Benefits and challenges of nanomaterials in assisted reproductive technologies
Abstract
Assisted reproductive technology (ART) have contributed to preserve fertility in humans and to increase multiplication of genetically superior animals. Despite being highly practiced worldwide, ART presents some challenges, especially because gametes and embryos are kept in vitro for a variable period of time, and the oxidative stress in vitro can have negative impact on oocyte competence and embryo development. Nanotechnology needs to be considered to help overcome some of those impairments, since it can provide strategies to deliver antioxidants and hormones to gametes and embryos in vitro. The application of nanotechnology to ART can allow the development of new protocols using nanomaterials to improve in vitro oocyte competence and embryo production. This review discusses the applicability of nanomaterials to improve sperm selection, to deliver antioxidants and hormones to preantral follicles, oocytes, and embryos in vitro, as well as the concerns about using nanotechnology in ART.
1 INTRODUCTION
The development of assisted reproductive technologies (ART) has contributed to reduce the incidence of infertility in human species and to increase the number of offspring from genetically superior animals and of those animals in risk of extinction (Hansen, 2020). The main reproductive biotechnologies routinely used over the years are sex-sorting sperm and artificial insemination, embryo transfer, in vitro maturation and fertilization of oocytes (IVF), in vitro embryo production (IVP), and in vitro culture of follicles. These biotechniques have revolutionized infertility treatments in human species, since over the last 4 decades, pregnancy rates following ART have increased from 6% to 35% (J. Wang & Sauer, 2006). However, the average live birth rate after ART still remains low, and does not exceed 30% per started cycle (Barkalina et al., 2015). In animals, IVP has created multiple opportunities for increasing the number of offspring from genetically valuable females. Live birth after fertilization of oocytes from in vitro cultured early follicles have been obtained in mice (O'Brien et al., 2003), but not for domestic species, in which early follicles need longer in vitro culture periods. In bovine species, the number of embryos produced by IVF has grown exponentially in recent years, but the percentage of blastocysts after IVF is still limited, around 40% (Lonergan et al., 2016).
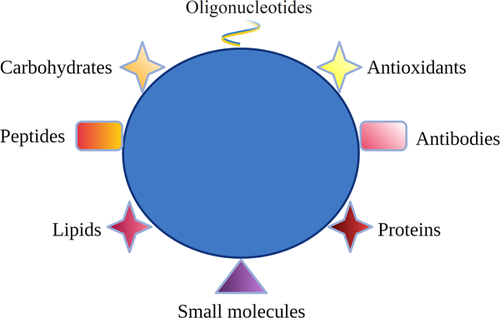
Several variables impact the success of in vitro embryo production and ART. Certainly, culture media, oxygen concentration, temperature, media pH, and the manipulations of embryos in the laboratory can impact its reproductive potential (Swain et al., 2016). Vanhoutte et al. (2009) reported that three-dimensional coculture systems influence the rate of oocyte fertilization and embryo production. Additionally, producing embryos in vitro involves exposing oocytes to nonphysiological environment that induces the production of reactive species of oxygen (ROS). Oocytes and embryos produce ROS as normal cell metabolism and under in vitro stress conditions produce excessive ROS, which can play a critical role in cell survival (Soto-Heras & Paramio, 2020). Thus, optimization of in vitro culture conditions, such as the supplementation of culture media with antioxidants, small molecules, and growth factors has been reported to increase gamete/embryo survival and to improve developmental potential (Barkalina et al., 2015). Nanotechnology is already employed in traditional drug delivery and tissue engineering (Shi et al., 2010), and offers the possibility to develop devices particularly adapted to improve in vitro culture systems. Nanomaterials have sizes between 1 and 100 nm and relatively high surface area. Due to their high loading capacity, stability, and selective affinity, they can represent a potential tool for delivering molecules into gametes and embryos (Barkalina et al., 2015). Recently, many nanodelivery systems loaded with plant-based bioactive antioxidants (nano-resveratrol, nano-curcumin, nano-quercetin, nanogenistein, nano-epigallocatechin-3-gallate), and with antioxidant hormones (melatonin) have been demonstrated to be efficacious in modulating cellular oxidative stress (Lombardo et al., 2019, Remião et al., 2016). Various factors can influence the efficiency of nanoparticles as carriers (Vaiserman et al., 2020), for example, size is an important parameter of nanoparticle that determines its pharmacokinetics and entry into the cell (Hoshyar et al., 2016). Surface properties of nanoparticles determine their hydrophilicity or hydrophobicity and biological responses, including cellular uptake (Ajdary et al., 2018). Surface charge is among the most important properties of nanoparticle substantially determining its cellular absorption (Fröhlich, 2012). Due to their properties, nanoparticles may protect loaded bioactive antioxidant substances from degradation, increase half-life and improve their beneficial effects in vitro. As illustrated by Figure 1, these active carriers can allow the delivery of substances to the target sites with reduced dosage and the controlled release of the transported biomolecules (antioxidants, RNAs, proteins, lipids, carbohydrates, and small metabolite molecules) (Conte et al., 2017). Another basic advantage of nanoparticles as drug carriers is their ability to increase the solubility of hydrophobic compounds, increasing the dissolution efficiency of the active substance (Długosz et al., 2020). Thus, these carriers have great potential to be used during in vitro culture of gametes and embryos and, consequently, improve the effectiveness of ART in animals and humans.
Various studies reported that nanoparticles have been used to improve the selection of sperm, semen sexing, and cryopreservation (Bisla et al., 2020; Domínguez et al., 2018; Isaac et al., 2017), as well as to improve survival of preantral follicles (Karime et al., 2020) and COC maturation (Abdel-Halim & Helmy, 2018). These observations indicate the potential of using nanomaterials to improve ART and emphasize the importance of knowing the recent advances about these smart nanocarriers. This review aims to discuss the characteristics of nanomaterials, the mechanism of actions, their beneficial effects on gametes and embryos in vitro, as well as the concerns about the use of these nanomaterials in reproductive biotechniques.
2 NANOPARTICLES AND THEIR CHARACTERISTICS
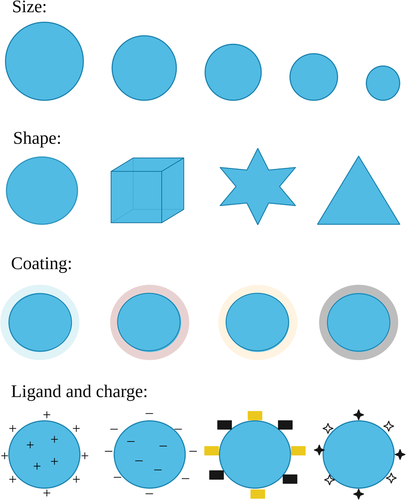
The use of nanometric materials has gained a lot of prominence and development in various areas of research (F. Ghorbani et al., 2020). The production and manipulation of materials in nanoscale is known as nanotechnology (Rizvi & Saleh, 2018). A material is considered in nanoscale if it is in the range between 1 and 100 nanometers. Nanoparticles, regardless of their constitution, shape, types of interactions, and applications, are nano-object with all external dimensions in the nanoscale where the lengths of the longest and the shortest axes of the nano-object do not differ significantly. On nanometric scale, objects have very different physical–chemical properties, as opposed to their constituents when they are in larger sizes (Zahmatkesh et al., 2020). The reason for such behavior is the great relationship between the surface/volume, providing a high reactivity of these compounds; and the domain of the quantum mechanics properties of matter in nanoscale materials (Kumar & Kumbhat, 2016). Particularly important for biomedical applications like those reviewed here is that the nanoscale is on the same order of magnitude over which most biochemical interactions take place, that is, they can be recognized by, or interact with, cells, organelles, biomolecules, and so forth. The factors that determine the effectiveness of nanoparticles to deliver drugs to cells or to cause toxicity are their size, shape, charge, material, and surface-coating (Figure 2; Hou & Zhu, 2017). The figure shows that dimensions (small or large sizes), shapes (sphere, rod, hyperbranched, multilamellar, or multilayered structures), and surface properties (functional groups, surface charge, PEGylation, coating processes, or attachment of targeting moieties) can influence the function of nanoparticles (Lombardo et al., 2019).
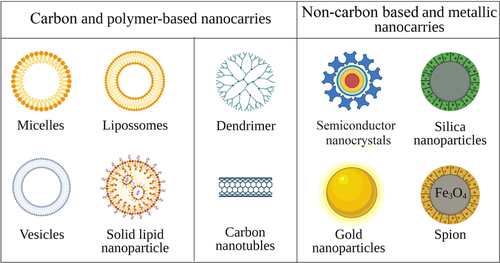
Nanostructured systems can be divided into carbon-based and non-carbon-based nanomaterials, while their physiochemical properties can be tuned by altering their compositions (carbon-based, non-carbon-based, or hybrid) (Figure 3). The carbon-based nanomaterials are generally characterized by high biocompatibility and improved drug loading capacity. They allow versatile control of both morphology and chemical composition, while their colloidal stability and relatively large size allow incorporating and carrying a wide combination of different (hydrophilic/hydrophobic) drugs (Souery & Bishop, 2018). Depending on the preparation methods, organic nanomaterials can be subdivided into two main categories: (1) nanostructures that exploit the self-assembly processes, such as amphiphilic systems; and (2) those obtained by specific synthesis methods, like dendrimers, hyperbranched polymers, chemical nanogels, and carbon nanotubes (Figure 3, Lombardo et al., 2019). The non-carbon-based nanocarriers are generally composed of two regions: a core containing the non-carbon-based component, such as gold, semiconductor nanocrystals, silica, or iron oxide, and an outer layer composed mainly of carbon-based components that provide a suitable substrate for the conjugation of biomacromolecules (Lombardo et al., 2019).
The mechanism for making nanoparticles depends on several factors, especially for the purpose of application and the specific type of nanoparticle. There are two approaches: top-down and bottom-up. The first uses physical methods to build the nanoparticles from the bulk material for stages of smaller scales. The second approach uses chemical reactions to create the atom-by-atom nanomaterial. On the other hand, the synthesis of nanoparticles with plant extract is eco-friendly and has advantages, like production of nanoparticles in large quantities, simplicity of reducing metal salts, rapid, cost effective, and safety for clinical research (Latha et al., 2018). Different types of metal (gold and silver) and metal oxide (copper oxide, and zinc oxide) nanoparticles can be synthesized using natural extracts (Singh et al., 2018). Nanotechnology is promoting new opportunities for the application of solutions to everyday problems; from information technologies to the field of medical sciences, like animal and human reproduction (Vishwakarma et al., 2013). A very promising field is the manipulation of nanostructured drugs to create systems known as drug delivery (Mu et al., 2014). Knowing and understanding the physical–chemical mechanisms of nanomaterials and how they can interact with living systems can help the development of new biotechnologies.
Nanoparticles are produced and then coated with drugs, polymers, peptides, proteins, oligonucleotides, or fluorophores; and eventually added to cells cultured in vitro. Functionalization of nanoparticles with these biomolecules can be done through noncovalent interactions, that is, electrostatic, hydrophobic, and affinity interactions (Yu et al., 2012). It is also possible to conjugate biomolecules with nanoparticles via covalent interactions by chemisorption of the biomolecules on the particle surface or through the use of a bifunctional linker (De et al., 2008). Chemisorption is a chemical reaction that occurs between nanoparticles and thiol group of biomolecules through cysteine residues present on the surface of biomolecules (Saallah & Lenggoro, 2018). Albanese et al. (2012) showed that the presence of ligands on nanoparticle surfaces at a given density over a specific curvature contributes to the overall avidity of these nanoparticles for cell receptors. Their interaction with serum proteins and cell membrane receptors is also determined by the nanoparticle design, and consequently, influences cell uptake, gene expression, and toxicity.
In the area of reproductive medicine, nanotechnology has been used successfully in the diagnosis and treatment of disorders related to infertility (Zegers-Hochschild et al., 2017). In the field of in vitro embryo production, some techniques have been applied to deliver certain molecules to improve the chances of successful implantation (Remião et al., 2018). Thus, nanotechnology has a great potential to be applied to ART in humans and animals (Arvizo et al., 2012). Therefore, the main challenges in the area of reproductive sciences are to understand how the use of nanotechnology can help the techniques already in application.
3 EFFECTS OF NANOPARTICLES ON SPERMATOZOA IN VITRO
The applicability of nanoparticles has been used to improve the selection of sperm, semen sexing, and cryopreservation in several species of farm animals (Bisla et al., 2020; Domínguez et al., 2018; Isaac et al., 2017). It is important to emphasize that their size, surface volume, composition, shape, and surface functionalization are very important for their efficiency, since different nanoparticles have different effects when exposed to sperm (Z. Li et al., 2015; Taylor et al., 2015; Truong et al., 2019). The use of magnetic iron oxide nanoparticles has benefic effects in the process of sexing semen in swine and bovine species, neither having cytotoxic effects nor interfering in the descendant generated from the magnetic nanoselection in swine species (Farine et al., 2016; Durfey et al., 2017). In addition, the use of magnetic iron oxide nanoparticles is also a tool that promises to improve fertility in males. Farine et al. (2016) reported separation of damaged sperm cells by binding to specific aptamer coupled to superparamagnetic nanoparticles improves the quality of semen. Additionally, conjugation of magnetic nanoparticles with annexin V or lectins successfully removed apoptosis and acrosome reacted spermatozoa (Durfey et al., 2019).
For semen preservation and storage, silver nanoparticles prove to be an antimicrobial agent in porcine sperm, being an alternative to the use of antibiotics (Pérez-Duran et al., 2020). In addition, the use of zinc nanoparticles increases mitochondrial activity, enhances membrane integrity, reduces lipid peroxidation, and improves total antioxidant capacity in bovine sperm (Jahanbin et al., 2021). Sperm uses ATP to maintain cell functionality, viability, acrosome reaction, and mobility required to reach and penetrate the oocyte (Piomboni et al., 2012). Therefore, Zn inclusion nanoparticles in semen extender have a beneficial influence on sperm structure, like membrane integrity, and mitochondria activity (Jahanbin et al., 2021).
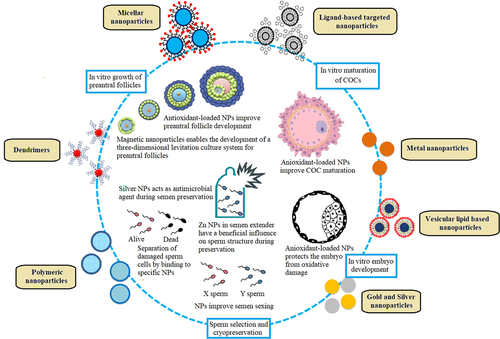
Nanoparticles can be used in methodologies to improve the quality of sperm in cryopreserved semen (Falchi, Galleri, et al., 2018). For example, cerium oxide (CeO2), zinc oxide (ZnO), and selenium nanoparticles demonstrated the ability to maintain the viability and motility of spermatozoa by decreasing ROS production and membrane lipid peroxidation when added in freezing media (Isaac et al., 2017; Falchi, Galleri, et al., 2018; Khalil et al., 2019). Jahanbin et al. (2021) inseminated cows with semen exposed to zinc nanoparticles and reported improved embryonic development. Taylor et al. (2015) also reported no evidence of gold nanoparticles absorption in bovine and porcine sperm. To emphasize the importance of associating nanoparticles with other biomolecules, pig sperm vitality (motility, membrane integrity, and morphology) was not affected by gold, silver, or gold-silver nanoparticles coated with bovine albumin serum (Tiedemann et al., 2014). Despite the potential of nanoparticles to improve male fertility, their use in animal husbandry still needs further studies for the application of these nanoparticles in vivo (Falchi, Khalil, et al., 2018; Feugang et al., 2019). It is very important to consider the time of exposure, since for human sperm, excessive exposure to silver nanoparticles caused DNA damage, structural anomalies, and increased ROS production (E. Wang et al., 2017). Figure 4 shows the benefits of nanotechnology to selection of sperm, semen sexing, and cryopreservation.
4 EFFECTS OF NANOPARTICLES ON IN VITRO CULTURED PREANTRAL FOLLICLES
Nanoparticles synthesized with plant extracts have successfully been used in culture systems for preantral follicles. Nascimento et al. (2019) reported that nanoparticles functionalized with hydroethanolic extract of red propolis increased glutathione (GSH) levels, mitochondrial activity, and antrum formation in ovine preantral follicles cultured in vitro. GSH is a tripeptide formed by amino acids cysteine, glycine, and glutamine, which plays an important role in combating oxidative stress (Adeoye et al., 2018). Thus, increasing GSH during in vitro culture of preantral follicles is very important to reduce oxidative stress in the cells and to improve follicles growth and viability (Soto-Heras & Paramio, 2020). Zhai et al. (2018) showed that zinc oxide (ZnO) nanoparticles improved the development of mice secondary follicles and had no deletions effects on ovarian follicle viability. Super-paramagnetic iron oxide (Fe3O4) nanoparticles loaded with curcumin prevented damage to mice preantral ovarian follicles in dehydroepiandrosterone-induced polycystic ovarian syndrome (Abhari et al., 2020). These authors showed that these nanoparticles increase the levels of B-cell lymphoma 2 (BCL2), reduces those of BCL2-associated X (BAX) and Caspase3, and consequently moderates apoptosis in granulosa cells of preantral follicles. In addition, magnetic nanoparticles improved development and antrum formation in bovine secondary follicles cultured in vitro (Antonino et al., 2019). These authors show that the use of magnetic nanoparticles enables the development of a three-dimensional levitation system with potential to be applied as an alternative method to culture follicles for long-term periods. An important indicator of preantral follicle development is the secretion of related hormones, such as estradiol and progesterone. Estradiol is synthesized in granulosa cells and is essential for follicle development and oocyte growth (Itoh et al., 2002). Recently, Abdollahi et al. (2020) showed that green gold nanoparticles synthesized with the Achillea millefolium extract increased the levels of estradiol in mice preantral follicles during in vitro culture. Thus, green gold nanoparticles synthesized with the Achillea millefolium extract have beneficial effects on the development and growth of preantral follicles (Abdollahi et al., 2020). Figure 4 shows the benefits of nanotechnology to in vitro preantral follicle culture.
5 EFFECTS OF NANOPARTICLES ON CUMULUS-OOCYTE COMPLEXES MATURED IN VITRO
Many studies have investigated the effects of nanoparticles during in vitro maturation of cumulus-oocyte complexes (COC). Recently, Jahanbin et al. (2021) revealed that addition of Zn nanoparticles to in vitro maturation media increase superoxide dismutase (SOD) activity in cumulus cells, decrease DNA damage, and apoptosis in COC and consequently enhance embryo development rate. Catalase (CAT) and SOD are enzymes that catalyze the conversion of superoxide into oxygen and hydrogen peroxide. Through its activity, SOD controls the levels of reactive oxygen species (ROS) and reactive nitrogen species, thus limiting the potential toxicity of these molecules (Wang et al., 2018). Lei et al. (2018) showed that fullerenol nanoparticles negatively regulated connexin-43 (CX43) expression and the resulting retraction of transzonal projections (TZPs) interrupted transport based on TZPs and consequently accelerated the resumption of rat oocyte meiosis. These authors also reported a perinuclear distribution of CX43 and EGFR in granulosa cells demonstrating that fullerenol nanoparticles can interfere in the process of resuming oocyte meiosis. Abdel-Halim (2018) showed that linoleic acid acts as an oxidative stressor and decreases the percentage of bovine oocytes reaching metaphase II stage, the rate of fully expanded cumulus cells, and the percentage of blastocyst. Surprisingly, the presence of 10 mg/ml chitosan nanoparticles in COC maturation medium completely alleviated the oxidative effects of linoleic acid on nuclear maturation of oocytes, cumulus cell expansion, and blastocyst rate. However, reduction in the percentages of fully expanded cumulus cells and developmental competence of bovine oocytes occurred with increasing chitosan nanoparticles to 60 and 100 mg/ml, showing that toxicity is dose dependent. These findings are in agreement with those reported by Hu et al. (2011) that demonstrated no apparent toxic effects of low concentrations of CSNPs on zebrafish embryos.
Bovine COCs matured in vitro in the presence of copper and ZnO nanoparticles had increased intracellular glutathione content of cumulus cells and consequently in vitro improved embryo development (Abdel-Halim, Moselhy, et al., 2018). The presence of nano-selenium and nano-ZnO nanoparticles during in vitro maturation of bovine COCs also increased both intracellular glutathione concentration and DNA integrity of cumulus cells (Abdel-Halim & Helmy, 2018). Increased levels of glutathione in cultured cells are very important to control oxidative stress (Adeoye et al., 2018). In pig, co-incubation of oocytes and gold nanoparticles coated with BSA had no impact on oocyte maturation and oocytes selectively absorbed gold nanoparticles, but no toxicity was reported (Tiedemann et al., 2014).
The presence of melatonin-loaded lipid-core nanoparticles in in vitro maturation medium of bovine COC was effective in decreasing ROS levels and the apoptotic cell numbers, upregulating glutathione peroxidase 1 (GPX1) and SOD genes, and downregulating CASP3 and proapoptotic BAX genes (Remião et al., 2016). SOD is a crucial antioxidant, catalyzing the dismutation of superoxide into oxygen and hydrogen peroxide, while GPX is a family of antioxidant enzymes that inactivates hydrogen peroxide and lipid hydroperoxide (Lin & Wang, 2021). Melatonin-loaded lipid-core nanoparticles penetrate into oocytes and remain inside the cells until they reach the blastocyst stage, highlighting that when melatonin is encapsulated in lipid-core nanoparticles during in vitro oocyte maturation improves the quality of blastocysts (Remião et al., 2016). These authors reported that Mel-LNC was more effective that nonencapsulated melatonin, probably because this configuration facilitates the release of melatonin throughout the cells. These melatonin-loaded lipid-core nanoparticles can trigger an antioxidant cascade that produces radical scavenging products, minimizing oxidative stress through a variety of mechanisms (Reiter et al., 2016) Gonçalves et al. (2021) showed that nanoparticles produced using biocompatible and biodegradable poly-lactic-co-glycolic acid (PLGA) can be taken up by cumulus cells and to a less extent by the enclosed oocytes during in vitro maturation of bovine COC. These authors reported that nanoparticles transfer to the oocyte appears to be via cumulus cells and transzonal projections, and that exposure of COC to PLGA nanoparticles increases embryo cleavage and blastocyst rates. PLGA nanoparticles themselves are formed of, and biodegradable to, lactic and glycolic acids (Makadia & Siegel, 2011). In ovarian follicles, production of lactate by granulosa cells is observed in early antral follicles and remains at relatively high levels during follicle growth and maturation (Boland et al., 1993). Lactate is an active metabolite involved not only in metabolism, but also in signal transduction and redox regulation (Philip et al., 2005). Thus, the lactic acid produced from PLGA nanoparticles degradation may be used as a metabolic substrate and may support the cellular antioxidant capacity in in vitro cultured COC (Gonçalves et al., 2021). These authors also emphasized that PLGA nanoparticles have promising applications as carriers for drug or molecule delivery targeting in cumulus cells and oocytes. For example, delivering antioxidants or mitochondrial-targeted compounds to oocyte and cumulus cells may be an important tool to regulate in vitro oxidative stress. Figure 4 shows the benefits of nanotechnology to in vitro maturation of COCs.
6 EFFECTS OF NANOPARTICLES ON IN VITRO EMBRYO DEVELOPMENT
Exogenous antioxidants associated with nanoparticles have been applied to the embryonic culture medium to allow rapid penetration and to protect the embryo from oxidative damage. In this context, lipid nucleic nanoparticles associated with melatonin improved in vitro development of bovine embryos, decreased cell apoptosis, the levels of ROS, and of mRNA for BAX and Caspase 3 (Komninou et al., 2016). Interestingly, it positively regulated transcripts of genes involved in antioxidant defense, as CAT and SOD2 (Komninou et al., 2016). This data shows that lipid nucleic nanoparticles, which have an organogel in their nucleus and acts as a second diffusional barrier for the release of melatonin, increases the protective effects of melatonin against oxidative stress and cell apoptosis during in vitro culture of bovine embryos (Jäger et al., 2009). These studies show the potential of using of nanoparticles in the delivery of different biomolecules during in vitro culture of embryos (Lucas et al., 2019).
Komninou et al. (2016) reported that bovine embryos cultured in the presence of melatonin-loaded lipid-core nanoparticles had the highest hatching when compared to nonencapsulated melatonin, melatonin-loaded polymeric nanocapsules. Melatonin-loaded lipid-core nanoparticles also increased embryo cell number, decreased cell apoptosis and ROS levels, downregulated mRNA levels of BAX and Caspase 3 genes, and upregulated mRNA levels of CAT and SOD enzymes (Komninou et al., 2016). These findings indicate that nanoencapsulation with lipid-core nanocapsules increases the protective effects of melatonin against oxidative stress and cell apoptosis during in vitro embryo culture in bovine species. Figure 4 shows the benefits of nanotechnology to in vitro embryo development.
7 CONCERNS ABOUT THE USE OF NANOMATERIALS
It is well known that biological structures, like amino acids, proteins, lipids, and so forth, have evolved into precise sizes, shapes, and chemistries to mediate interactions and functions. These biological molecules and structures are in the nanometer-size range and have specific functions to regulate cell growth and viability (Albanese et al., 2012). In addition to size, shape, and ligand density, the surface charge of molecules is also important in dictating cellular function. Thorek and Tsourkas (2008) reported that positively charged nanoparticles are taken up by cells at a faster rate than nanoparticles with a neutral or negative charge. Thus, it will be important to understand how the physicochemical properties of nanostructures relate to biological structures to adequately materialize the potential of nanoparticles to interact with sperm cells, COCs, and embryos and, consequently, improve the efficiency of ART.
Given that the complex interactions of nanomaterials with sperm cells, COCs, and embryos are still not fully understood, it is very important to discuss the risk of exposure of gametes and embryos to these molecules. The use of nanocarriers based on metal and metal oxide nanoparticles, despite their great potential, has some negative effects. The problems resulting are associated with their reduced stability, tendency to agglomerate, the possibility of releasing metal ions, or changing the composition by oxidizing their surface. However, as well discussed by Długosz et al. (2020), the level of cytotoxicity depends on the type of nanoparticles, that is, their morphology (shape, size), chemical purity, and type of solvent used, which are associated with the choice of the preparation method, type of functionalization, and type of biofunctionalization of nanocarriers, as well as their stability and aggregation susceptibility and agglomeration (Oberdörster et al., 2005; Asati et al., 2010).
Regarding male gametes, excessive-time exposure of human sperm to silver nanoparticles caused DNA damage, structural anomalies, and increased ROS production (E. Wang et al., 2017). In the same way, Taylor et al. (2015) showed that gold nanoparticles having surface properties that allow direct contact with the sperm plasma membrane can have toxic effects on bovine sperm in a concentration and time-dependent manner. Co-incubation of gold nanoparticles without association or in association with oligonucleotides can also decrease sperm motility (Taylor et al., 2015). Bioaccumulation of silver nanoparticles was observed in in vitro cultured Sertoli cells. These cells had autophagosomes and silver nanoparticles were present around these vacuoles, on the membrane, and in the cytoplasm, which caused signs of cell degradation.
For female gametes, after culturing early follicles enclosed in sheep ovarian tissues in vitro, Karimi et al. (2020) showed that uncoated iron oxide nanoparticles induce oxidative stress on the ovarian cortex while tissues treated with PEGylated iron oxide nanoparticles exhibit no significant change in oxidative stress. M. Ghorbani et al. (2019) showed that green synthesized zinc oxide nanoparticles and commercial zinc oxide nanoparticles have negative effects on preantral follicles through increasing the production of free radicals, reducing the expression of estradiol and testosterone, reducing expression of GDF-9 and BMP-15, as well as increasing the expression of Foxo1 and VNN1. However, when Galbanum extract covered the surface of zinc oxide nanoparticles, most of adverse effects of these nanoparticles were blocked. Additionally, zinc oxide nanoparticles synthesized with aqueous extract of Ferula gummosa gum resin (Galbanum) had negative effects on preantral follicles through increasing the production of free radicals, reducing the expression of estradiol, testosterone, GDF-9, and BMP-15.
In COC matured in vitro, bioaccumulation of gold nanoparticles was seen within porcine oocytes, without causing damage, but silver nanoparticles accumulated in cumulus cells causing toxic effects and decreasing maturation rates (Tiedemann et al., 2014). Huang et al. (2018) showed that silver nanoparticles can impair the maturation of mouse oocytes, decreasing the rates of in vitro fertilization and further inducing injury on embryonic development. Although the toxicity of silver nanoparticles appears to be driven by oxidation, it has not yet been shown whether silver, in its nanoparticulate form, is responsible for toxic effects (Kawata et al., 2009; Mathias et al., 2015) or whether the dissolution of silver ions in the course of metal oxidation would be the cause of the harmful effects (Chen et al., 2017). During in vitro culture of COC, cerium dioxide (CeO2) nanoparticles aggregated, but the crystal structure of these nanoparticles remained stable in the culture medium. In oocytes, aggregates of CeO2 nanoparticles were observed only around the zona pellucida, but an increase in DNA damage was seen when using higher concentrations of CeO2 nanoparticles (Courbiere et al., 2013). Likewise, Liu et al. (2010) reported that calcium phosphate nanoparticles can penetrate human granulosa cells and enter the lysosome and mitochondria. Recently, superparamagnetic iron oxide nanoparticles have shown dose-dependent toxicity, causing vacuolization in oocyte mitochondria and disorganized granulosa cells in murine species (Bakhtari et al., 2020).
During in vitro culture of embryos, Ag nanoparticles decreased the number of cells and promoted apoptosis in the internal cell mass and trophectoderm of blastocysts, reducing the postimplantation development of embryos in recipient mice (P. Li et al., 2010). Such impact can be attributed to the Ag ions that dissolve in the course of metal oxidation (Chernousova & Epple, 2013; Taylor et al., 2015). Concerning gold nanoparticles, Taylor et al. (2014) showed that gold nanoparticles at a concentration of 50 µg/ml did not elicit effect on mice embryonic development, apoptotic activity and did not affect the transcript profiles. Park et al. (2013) investigated the influence of chitosan and gold nanoparticles during the in vitro culture of murine embryos and no effect of gold nanoparticles on the development of blastocysts was observed, but embryos cultured with chitosan nanoparticles showed less developmental competence, reduction in mitochondrial activity, and less expression of pluripotency markers, as well as of transcripts associated with trophectodema.
In general, the toxicity of nanoparticles depends to a large extent on the type of surface coating, the size and concentration of nanoparticles, as well as the time of exposure, since the use of biocompatible materials can reduce their toxicity (Elsaesser & Howard, 2012). As nicely discussed by Długosz et al. (2020), there are many methods to prevent or limit the toxic effects of metallic nanoparticles and metal oxides. For example, changing the shape and size of particles, as well as modification of their surface, can lead to the formation of nanoparticles with the desired properties, but without a toxic effect (Długosz et al., 2020). Understanding the behavior of nanoparticles in in vitro systems, and their interactions with biological compounds, is crucial for the safe implementation of these materials in ART. Identifying how size, shape, and chemistry of nanoparticles influence the reproductive cells will allow researchers to redesign the nanoparticles to potentiate their beneficial effects and to improve ART. The fundamental studies on interactions of nanoparticles with biological structures will enable nanotechnology to create specific design rules.
8 FINAL CONSIDERATIONS
Current achievements in nanobiotechnology show that nanoparticles can successfully be used for the selection of alive sperm, semen sexing, and to deliver antioxidants to control oxidative stress during the culture of preantral follicles, COCs, and embryos. Despite the advances in ART, some challenges remain, mainly related to improvement culture systems to increase the rates of early follicle development in vitro, the acquisition of oocyte competence after IVM, and the percentage of blastocysts after IVF. To achieve these goals, nanotechnology represents a valuable tool that must be explored further to help researchers to optimize in vitro culture systems of gametes and embryos. Nanomaterials can bring specificity, practice, and sensibility to the next generation of biotechniques that can be used to improve the multiplication of animals and to solve fertility problems in humans. It is very important to develop more in vitro experiments to test safety and efficiency of these new methodologies that can accelerate new advances in ART. The development of novel nanocarriers with specific size, shape, ligand density, and charge to deliver biomolecules to gametes and embryos in vitro can have a positive impact for ART in humans and animals. Special attention is still required for the known toxicological aspects of nanomaterials before application.
ACKNOWLEDGMENTS
José Roberto Viana Silva is a researcher of Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grant no. 308737/2018-0).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available.