (Figure 3b). The top 10 most abundant proteins in each proteome accounted for 60.0%–65.6% of total protein composition. Second, the single most abundant spermatostyle protein in all Dineutus species was an S-LAP (Dorus et al., 2006, 2011; Wasbrough et al., 2010) that accounted for an average of 17.2% of total protein abundance. In O. villosus, the most abundant protein (19.1% of total protein abundance) shared sequence homology with a major limpet shell matrix protein (ELDP2_LOTGI; Mann & Edsinger, 2014), an extracellular matrix-related protein that might contribute to shell mineralization (Marie et al., 2013); an S-LAP was the third most abundant protein. When considering all six species, the top 10 proteins consistently included 1−3 copies of an S-LAP family member, a non-S-LAP M17 leucine aminopeptidase (M17 LAP; Drinkwater et al., 2019), a chymotrypsin and a homolog of a limpet shell matrix protein. Third, a comparison of high abundance spermatostyle proteins (i.e., those accounting for 75% of the total protein content per species) revealed high levels of proteome conservation (typically >50%; range 44%−85%) among species with an average conservation percentage of 66% for all pairwise comparisons among Dineutus species (Figure 3c).
3.3 S-LAP localization in spermatostyles and S-LAP diversification
Drosophila S-LAPs are testis-specific in expression and are amongst the most abundant integral sperm proteins (Dorus et al., 2011; Garlovsky et al., 2022; Wasbrough et al., 2010). To visually confirm S-LAP presence in spermatostyles, in addition to whirligig sperm, we conducted immunohistochemical confocal imaging of D. assimilis spermatostyles and sperm stained with a polyclonal S-LAP1 antibody (Figure 4a−g), DAPI, and the actin stain phalloidin. As expected, the antibody detected S-LAP proteins in sperm, particularly their flagella. It was also evident from composite images that spermatostyles exhibited anti-S-LAP signal in regions that were absent of bound sperm heads, as judged by the lack of DAPI signal. This direct visualization confirmed the presence of S-LAP proteins in the spermatostyle, thus corroborating our proteomic results. Phalloidin staining was also observed in both sperm and spermatostyles, which is consistent with the identification of actin in our spermatostyle proteomes and the presence of actins in insect sperm more generally (Degner et al., 2019; McCullough et al., 2022; Whittington et al., 2017).
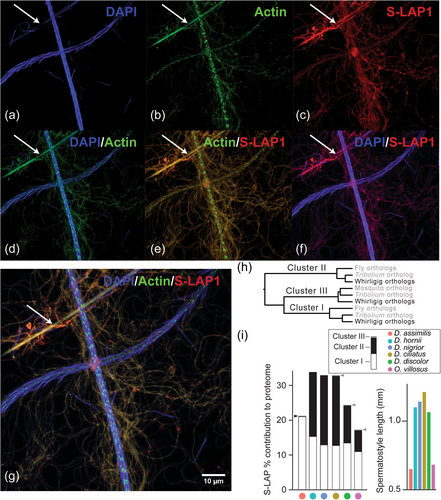
To determine if the contribution of members of the S-LAP protein family to spermatostyles was conserved, we investigated S-LAP abundance variation in a phylogenetic framework. This revealed that S-LAPs were distributed across three major phylogenetic clusters that were variable in their contribution to spermatostyle composition (Figure 4h,i; Dorus et al., 2011). All whirligig spermatostyles except D. assimilis include cluster I and cluster II orthologs in high abundance, and three out of the six proteomes included additional S-LAP copies from a third previously uncharacterized cluster (Figure 4h,i). S-LAP abundance may also be associated with spermatostyle structure as the short spermatostyles of Orectochilus and D. assimilis both feature smaller quantities of cluster II S-LAPs (Figure 4i). We conducted phylogenetic ANOVA and PGLS to determine if spermatostyle lengths differ significantly with the abundance of their included S-LAPs. Linear regression did not support a significant relationship between spermatostyle length and S-LAP cluster II protein abundance (F1,4 = 6.18, p = 0.06). However, discretization of spermatostyle length data into two groups permitted a phylogenetic ANOVA that revealed a weakly significant relationship (F1,4 = 7.11, p = 0.035) between S-LAP cluster II protein abundance in species possessing either short or long spermatostyles. Drosophila S-LAPs are notable among M17 aminopeptidases for possessing putatively disruptive substitutions at catalytic sites (Dorus et al., 2011). The absence of enzymatic activity has subsequently been confirmed for S-LAP 1 and 6 using in vitro assays (Laurinyecz et al., 2019). These novel substitutions have led authors to question the function of these proteases given their extreme abundance in sperm (Dorus et al., 2011), and novel functionality of S-LAPs can readily be envisioned as M17-LAPs in other organisms perform a host of aminopeptidase-independent functions (Drinkwater et al., 2019). Sequence alignment with Drosophila and beetle S-LAP amino acids revealed that whirligig S-LAPs have conserved catalytic site residues but have experienced novel amino acid substitutions in key residues involved in coordinating metal ion binding (Supporting Information S1: Figure 2a). Specifically, these involve the replacement of negatively charged residues with those that are neutral or hydrophobic (Supporting Information S1: Figure 2b; Maric et al., 2009).
3.4 Spermatostyle proteome functionality and proteolytic activity
GO enrichment analyses of molecular function were conducted independently for each of the six species-specific spermatostyles proteomes (Supporting Information S1: Table 3). Given the substantial conservation across proteomes studied, it was not unexpected to observe consistent significant enrichments of proteases (and other catalytic classes of proteins) and structural proteins (primarily tubulins and actins). In total, 9.7%–12.3% of the proteome was predicted to be catalytic. Although proteases were not the largest class in number (~2%–3% of all proteins), they consistently accounted for a large proportion of the total amount of protein (31%–51% of cumulative abundance). In contrast, structural proteins were similar in absolute number but far less abundant (4%–6% of all proteins; 3%–7% cumulative abundance). To explore overall interspecific variation in functional conservation, we plotted spermatostyle GO terms by their average abundance and significance of enrichment. This highlighted the following two groups of significantly enriched terms: those that comprised highly abundant proteins and those comprised of lower abundance proteins (e.g., the top two quadrants in Figure 5). Among the highly abundant proteins (see Figure 5) were terms for peptidase activities, manganese ion binding activity, transaminase activity, and transferase activity, and among the lower abundance proteins were a wide variety of additional enzymes and proteins characterized in seminal fluid and in reproduction (e.g., spermine and prostaglandins; Supporting Information S1: Table 2; Mann, 1974; Mayoral Andrade et al., 2020).
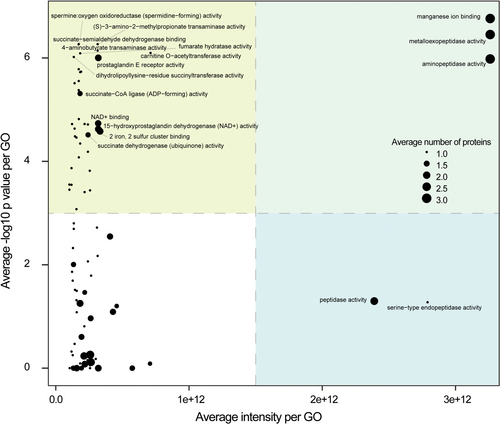
The enrichment and abundance of proteolytic enzymes in spermatostyles was perhaps unexpected in light of the assumed role of spermatostyles as the structural scaffold for sperm during transfer to and migration through the FRT (Higginson & Pitnick, 2011). To test whether proteases within spermatostyles retain enzymatic activity, we conducted proteolytic assays with spermatostyles isolated from D. assimilis SV. These assays revealed that one male equivalent of D. assimilis spermatostyles had enzymatic activity comparable to approximately 0.01–0.02 ng/mL of trypsin, which is similar to the concentration of trypsin found in mosquito midguts immediately following a bloodmeal (Noriega et al., 1996; Pennington et al., 1995). We note that this assay does not provide information about the specific proteins contributing to this activity, including whether S-LAPs may have enzymatic capacity.
3.5 Proteome co-diversification with spermatostyle and FRT structure
Spermatostyle structure and composition are predicted to covary with the FRT, with which they interact. We used a PCA to characterize compositional variation in the proteome underlying spermatostyle evolution across the phylogeny (herein referred to as a “phyloproteospace” [Figure 6a], a proteomic application of a phylomorphospace approach (see Sidlauskas, 2008)). The first principal component (PC) captured 59.4% of the variation and was almost entirely accounted for by abundance variation of the S-LAP and shell matrix protein families (Figure 6b). PC2 captured 31.9% of the variation and was largely accounted for by M17 LAP abundance differences. PC3 and PC4 described 5% and 2.4% of the variation, respectively. We note that the clustering of several Dineutus species in phyloproteospace was due to the high abundance of S-LAPs and lower quantities of a shell matrix protein, which was absent from the D. hornii proteome altogether.
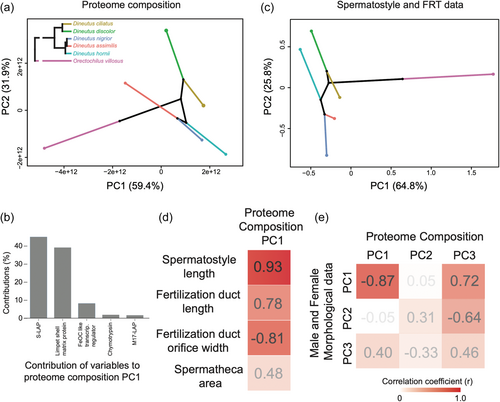
PCA of spermatostyle and FRT traits revealed a robust divergence between Orectochilus and Dineutus. Both clades of Dineutus localized to a similar region of phylomorphospace (Figure 6c). The major contributors to PC1 included (i) the width of the apical orifice of the fertilization duct (accounting for 58.1% of PC1 variance), (ii) sperm head length (16.5%), (iii) spermatheca length (8.9%), (iv) spermatheca area (6.7%), and (v) spermatostyle length (4.3%). PC2 was largely accounted for by (i) spermatheca area (accounting for 52.5% of PC2 variance), (ii) spermatheca width (28.1%), and (iii) width of the apical orifice of the fertilization duct (14.5%).
Phylogenetically controlled regressions between major PCs (Supporting Information S1: Table 4; Figure 6d,e) indicated evolutionary covariation between the composition of the spermatostyle proteome and spermatostyle and FRT morphometry. It is important to note that the power of these analyses is limited by our sample size, and none of the p-values remained significant after multiple test corrections (Supporting Information S1: Table 4). Nonetheless, consistently high goodness-of-fit measures (R2 > 40%) were suggestive of functional and/or evolutionary relationships between the scores and loadings of major PCs and studied traits (Supporting Information S1: Table 4; Figure 6d,e). Specifically, models predicted that as spermatostyles elongate and fertilization ducts lengthen and narrow, S-LAPs increase in abundance with a corresponding decrease in the shell matrix protein. This pattern was similarly reflected in the strongly negative correlation found between PC1 of both datasets (r = −0.87; PGLS p = 0.02 before correction; Figure 6e; Supporting Information S1: Table 4). Covariation was also observed between morphometric PC2 and proteome composition PC3 (r = −0.64; PGLS p = 0.16 before correction; Figure 6e; Supporting Information S1: Table 4). This relationship suggests that spermatheca size decreases are associated with increasing chymotrypsin abundance. Despite our limited taxon sampling, these patterns suggest evolutionary co-diversification at the molecular and morphological levels between spermatostyles and the environments in which they function.
4 DISCUSSION
The remarkable structural and biochemical diversification of ejaculates is almost certainly attributable to the diversity of postcopulatory roles they contribute to in the FRT (Dorus et al. 2004; Higginson et al., 2012b; McCullough et al., 2022; Pitnick, Hosken, et al., 2009; Pitnick et al., 2020; Ravi Ram & Wolfner, 2007). In this study, we characterized the proteomic composition and evolution of spermatostyles, which to date have received little attention beyond morphological examination (Breland & Simmons, 1970; Chawanji et al., 2005, 2006; Chevaillier, 1963; Chevaillier & Maillet, 1965; Dallai et al., 2019, 2020; Folliot & Maillet, 1970; Giglio et al., 2024; Gómez & Maddison, 2020; Hayashi & Kamimura, 2002a, 2002b; Maillet, 1959; Mercati et al., 2023; Roberston & Gibbs, 1937; Salazar et al., 2022, 2023; Sodré et al., 2024). Although functional data are generally lacking, it has been assumed that the adaptive value of spermatostyles is the facilitation of cooperative sperm migration through the FRT (Higginson & Pitnick, 2011; Immler, 2008; Pizzari & Foster, 2008). As such, we anticipated that spermatostyles would be largely composed of structural proteins (e.g., tubulins, actins, dyneins) and that other types of proteins (e.g., enzymatic or metabolic) would be underrepresented. On the contrary, our proteomic characterization revealed that spermatostyles are comprised of a conserved repertoire of highly abundant proteolytic enzymes. Notably, there are only a limited number of putatively structural proteins found in high abundance in all species such as a homolog of a limpet shell matrix protein. Biochemical analyses further confirmed that spermatostyles have proteolytic activity, although the precise identity of the protein(s) contributing to this activity remains unknown. Nothing is known about the mechanisms responsible for sperm dissociation from the spermatostyle. Based on the results of the present study, we speculate that spermatostyles may contain the molecular cargo (e.g., proteolytic enzymes and other catalytic proteins) responsible for the precise regulation of sperm dissociation (perhaps in conjunction with female-derived factors). When considered with the observation that spermatostyles persist in the FRT for a prolonged period of time following sperm dissociation (Breland & Simmons, 1970; Gustafson & Miller, 2017; R. A. G. and S. P., personal observation), these results are also consistent with the general hypothesis that spermatostyles are involved in other postmating functions (Pitnick et al., 2020). It is noteworthy that the enrichment in proteolytic pathways in spermatostyles mirrors similar enrichments in both SFPs and FRT secretions in other insects (McCullough et al., 2022; McDonough-Goldstein et al., 2021; Meslin et al., 2017; Plakke et al., 2019; Qian et al., 2023; Rogers et al., 2009).
The localization and persistence of SFPs in the FRT have not been widely investigated (but see Avila et al., 2011; McCullough et al., 2022; Ravi Ram et al., 2005; Ravi Ram & Wolfner, 2007), but such information is critical for understanding their function and evolution. In D. melanogaster, only a restricted subset of SFPs accompany sperm into the female's sperm-storage organs (McCullough et al., 2022; Wolfner, 2011). The persistence of only a few male-derived molecules may be dependent upon specific interactions between these molecules and sperm. For example, the SFP known as “Sex Peptide” is transported to the sperm-storage organs after binding to the plasma membrane of sperm flagella. It is then gradually released by cleavage from sperm over the course of days to impact female remating and other postmating responses (Hopkins & Perry, 2022; Kubli, 2003; McCullough et al., 2022; Peng, Chen, et al., 2005; Peng, Zipperlen, et al., 2005; Ravi Ram & Wolfner, 2007, 2009; Wainwright et al., 2021). In contrast, the vast majority of Drosophila SFPs are greatly reduced in abundance or undetectable across FRT tissues within hours of insemination (McCullough et al., 2022). Clearly, the robust physical association of spermatostyles with sperm (Figure 1) ensures that spermatostyles accompany sperm during their migration through the FRT and into the spermatheca (Breland & Simmons, 1970). After sperm dissociation, whirligig spermatostyles are known to exhibit prolonged persistence within female sperm-storage organs (Breland & Simmons, 1970; Gustafson & Miller, 2017; but see Salazar et al., 2023). Bare spermatostyles have also been observed in both the fertilization ducts and spermathecae of wild-caught female D. assimilis (Figure 1e; R. A. G., personal observation). Spermatostyles are thus available to participate in prolonged physical and biochemical interactions with the FRT. Although resolving the precise nature of these interactions requires further investigation, the existence of such functional interactions is supported by our observation that spermatostyle proteome composition covaries with various axes of FRT morphology.
The redeployment and specialization of existing molecular systems in novel contexts, oftentimes through gene duplication and gene family diversification (Conant & Wolfe, 2008), is prevalent in the evolution of reproductive systems (Begun et al., 2006, 2007; Chen et al., 2013; Dorus et al., 2011; Laurinyecz et al., 2019; Levine et al., 2006; Loppin et al., 2005; Luis Villanueva-Canas et al., 2017; Meslin et al., 2017). S-LAPs are some of the most abundant proteins in insect sperm (Degner et al., 2019; Dorus et al., 2006, 2011; McCullough et al., 2022) and a primary component of the paracrystalline component of the mitochondrial derivatives that flank the axoneme of insect sperm flagella (Jamieson et al., 1999; Laurinyecz et al., 2019). This material has been credited with the unusual elasticity observed in swimming insect sperm (Baccetti et al., 1977; Werner & Simmons, 2008). Drosophila S-LAPs have experienced disruptive amino acid substitutions at key catalytic and co-factor binding residues, which likely explain the lack of enzymatic activity in S-LAP mutants (Laurinyecz et al., 2019) and may also contribute to their functional specialization in sperm (Dorus et al., 2011). Our discovery of S-LAPs in whirligig spermatostyles provides a new example of functional redeployment in which these insect sperm proteins have been co-opted to function within an ejaculate structure (Figures 4 and 5). It is noteworthy that our analyses revealed covariation between S-LAP abundance and spermatostyle length. Whirligig S-LAPs, unlike those of Drosophila, retain many of the core catalytic residues (Figure 4, Supporting Information S1: 2). In contrast, they have experienced substitutions in cation cofactor binding residues (Figure 4, Supporting Information S1: 2), which suggests they may lack canonical M17 aminopeptidase activity (i.e., typical M17 aminopeptidases digest proteins following binding of a metal cofactor; Cadavid-Restrepo et al. 2011; Drinkwater et al., 2019; Lowther & Matthews 2002; Modak et al. 2016).
Although S-LAPs and other metal-dependent M17 aminopeptidases were consistently the most abundant spermatostyle proteins, our comparative proteomic analyses also revealed divergence in family member contribution to spermatostyle composition. These findings support a model in which the S-LAP family (and other M17 aminopeptidases) serves as a molecular toolbox of interchangeable components that facilitates the evolutionary diversification of spermatostyles. Although it is unclear if S-LAPs are proteolytically active, the potential for evolutionary variation in S-LAP functionality may provide the mechanistic link that explains the observed covariation between spermatostyle length and this class of predominant spermatostyle proteins (Figure 6). In addition to biochemical and structural analyses, proteomic characterization of spermatostyles (and sperm) in hemipterans, which diverged from our current study species over 400 million years ago and include lineages with independent evolutionary origins of spermatostyles, will further inform our understanding of the deployment of the S-LAP molecular toolbox across distinct and rapidly evolving male reproductive cells and structures.
AUTHOR CONTRIBUTIONS
R. Antonio. Gomez, Romano Dallai, Yasir Ahmed-Braimah, Scott Pitnick, and Steve Dorus conceived and designed the research study. R. Antonio Gomez, Romano Dallai, Dylan J. Sims-West, and David Mercati collected the data. Romano Dallai, David Mercati, and Rita Sinka contributed valuable imaging resources, analytical tools, and feedback on the manuscript. R. Antonio Gomez, Scott Pitnick, and Steve Dorus analyzed the data and wrote the manuscript.
ACKNOWLEDGMENTS
We thank Benjamin Sterrett for providing access to the Cornell Experimental Ponds; Zeeshan Syed, Abelard Cong, Stephanie Nguyen, Peter Wengert, Alexander Newman, and Sloan Cochran for field and laboratory assistance; and Heidi Hehnly for critical guidance on staining and confocal microscopy in collaboration with the Blatt Bioimaging Center. Thanks to Andrea Pauli, Noritake Hirohashi, and the Biology of Spermatozoa 2023 meeting delegates for thought-provoking discussions. We also thank two anonymous reviewers for their insightful comments on an earlier version of the manuscript. This research was funded by an NSF Postdoctoral Research Fellowship in Biology (DBI-2011045 to R. A. G.), the National Institutes of Health (NIH-R35GM147454 to Y. A.-B.), and a generous gift by Mike and Jane Weeden to Syracuse University.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the ProteomeXchange Consortium at https://www.ebi.ac.uk/pride/, reference number PXD048928. Raw sequence reads are deposited in the NCI SRA (BioProject Accession PRJNA1063663). RAW mass spectroscopy files are archived with the ProteomeXchange (Project accession PXD048928). Benefits from this research arise from the sharing of our data and results on public databases as described above.




