Isolation, proliferation, cytogenetic, and molecular characterization and in vitro differentiation potency of canine stem cells from foetal adnexa: A comparative study of amniotic fluid, amnion, and umbilical cord matrix
Abstract
The possibility to isolate canine mesenchymal stem cells (MSCs) from foetal adnexa is interesting since several canine genetic disorders are reported to resemble similar dysfunctions in humans. In this study, we successfully isolated, cytogenetically and molecularly characterized, and followed the differentiation potency of canine MSCs from foetal adnexa, such as amniotic fluid (AF), amniotic membrane (AM), and umbilical cord matrix (UCM). In the three types of cell lines, the morphology of proliferating cells typically appeared fibroblast-like, and the population doubling time (DT) significantly increased with passage number. For AF- and AM-MSCs, cell viability did not change with passages. In UCM-MSCs, cell viability remained at approximately constant levels up to P6 and significantly decreased from P7 (P < 0.05). Amnion and UCM-MSCs expressed embryonic and MSC markers, such as Oct-4 CD44, CD184, and CD29, whereas AF-MSCs expressed Oct-4, CD44. Expression of the hematopoietic markers CD34 and CD45 was not found. Dog leucocyte antigens (DLA-DRA1 and DLA-79) were expressed only in AF-MSCs at P1. Isolated cells of the three cell lines at P3 showed multipotent capacity, and differentiated in vitro into neurocyte, adipocyte, osteocyte, and chondrocyte, as demonstrated by specific stains and expression of molecular markers. Cells at P4 showed normal chromosomal number, structure, and telomerase activity. These results demonstrate that, in dog, MSCs can be successfully isolated from foetal adnexa and grown in vitro. Their proven stemness and chromosomal stability indicated that MSCs could be used as a model to study stem cell biology and have an application in therapeutic programs. Mol. Reprod. Dev. 78:361–373, 2011. © 2011 Wiley-Liss, Inc.
Abbreviations:
AF, amniotic fluid; AM, amniotic membrane; MSCs, mesenchymal stem cells; P#, passage number; TRAP, telomere repeat amplification protocol; UCM, umbilical cord matrix.
INTRODUCTION
Mesenchymal stem cells (MSCs) are defined to be multipotent stem cells that can be differentiated into various types of cells in vitro and in vivo, under controlled conditions (Jiang et al., 2002; Bossolasco et al., 2006; Ilancheran et al., 2007; Weiss et al., 2008; Cao and Feng, 2009). These cells can be isolated from many kinds of adult tissues, such as fat, skin, and brain (reviews by Hipp and Atala, 2008 and Gianaroli et al., 2009). However, their most common source is bone marrow, from where these cells were first isolated (Jiang et al., 2002; review by Marcus and Woodbury, 2008).
Important sources of MSCs could also be foetal adnexa, such as amniotic fluid (AF), amniotic membrane (or amnion; AM), and umbilical cord matrix (UCM), as demonstrated in previous studies in humans (for AF, see: Bossolasco et al., 2006; Roubelakis et al., 2007; Sessarego et al., 2008; and reviews by Marcus and Woodbury, 2008; Gucciardo et al., 2008; Pappa and Anagnou, 2009; for AM, see: Ilancheran et al., 2007; Izumi et al., 2009; and reviews by Marcus and Woodbury, 2008; Gucciardo et al., 2008; Pappa and Anagnou, 2009; for UCM, see: Karahuseyinoglu et al., 2007; Kestendjieva et al., 2008; and reviews by Marcus and Woodbury, 2008; Troyer and Weiss, 2008; Pappa and Anagnou, 2009).
Although MSCs from human adult tissues are promising for clinical applications, some limitations exist in terms of access to and use of these sources (Soncini et al., 2007). On the other hand, MSCs isolated from AF, AM, and UCM circumvent all of these limitations. These tissues are routinely discarded at parturition, so limited ethical controversy is associated with the harvest of the resident MSCs. Thanks to their extracorporeal nature, these cells are therefore easy to obtain and, moreover, are available in large supply. The comparatively large volume of extra-embryonic tissues and easy handling theoretically increases the number of stem cells that can be isolated (Soncini et al., 2007; review Marcus and Woodbury, 2008).
The hope of cell therapy, as a new clinical approach to repair tissue damage, relies on the characteristics of the foetal stem cells (Soncini et al., 2007; Gianaroli et al., 2009). In fact, these cells, as observed for adult MSCs, do not express the polymorphic antigens HLA-A, HLA-B, HLA-C (class IA), and HLA-DR (class II) on their surfaces, thereby evading immune defence against them (Terada et al., 2000; Ilancheran et al., 2007; review by Marcus and Woodbury, 2008; Weiss et al., 2008). Moreover, their low expression of costimulatory molecules, such as CD40, CD80, and CD86, seems to enhance transplantation tolerance, making these cells useful for allotransplant and xenotransplant (Weiss et al., 2008). These findings suggest that MSC derived from these tissues may be immunogenically inert and have a reduced risk of rejection upon transplantation (Ilancheran et al., 2007). The main applications of MSCs are in the therapy of haematological disorders, cardiovascular degenerative diseases, genetic and neurological disorders, and in tissue engineering (review by Gucciardo et al., 2008).
The dog has been considered an attractive animal model to evaluate new drugs or medical trials for preclinical purposes. An advantage of using the canine model is the possibility to perform MSCs transplantation in large size animals (Seo et al., 2009). The scientific interest in using the canine species as an animal research model to study human diseases is exponentially increasing (Ostrander et al., 2000; review by Songsasen and Wildt, 2007; Travis et al., 2009). More than 370 canine genetic disorders are reported, most of them resembling similar diseases and dysfunctions occurring in humans (review by Songsasen and Wildt, 2007). Retaining different canine genotypes can be invaluable for studying the mechanism and etiology of human genetic diseases, especially rare recessive disorders with complex inheritance that are difficult to study in human population (Patterson, 2000).
Considering the reported context, it is of interest to establish AF-MSCs, AM-MSCs, and UCM-MSCs to use for different purposes, such as proliferation/freezing trials, characterization studies, drug testing, and auto/allo/xenogenic transplantation. For these reasons, the animal model takes on capital importance. In the present study, we isolated, molecularly characterized and followed the differentiation potency of AF-MSCs, AM-MSCs, and UCM-MSCs in dogs. Chromosomal stability and telomerase activity were also demonstrated.
RESULTS
Cell Morphology, Population Doubling Time, and Cell Viability
In all three cell types, the morphology of proliferating cells typically appeared fibroblast-like (Fig. 1A–C for AF-MSCs, AM-MSCs, and UCM-MSCs, respectively), and clusters of rapidly expanding cells were observed. In Figure 1D, a cluster of AF-MSCs is shown.
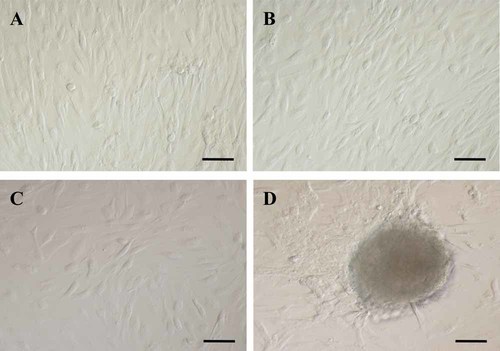
Photomicrographs of undifferentiated mesenchymal stem cells (MSCs) in culture. In all three cell lines, a spindle-shaped fibroblast-like appearance can be observed: amniotic fluid-MSCs (A), amniotic membrane-MSCs (B), and umbilical cord matrix-MSCs (C). Three-dimensional, relief contrast image of cell cluster with rapidly expanding adherent spindle-shaped fibroblast-like cells compatible with undifferentiated mesenchymal stem cell morphology (D). Cells were observed on day 3 of P2. Scale bars = 30 µm.
The population doubling time (DT) was measured, calculated, and drawn as a graph (Fig. 2A–C). In all three cell lines, the proliferation rate was reduced with passages. In particular, a significant increase in DT was found in AF-MSCs as compared to passage 1 (P1) versus P3/P4 (P < 0.05) and P1 versus P6/P7/P8 (P < 0.001). At P1, 1.12 ± 0.04 days were necessary to double the cell number, whereas at P8, DT was 1.50 ± 0.05 days (Fig. 2A). In AM-MSCs, 1.37 ± 0.05 days were necessary at P1 to double cell number, whereas at P6, DT was 1.48 ± 0.04 days (P < 0.01), and from P7, the significance level increased to P < 0.001 (1.67 ± 0.13 and 1.66 ± 0.1 for P7 and P8, respectively; Fig. 2B). In UCM-MSCs, DT significantly increased in P2/P4 versus P1 (P < 0.01) and P3/P5 versus P1 (P < 0.05). The significance level increased to P < 0.001 in P6/P7/P8 versus P1 (Fig. 2C). At P1, 1.14 ± 0.04 days were necessary to double cell number, whereas at P8, DT was 2.42 ± 0.72 days.
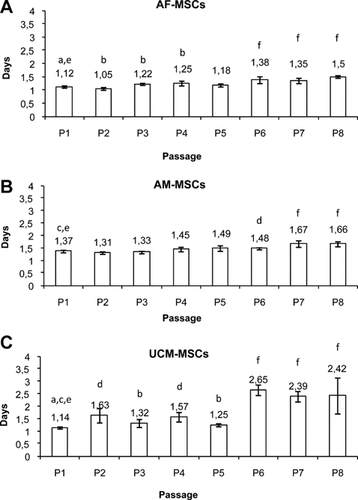
Doubling time of canine foetal adnexa-derived MSCs, expressed as number of days required for cell number doubling. Doubling times increased with passages for all three cell lines. AF-MSCs (A), AM-MSCs (B), and UCM-MSCs (C). Data are expressed as mean ± standard deviation of values obtained by four determinations. Student's t-test: a,b P < 0,05; c,d P < 0,01; e,f P < 0,001.
In addition, cell viability was measured. In particular, cell viability in AF-MSCs and AM-MSCs did not change with the passages (percentage of viable cells: mean values = 95.51 ± 2.81 and 91.97 ± 2.34, respectively; Fig. 3A,B). In UCM-MSCs, cell viability remained at approximately constant levels up to P6, and significantly decreased from P7 (P < 0.05; Fig. 3C).
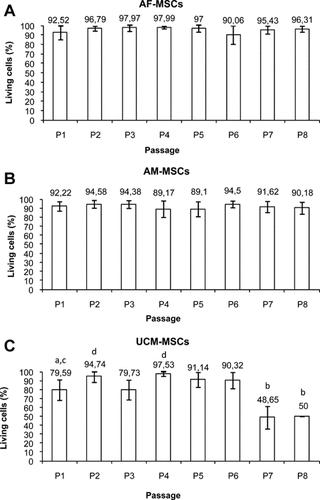
Viability of canine foetal adnexa-derived MSCs, expressed as percentage of living (unstained) cells after trypan blue staining. Cell viability remained at constant values in AF-MSCs (A) and in AM-MSCs (B), whereas it decreased in UCM-MSCs (C). Data are expressed as mean ± standard deviation of values obtained by four determinations. Student's t-test: a,b P < 0.05; c,d P < 0.01.
Stem Cell and Hematopoietic Markers mRNA Expression
The expression of stem cell markers was evaluated in all three cell lines at P1 and P2 (Fig. 4). In P1, AF-MSCs expressed Oct-4, CD44, DLA-DRA1, and DLA-79. In P2, only CD44 was expressed. CD184 and CD29 expressions were not observed in this cell line. For AM-MSCs, cells in P1 expressed Oct-4, CD44, CD184, and CD29. In P2, these cells expressed Oct-4 and CD184, but they did not express CD44 and CD29. For UCM-MSCs, P1 cells expressed Oct-4, CD44, CD184, and CD29, as in the P1 of AM-MSCs. In P2, CD44 and CD184 were expressed; Oct-4 and CD29 were not observed. The housekeeping gene HPRT1 was expressed in all cell lines and passages. Expression of the embryonic stem cell marker Nanog, the hematopoietic markers CD34 and CD45, and the major histocompatibility complex (MHC) genes DLA-DQA1 and DLA-88 were never found in any cell lines or passages. Canine subcutaneous fibroblasts, used as negative controls, did not express stemness or MHC markers. Dog leucocytes, used as positive controls for hematopoietic and MHC markers, expressed hematopoietic and MHC markers but they did not express embryonic or mesenchymal stemness markers. In order to confirm the identity of Oct-4 in all three cell lines, sequence analysis of the RT-PCR-amplified fragments has been performed (Ceinge Biotecnologie Avanzate—Nucleic Acid Sequencing Services—University of Naples, Naples, Italy) and amplifications products show identity to canine Oct-4 (NCBI Nucleotide Database: XM_538830.1).
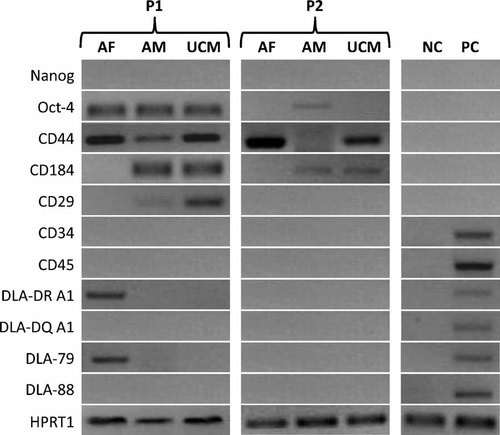
Reverse transcription–polymerase chain reaction (RT-PCR) data comparing canine AF-MSCs, AM-MSCs and UCM-MSCs with canine differentiated subcutaneous fibroblasts (negative controls, NC). Cells at P1 and P2 were examined for expression of markers for embryonic pluripotent cells (Nanog: 185 bp; Oct-4: 200 bp), markers for MSCs (CD44: 268 bp; CD184: 114 bp; CD29: 188 bp), markers for hematopoietic stem cells (CD34: 255 bp; CD45: 159 bp), and markers for major histocompatibility complex (DLA-DRA1: 246 bp; DLA-DQA1: 163 bp; DLA-79: 333 bp; DLA-88: 355 bp). In P1, all cell lines expressed at least one embryonic and MSC marker. In detail, AM-MSCs and UCM-MSCs expressed Oct-4, CD44, CD184, and CD29, whereas AF-MSCs expressed Oct-4 and CD44. Expression of the hematopoietic markers CD34 and CD45 was never found, whereas dog leucocyte antigens (DLA-DRA1 and DLA-79) were expressed in only AF-MSCs. In P2, expression of at least one MSC marker was maintained in all samples. In detail, AM-MSCs expressed Oct-4 and CD184, AF-MSCs expressed CD44, and UCM-MSCs expressed CD44 and CD184. All cell lines expressed the housekeeping gene HPRT1 (144 bp). Dog leucocytes were used as positive controls (PC) of hematopoietic and MHC markers expression.
Karyotype and Telomerase Activity
It is known that the karyotype of stem cells can undergo changes during passaging, and show rates of chromosomal abnormalities (Buzzard et al., 2004); for this reason, we performed karyotype analysis on all our samples. All observed metaphases of AF-MSCs (n = 15), AM-MSCs (n = 23), and UCM-MSCs (n = 13) were normal in chromosomal number and structure, thus confirming the genomic stability of the cells in culture (Fig. 5). Telomerase activity and telomere maintenance are associated with the immortality of cancer cells, germ-line cells, MSCs, and embryonic stem (ES) cells (Shay and Wright, 1996; Shay et al., 1996; Hiyama and Hiyama, 2007). Accordingly, a telomerase activity detection assay was used to distinguish immortal cells from differentiated somatic cells. We performed a Telomere Repeat Amplification Protocol (TRAP) assay (TRAPeze® Telomerase Detection Kit; Chemicon International Inc., Temecula, CA) on AF-MSCs, AM-MSCs, and UCM-MSCs, and analyzed telomerase expression and activities in comparison with canine subcutaneous tissue, canine immortalized fibroblasts, canine newly isolated fibroblasts and canine tumor cells (Materials and Methods section). The TRAP assay is a two enzyme-based system in which the extracted and to-be-tested telomerase adds telomeric repeats to a substrate first, and then Taq polymerase amplifies it. All of our samples showed high telomerase activity except for the subucutaneous tissue (Fig. 6). Since telomerase is a heat-sensitive enzyme, every sample was tested for heat sensitivity as a control. All the heat-treated test extracts confirmed the inactivation of enzyme except for the one corresponding to the canine breast adenoma ZMTH3 (smear detected), even after increasing the length of heat-inactivation two-fold and three-fold (Fig. 6). The extra 36-bp band representing the internal control (standard internal control; S-IC) was visible in all assays except for AF and AM, probably due to the excessively high telomerase activity in these samples.
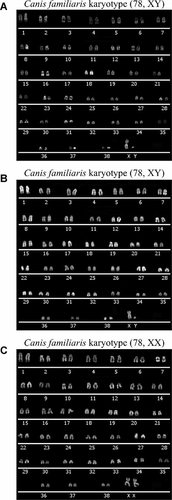
Quinacrine band karyogram showing chromosomes of the amniotic fluid (A), amniotic membrane (B), and umbilical cord matrix-mesenchymal stem cells (C). Cells were analyzed at P4 and show a normal diploid karyotype of 78 chromosomes.
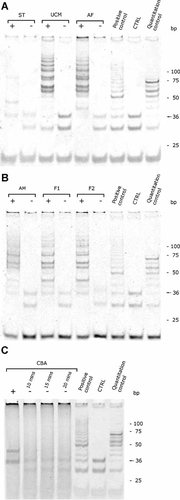
Telomere repeat amplification protocol (TRAP) results. Telomerase extraction and activity tests were performed on canine subcutaneous tissue (ST), UCM and AF (A); AM, canine immortalized fibroblast (F1) and canine newly isolated subcutaneous fibroblast cell culture (F2) (B); canine breast adenoma (CBA) ZMTH3 (C). For each sample, a negative control (−), generated by thermic heat shock of telomerase complex according to protocol, has been carried out. An internal positive control provided by the TRAPeze® Telomerase Detection Kit has been used for each gel. As a general negative control (CTRL), lysis buffer was used to test the potential generation of primer-dimer PCR artifacts and contamination. If the assay worked optimally, the internal control band (S-IC, 36bp) is present in the control lane. Cell samples of AF, AM, and UCM were analyzed at P4.
In Vitro Differentiation
When induced to neuronal differentiation, cells of the three cell lines were positive for nestin expression (Fig. 7A). These cells showed the presence of Nissl bodies and a neuronal-like morphology (Fig. 7B) as compared to the fibroblast-like appearance observed in cells cultured in basal conditions (Fig. 1).
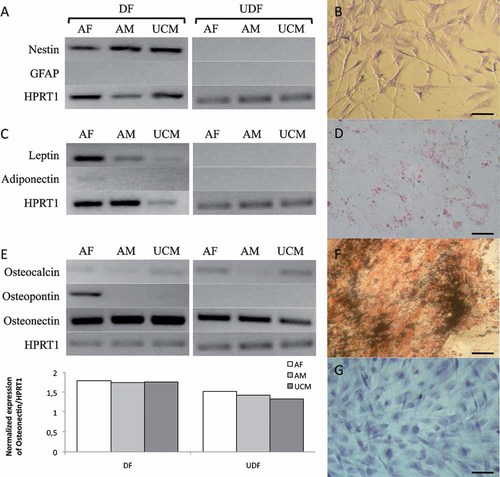
Neurogenic, adipogenic, osteogenic, and chondrogenic differentiation of canine AF-MSCs, AM-MSCs, and UCM-MSCs at P3. For each cell line, expression of tissue-specific genes is shown in differentiated (DF) and undifferentiated (UDF) samples (panel A, C, E). Photomicrographs (B, D, F, G) are representative of morphological appearance and specific staining (B, D, F, G) of differentiated cells. Expression of the neurogenic gene nestin (A) and presence of Nissl bodies in cells with neuronal-like morphology (B; UCM-MSCs; scale bar: 50 µm) after neurogenic differentiation. Expression of adipogenic genes leptin and adiponectin (C), and the presence of lipid droplets (D; AM-MSCs; scale bar: 30 µm) after 25 days of adipogenic differentiation. Expression of osteogenic gene osteopontin (E) after 14 days of osteogenic differentiation; overexpression of osteonectin gene (E, 1.2-, 1.2-, and 1.3-folds in DF compared with UDF cells for AF, AM and UCM, respectively); presence of Ca++ mineralization by Von Kossa staining (F; UCM-MSCs; scale bar: 30 µm). Presence of acidic proteoglycans demonstrated after 14 days of chondrogenic differentiation by Alcian blue straining (G; AF-MSCs; scale bar: 30 µm).
In cells induced to differentiate in an adipogenic medium, leptin expression (Fig. 7C) and the presence of lipids droplets (Fig. 7D) were evident after 25 days of adipogenic differentiation in all three cell lines. Adiponectin expression was found only in AF-MSCs (Fig. 7C).
In cells induced to differentiate in an osteogenic medium for 14 days, semi-quantitative RT-PCR analysis revealed over-expression of the osteogenic gene osteonectin. In AF-MSCs, AM-MSCs, and UCM-MSCs, expression of this gene was found to be 1.2-, 1.2-, and 1.3-fold higher, respectively, in differentiated as compared to undifferentiated cells. Expression levels of osteocalcin between differentiated cells and controls did not change in any of the three cell lines. Osteopontin was expressed only in AF-MSCs (Fig. 7E). Calcium mineralization was also found in all three cell lines, as revealed by Von Kossa staining (Fig. 7F).
After 14 days of in vitro culture in a chondrogenic medium, all cell lines were stained with Alcian blue, showing the typical metachromasia of cartilage, thus chondrogenic differentiation was demonstrated by the mucopolysaccharide-rich extracellular matrix (Fig. 7G).
DISCUSSION
The possibility of stem cell isolation for use in cell therapy drives researchers to direct their studies towards new sources in order to obtain relevant numbers of cells and to minimize risks for donors and recipients. In this study, we successfully isolated stem cells from foetal adnexa: AF, AM, and UCM. Cells from these tissues were capable of self-renewal and could be used to obtain in vitro or in vivo differentiation into various cell lines.
Cells were spindle-shaped, plate-adherent and able to be substantially subcultured in vitro. The results of the proliferation study showed that the three cell lines behaved similarly since DT values increased with the passage number. Cell viability analysis showed that in UCM-MSCs, the percentage of living cells decreased with the passage number, whereas in AF-MSCs and AM-MSCs the viability remained at a constant level. These data are in agreement with those reported in previous studies in humans (references cited in Introduction section) and in few studies reported to date in dogs: Seo et al. (2009) isolated MSCs from umbilical cord blood (UCB). They observed that these cells could be cultured for 11 passages, and their doubling time increases with passage number. Zucconi et al. (2010) reported that MSCs from umbilical cord veins could be cultured for at least seven passages without altering their morphology and proliferating capacity.
Our cells also had normal karyotypes after in vitro culture. This proves that tissue culture procedures employed in the present study do not alter chromosomal organization or stability, at least for rearrangements that can be detected by classical cytogenetic approaches. In addition, the finding of male karyotypes provided further confirmation of the foetal origin of examined cell lines. To our knowledge, this is the first study reporting normal chromosomal number and structure of canine MSCs from AF, AM, and UCM. A previous study (Zucconi et al., 2010) reported normal chromosomal number in MSC from umbilical cord vein.
The activity of telomerase, a reverse transcriptase that can elongate telomeric repeats, is usually diminished after birth so that telomere length is gradually shortened with cell divisions, and triggers cellular senescence (Hiyama and Hiyama, 2007) and apoptosis (Rodriguez-Brenes and Peskin, 2010). Germ cells, stem cells, and the majority of cancer cells express telomerase. Cells that express the enzyme at sufficient levels maintain a stable telomere length and have an unlimited replication capacity (Rodriguez-Brenes and Peskin, 2010). As expected, the isolated cells showed telomerase activity. Noteworthy, a faint 36-bp internal control band was visible in the UCM telomerase sample, whereas this band was absent in the AF and AM samples, suggesting that reduced telomerase activity might contribute to the compromised doubling time and viability of this source of foetal MSCs. To our knowledge, this is the first study reporting that canine MSCs isolated from foetal adnexa show telomerase activity. All the heat-treated test extracts confirmed the enzyme inactivation, except for the one corresponding to the canine breast adenoma ZMTH3 (smear detected). This can be explained by the extremely high protein concentration of the tumor sample, which can produce PCR artifacts. Moreover, TRAP-assay positive results from canine fibroblasts were obtained because of the residual activity of the telomerase complex in stable cell lines (F1) and the obvious selection of non-terminally differentiated cells from the primary tissue samples collected using the cell isolation and cell culture protocol (F2), as previously reported by Kim et al. (1994) and Shay and Wright (1996). All the positive samples have been compared to and displayed higher activity than the quantification control.
Molecular characterization showed us that stemness marker expression varies among the three cell lines: AM-MSCs and UCM-MSCs expressed the embryonic marker Oct-4 and the three tested mesenchymal markers (CD44, CD184, and CD29) at P1. AF-MSCs expressed only the embryonic marker Oct-4 and the mesenchymal marker CD44. In P2, AM-MSCs lost the expression of CD44 and CD29, UCM-MSCs lost the expression of Oct-4 and CD29, and AF-MSCs lost the expression of Oct-4 while maintaining the mesenchymal marker CD44. Further studies are needed to better understand the variability of marker expression between lines and passages. The finding of Oct-4 expression in stem cell lines of fetal origin is in agreement with previous studies (Bossolasco et al., 2006, human AF; Mauro et al., 2010, ovine AF; Ilancheran et al., 2007, human AM; Mauro et al., 2010, ovine AM; Hoynowski et al., 2007, equine UCM; Cremonesi et al., 2008, equine UCM; Seo et al., 2009, dog UCB). In our study, the identity of Oct-4 in all three cell lines has been confirmed by sequence analysis of the RT-PCR fragments. On the other hand, little information is available to date on Nanog expression in fetal stem cell lines. As reviewed by Abdulrazzak et al. (2010), Nanog was found to be weakly expressed in human Wharton's Jelly, expressed in trace in human AF-MSCs and was not determined in human amnion-derived MSCs. To our knowledge, only two studies examined stemness markers in canine foetal adnexa, and their findings are in line with our results. Seo et al. (2009) in UCB cells at P1 reported Oct-4, CD29, CD184 expression, and the lack of expression of CD34, CD45, DLA-DR. Zucconi et al. (2010) demonstrated that umbilical cord vein cells at P3 express CD29, but do not express CD34 and CD45. Expression of the hematopoietic markers CD34 and CD45 were never seen in our cell lines. This result was expected, and it confirmed that isolated cells do not belong to a hematopoietic lineage.
The observed reduced expression of MHC genes (only DLA-DRA1 and DLA-79 were expressed at P1 in AF-MSCs) and lack of expression in all the three cell lines at P2 hints at a potential application of these cells to allo- and xeno-transplantation. Our data are in agreement with the study by Seo et al. (2009), which reported the lack of expression of DLA-DR in umbilical cord blood MSCs.
Cell lines undergoing several passages can be easily transformed and acquire proliferative advantages, as compared to other cells, and resemble cancer cells. Other than the molecular markers we used, cancer and normal stem cells can be distinguished based on chromosomal stability. Our analysis clearly showed the stable nature of the isolated cell karyotype.
Nestin expression and Nissl staining suggested that, under the neurogenic culture condition used in this study, AF-MSCs, AM-MSCs, and UCM-MSCs may be induced to differentiate into an ectodermal lineage. Furthermore, under in vitro induction conditions, we were able to differentiate AF-MSCs, AM-MSCs, and UCM-MSCs into mesodermal lineages such as adipocytes, osteoblasts, and chondrocytes. Similar results were reported by Seo et al. (2009) and Zucconi et al. (2010). Seo et al. (2009) observed that umbilical cord blood MSCs cultivated in specific media could differentiate in neurocytes, osteocytes, and chondrocytes. Zucconi et al. (2010) demonstrated the umbilical cord vein cells' capacity to differentiate in vitro into adipocytic, chondrocytic, and osteocytic lineages when subject to specific protocols. The findings of these studies were confirmed by the analysis of gene line-specific expression.
Studies on the isolation and characterization of MSCs from foetal adnexa in humans are rapidly advancing (references cited in Introduction section). Some authors reported that MSCs isolated from human AF, AM, and UCM could be used for the study and therapy of some diseases such as amyotrophic lateral sclerosis and Parkinson disease (Fu et al., 2006; review by Gucciardo et al., 2008).
Studies in animal models are still in their infancy. In the equine, MSCs were isolated from UCM (Hoynowski et al., 2007; Cremonesi et al., 2008; Passeri et al., 2009). Some authors (Marcus et al., 2008; Wang et al., 2008) isolated MSCs from the AM in rats. Wang et al. (2008) demonstrated that these cells, when topically injected in the brain of rats with different deficits, contributed to the partial improvement of the rats' conditions. Some authors reported studies in mouse isolating AF-MSCs (Nadri and Soleimani, 2007; Rehni et al., 2007). Rehni et al. (2007) demonstrated how AF-MSCs ameliorate focal cerebral ischemia-reperfusion injury-induced behavioral deficits in mice. Mauro et al. (2010) isolated, expanded in vitro, characterized and followed differentiation potential of ovine stem cells collected from AF and AM.
The canine model could have a critical role in studying human diseases, and possible therapies, with similar transmission. Only few articles on MSC isolation were reported in the literature prior to submission; to our knowledge, our study is the first with the specific aim to isolate, to expand in vitro, and to characterize MSCs from AF, AM, and UCM in the canine model. Hatoya et al. (2006) observed that embryonic stem cells can be successfully isolated from canine blastocysts. These cells formed embryoid bodies in suspension culture and differentiated into several types of cells, including neuron-, epithelium-, fibroblast-, melanocyte-, and myocardium-like. Vaags et al. (2009) reported the derivation of permanent canine cell lines from pre-implantation stage embryos and their characterization for several genomic, proteomic and functional markers. Seo et al. (2009), as previously mentioned, isolated and characterized MSCs from umbilical cord blood, and Zucconi et al. (2010) reported the isolation of MSCs from umbilical cord vein, succesfully growing them in culture and showing a capacity for pluripotent differentiation. Vieira et al. (2010) observed that canine adipose tissue contains multipotent stem cells able to be maintained in vitro for extended periods with stable population doubling and low levels of senescence. These cells were also able to differentiate in vitro in adipogenic, chondrogenic, myogenic, and osteogenic cells.
In conclusion, this study provides a simplified isolation and characterization procedure for MSCs derived from AF, AM, and UCM in the dog and suggests that AF-MSCs, AM-MSCs, and UC-MSCs have the potential to be a resource for stem cell therapy and regenerative medicine in the canine animal model system.
MATERIALS AND METHODS
Cell Isolation and Culture
Samples were recovered after elective ovariohysterectomy in four bitches at 25–40 days of gestational age. AF, AM, and UCM of canine fetuses were used for the isolation of mononuclear cells. Pieces of the AM and UCM were placed in two different Petri plates with sterile and pre-warmed phosphate buffered saline (PBS; P4417, Sigma, Milan, Italy) with antibiotics (penicillin 100 U/mL, streptomycin 100 µg/mL solution, P0781; Sigma). After three flushes with PBS, pieces were placed in two new Petri plates. Pieces where cultured at the temperature of 38.5°C with 5% CO2 in culture medium: Dulbecco Modified Eagles Medium (DMEM; D5546; Sigma), fetal bovine serum (FBS; 10%; F3018, Sigma), L-glutamine (2 mM; G7513; Sigma), penicillin (100 U/mL) streptomycin (100 µg/mL), and amphotericin (0.25 µg/mL) solution (A5955, Sigma), and epidermal growth factor (EGF; 10 ng/mL; E4127, Sigma). After 5 days, pieces were removed and cells were cultured until their isolation. The collected AF was delivered in Falcon tubes and spun to separate cells from the liquid fraction. The recovered cells were re-suspended in culture medium, as previously described for AM and UCM, and placed into T25 cell culture flasks. Two cell lines were evaluated for each tissue type (AF, AM, and UCM).
Cell Expansion
Plated cells were monitored during the first expansion period (passage 0 = P0), and after 48–72 hr, the medium was changed to remove non-adherent cells. Approximately 10 days later, when well-developed colonies of fibroblast-like cells appeared, cultures were washed with PBS, harvested with 0.05% trypsin–0.02% EDTA (ECM0920D; Euroclone, Milan, Italy), and re-plated in six-well plates (3516; Corning, New York, NY). Cells were counted for each passage, with Burker's chamber by dilution (1:1) in trypan blue (T8154; Sigma), starting to P1. Cell viability was expressed as a percentage of unstained cells. Since then, cells were maintained for some passages to perform growth and proliferation studies.
The three types of cell lines were seeded in six-well plates at a density of 1,000 cells/cm2 and the doubling-time analysis was performed. Culture passages were performed every 4 days and cell counts were performed for each culture passage. Obtained values are used to allow the calculation of doubling time (DT). Population DT was calculated using the formula DT = CT/CD where CT (culture time) is the time between passage “n” and passage “n + 1,” and CD (cell doubling) = ln(nf/ni)/ln2 where “nf” is the harvested cell number and “ni” the seeded cell number (Lei et al., 2007). On the days of the first and second counts, aliquots of the cells were cryopreserved for each cell line for molecular characterization; aliquots of fresh samples from the third passage were used to perform in vitro differentiation, and aliquots of fresh samples of the fourth passage were used for karyotype analysis and the TRAP assay.
Karyotyping
Karyotype analysis of AF-MSCs, AM-MSCs, and UCM-MSCs at P4 was performed according to the standard procedures of Q banding. Briefly, the cell line was processed with colcemid (0.1 µg/mL in culture medium; D1925; Sigma) for 4 hr followed by three washes in a fixative (3 methanol:1 acetic acid). Chromosomes were banded by immersion in quinacrine mustard (Q2876; Sigma), fully washed with tap water, and mounted in 1× PBS. Metaphases were acquired by CCD camera using a Leica fluorescence microscope. Chromosomes were counted, and the banding pattern was compared to standard Canis familiaris karyotype according to Breen et al. (1999).
Telomere Repeat Amplification Protocol Assay
The TRAP assay is based on an improved version (Kim and Wu, 1997) of the original method described by Kim et al. (1994), and assesses the telomerase activity on samples by measuring the capacity of the extracted enzyme to carry out the amplification of the target telomeric repeat (TS) available in the commercial kit. This assay works as a two-enzyme system, utilizing telomerase activity and the polymerase chain reaction (PCR). During the first step of the reaction, telomerase adds a number of telomeric repeats (GGTTAG) onto the 3′ end of a substrate oligonucleotide (TS). In the second step, the extended products are amplified by PCR using the TS and RP (reverse) primers, generating a ladder or smear of products with six base increments starting at 50 nucleotides. Further, since telomerase is a heat-sensitive enzyme, a negative control can be obtained for every sample after heat inactivation of the sample itself.
A 36-bp amplification product represents a standard internal control (S-IC) for the experiment and should always be visible in all the samples (+ and −). Many cell/tissue extracts contain inhibitors of Taq polymerase and can give potentially false-negative results. To distinguish this from other problems, the TRAPeze® Primer Mix contains the internal control oligonucleotides K1 and TSK1 that, together with TS, produce a 36-bp band (S-IC) in every lane. The S-IC band also serves as a control for amplification efficiency in each reaction, and can be used for quantitative analysis of the reaction products. Cell extracts of AF-MSCs, AM-MSCs, and UCM-MSCs at P4 were assayed for telomerase activity following the instruction of the TRAPeze® telomerase detection kit (Chemicon International Inc.). Cell extracts were prepared by lysing around 1 × 106 cells with 1× CHAPS lysis buffer. For each sample, 10 µl of cell extract were heat treated by incubating at 85°C for 10 min prior to the TRAP assay to inactivate telomerase. ZMTH3 cell line, canine immortalized fibroblasts, canine newly isolated fibroblasts and canine subcutaneous tissue were used as controls. Cell extracts were used for PCR in a mix composed as follows: cell extract (2 µl), 10× TRAP buffer (5 µl), 50× dNTP mix (1 µl), TS primer (1 µl), TRAP primer mix (1 µl), Taq polymerase (2 U, 0.4 µl), and PCR-Grade Water (39.6 µl) for a final volume of 50 µl. Reaction products were analyzed using 12.5% polyacrylamide gel.
Neurogenic Differentiation
Cells at P3 of the three cell lines were plated in six-well plates. Twenty-four hours prior to neuronal induction, culture media were replaced with pre-induction media consisting of DMEM, 20% FBS and 1 mM β-mercaptoethanol (M7522, Sigma; Seo et al., 2009). For neuronal differentiation, the pre-induction medium was removed and the cells were washed with PBS and transferred to neuronal induction media composed of DMEM, 20% FBS, 2% dimethyl sulfoxide (D5879, Sigma), and 200 µM butylated hydroxyanisole (B1253, Sigma; Woodbury et al., 2000). Cells were cultured for 5 days and, after differentiation, Nissl staining was performed as reported by Hou et al. (2003) and Lu et al. (2010). Molecular tests were performed for nestin and glial fibrillary acidic protein (GFAP).
Adipogenic Differentiation
For adipogenic differentiation, cells at P3 of the three cell lines were cultured for 25 days in a differentiation medium composed as follows: DMEM, 10% FBS, 2 mM L-glutamine, 1× penicillin (100 U/mL), streptomycin (100 µg/mL), and amphotericin (0.25 µg/mL) solution, 1 µM dexamethasone (D2915, Sigma), 200 mM indomethacin (I7378, Sigma), and 10 µg/mL insulin (I6634; Sigma). The medium was changed twice weekly. Differentiation was evaluated by Red Oil-O staining (0.1% in 60% isopropanol; Bossolasco et al., 2006; Hoynowski et al., 2007) to visualize lipid droplets, and by molecular tests for adiponectin and leptin expression.
Osteogenic Differentiation
For osteogenic differentiation, cells at P3 of the three cell lines were cultured for 14 days in a differentiation medium composed of DMEM, 10% FBS, 1× penicillin (100 U/mL), streptomycin (100 µg/mL), and amphotericin (0.25 µg/mL) solution, 0.1 µM dexamethasone, 0.25 mM L-ascorbic acid 2-phosphate (A8960, Sigma), and 10 mM β-glycerophosphate (50020, Sigma). The medium was changed twice weekly. Differentiation was evaluated by Von Kossa staining (Ilancheran et al., 2007; Mauro et al., 2010) with some modifications (1% silver nitrate and 5% sodium thiosulfate) to detect calcium deposits, and by molecular tests for osteopontin, osteocalcin and osteonectin.
Chondrogenic Differentiation
Chondrogenic differentiation was assessed in a monolayer culture by incubating cells at P3 for 14 days in DMEM containing 100 nM dexamethasone, 50 µg/mL L-ascorbic acid 2-phosphate, 1 mM sodium pyruvate, 40 µg/mL proline, ITS (insulin 5 µg/mL, transferrin 5 µg/mL, selenious acid 5 ng/mL; I3146, Sigma), and 5 ng/mL TGF-β1 (100-36E, Peprovet, DBA, Milan, Italy). Differentiation was demonstrated by staining with Alcian blue (pH 2.5; Wang et al., 2004; Ghosh et al., 2010).
Reverse Transcription–Polymerase Chain Reaction (RT-PCR) Analysis
Total mRNA was isolated from AF-MSCs, AM-MSCs, and UCM-MSCs using NucleoSpin RNA II (FC140955L; Macherey-Nagel, Düren, Germany), and converted into cDNA using an Enhanced Avian HS RT-PCR kit (HSRT100-1KT, Sigma). The cDNA was amplified by using the PCR mix reaction: cDNA (100 ng), PCR buffer (1×; 5 µL), dNTP mix (10 mM each; 1 µL), PCR primers (mixture of 50 µM each primer; 1 µL), Hot Master Taq DNA Polymerase (1U; 2200320; 5Prime, Milan, Italy) and nuclease-free water for a final volume of 50 µl. The expression of the following set of genes was evaluated for molecular characterization before in vitro differentiation: canine Nanog homebox, canine Pou class 5 homebox 1 (POU5F1 alias Oct-4), canine CD44, canine Chemokine (C-X-C motif) receptor 4 (CXCR4 alias CD184), canine 1D Isoform of Integrin 1β (CD29), canine CD34, canine CD45, canine MHC class II DLA-DRA1 and DLA-DQA1and canine MHC class I DLA-79 and DLA-88. To test cell differentiation, the following markers were used: canine nestin and canine glial fibrillary acidic protein (GFAP) for neurogenic differentiation; canine adiponectin (ADIPOQ) and canine leptin for adipogenic differentiation; canine bone gamma-carboxyglutamate protein osteocalcin, canine osteopontin and canine osteonectin for osteogenic differentiation. Canine hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as an internal control for RNA extraction, RT procedure, and PCR. All oligonucleotide primers spanned a least one intron. Table 1 shows primer sequences, and the expected length of corresponding amplified fragments. The RNA templates of all analyzed genes were amplified for 35 cycles of 94°C (45 sec, denaturation), 59°C (45 sec, annealing), 72°C (40 sec, elongation), followed by one cycle at 72°C for 5 min, except CD45 and DLA-88, which were amplified with a 57°C annealing temperature. Negative controls (PCR without template and RNA without RT) were performed in each experiment (data not shown). Subcutaneous fibroblasts were used as negative controls for embryonic, mesenchymal, hematopoietic, and MHC markers. Dog leucocytes were used as positive controls for hematopoietic and MHC markers expression. PCR products were visualized with ethidium bromide on a 2% agarose gel.
| Gene | Amplification product (bp) | Forward primer | Reverse primer |
|---|---|---|---|
| Nanog | 185 | 5′-GGCCCCCAAGCACCCAACTC-3′ | 5′-ACCCGCGGACTGGTGGAAGA-3′ |
| Oct4 | 200 | 5′-TCAGGCGGATGTGGGGCTCA-3′ | 5′-GGGCCTGCACGAGGGTCTCT-3′ |
| CD44 | 268 | 5′-GCCCTGAGCGTGGGCTTTGA-3′ | 5′-TCTGGCTGTAGCGGGTGCCA-3′ |
| CD184 | 114 | 5′-GCGGGCGAGCGGTTACCAT-3′ | 5′-CCTCCCGGAAGCAGGGTTCCTT-3′ |
| CD29 | 188 | 5′-TGGGCTTGCGTTGCTGCTGA-3′ | 5′-CACCGGCAACTTAGAGACCAGC-3′ |
| CD34 | 255 | 5′-GCCTGCTCAGTCTGCTGCCC-3′ | 5′-TGGTCCCAGGCGTTAGGGTGA-3′ |
| CD45 | 159 | 5′-CTCACGCACACAGGCTCGCA-3′ | 5′-CCCACCCACTGGCACTGCTG-3′ |
| DLA-DRA1 | 246 | 5′-CGCTCCAACCACACCCCGAA-3′ | 5′-GGCTGAGGGCAGGAAGGGGA-3′ |
| DLA-DQA1 | 163 | 5′-GCACTGGGGCCTGGATGAGC-3′ | 5′-ACCTGAGCGCAGGCCTTGGA-3′ |
| DLA-79 | 333 | 5′-CTTCCTGGAGGGCAGGTGCT-3′ | 5′-GGCTTGGGCAGGCTCTTGTG-3′ |
| DLA-88 | 355 | 5′-GTGTGAGACCAGCTGCCTATGG-3′ | 5′-ACCTCATCAGCCTCACATCCCA-3′ |
| Nestin | 246 | 5′-CAGGTCCTGGAAGGTCGGCA-3′ | 5′-AGGCAAGGGCAAGGAGAGGG-3′ |
| GFAP | 150 | 5′-AACTTGCAGATCCGAGGGGGC-3′ | 5′-GGCAGGCTGCTAACCGAGAGC-3′ |
| ADIPOQ | 250 | 5′-GATGCAGGCATCCCAGGGC-3′ | 5′-CCGGCTCTCCAACCCCACAC-3′ |
| Leptin | 100 | 5′-GAAGCTGTGCCAATCCGAAA-3′ | 5′-AGGAGACAGACTGCGTGTGTGA-3′ |
| Osteocalcin | 174 | 5-GTGGCCGCACTCTGCCTCTG-3′ | 5′-GGGATCCGGGTAGGGGACCG-3′ |
| Osteopontin | 247 | 5′-GCAGAATGCTGTGCTGACTGAGG-3′ | 5′-ACCTCTTGTGGGGACAGCTGGA-3′ |
| Osteonectin | 206 | 5′-CTGCCCTGGTCCCATTGGCG-3′ | 5′-CCCGCATGCGCAGAGGGAAT-3′ |
| HPRT1 | 144 | 5′-CGCAGCCTTGGCGTCGTGAT-3′ | 5′-TCGAGCAAGCCGCTCAGTCC-3′ |
Semi-Quantitative RT-PCR Analysis
Semi-quantitative RT-PCR analysis was performed on AF-MSCs, AM-MSCs, and UCM-MSCs before and after osteogenic differentiation considering that, in these cell lines, osteogenic markers osteonectin and osteocalcin were found to be expressed in differentiated and undifferentiated cells. To check the exponential phase amplification of these two genes, we conducted a test with 120-µL final-volume PCR mixture (200 ng of cDNA, 1 U of HotMaster taq polymerase Eppendorf, 0.25 mM dNTPs, 1 mM each primer), taking 10 µl from the tube starting from cycle 27 up to 35. The same procedure was followed for the house-keeping HPRT1 gene. PCR amplification was conducted up to the optimal cycle identified in the previous step for both the targets and house-keeping gene. The obtained amplification products, together with a 100-bp DNA ladder, were run on 2% agarose gel stained with ethidium bromide and images were digitally captured with a CCD camera (Gel Doc 2000 Gel Documentation System; BioRad, Milan, Italy). Densitometric analysis was performed using the QuantityOne software (BioRad), and values were expressed as relative absorbance units. Messenger-RNA expression was normalized for each gene against the amount of HPRT1 mRNA. Three replicates were performed.
Statistical Analysis
The Student's t-test was used to evaluate the statistical significance of the results. Values with P < 0.05 were considered as statistically different.
ACKNOWLEDGMENT
Grant support: Progetto D'Ateneo 2009, University of Bari “Aldo Moro”, Italy (Code: ORBA09UDWX).