Morphological abnormalities, impaired fetal development and decrease in myostatin expression following somatic cell nuclear transfer in dogs
Abstract
Several mammals, including dogs, have been successfully cloned using somatic cell nuclear transfer (SCNT), but the efficiency of generating normal, live offspring is relatively low. Although the high failure rate has been attributed to incomplete reprogramming of the somatic nuclei during the cloning process, the exact cause is not fully known. To elucidate the cause of death in cloned offspring, 12 deceased offspring cloned by SCNT were necropsied. The clones were either stillborn just prior to delivery or died with dyspnea shortly after birth. On gross examination, defects in the anterior abdominal wall and increased heart and liver sizes were found. Notably, a significant increase in muscle mass and macroglossia lesions were observed in deceased SCNT-cloned dogs. Interestingly, the expression of myostatin, a negative regulator of muscle growth during embryogenesis, was down-regulated at the mRNA level in tongues and skeletal muscles of SCNT-cloned dogs compared with a normal dog. Results of the present study suggest that decreased expression of myostatin in SCNT-cloned dogs may be involved in morphological abnormalities such as increased muscle mass and macroglossia, which may contribute to impaired fetal development and poor survival rates. Mol. Reprod. Dev. 78:337–346, 2011. © 2011 Wiley-Liss, Inc.
Abbreviations:
BWS, Beckwith–Wiedemann syndrome; IGF, insulin like growth factor; IVP, in vitro-produced; LOS, large offspring syndrome (also, abnormal offspring syndrome); MFD, mean lesser fiber diameter; MRF, myogenic transcriptional regulatory factor; MRF4, myogenic factor 6; Myf-5, myogenic factor 5; MyoD, myogenic differentiation 1; SCNT, somatic cell nuclear transfer.
INTRODUCTION
Mammals have been successfully cloned for several years by transferring nuclei from somatic cells into enucleated oocytes. Cloning dogs by somatic cell nuclear transfer (SCNT) has been well described since the successful birth of “Snuppy,” the first SCNT-cloned dog (Lee et al., 2005), but this technology has not been routinely successful with dogs due to the difficulty of maturing canine oocytes in vitro. Despite the success of cloning various mammals, SCNT-cloned animals have abnormalities in morphology, gene expression, and metabolism. These phenotypic instabilities include “large offspring syndrome” (LOS) or “abnormal offspring syndrome” (AOS) (Wilson et al., 1995). These syndromes are well recognized in cattle and sheep SCNT embryos, and in pregnancies from in vitro-produced (IVP) embryos (Wilson et al., 1995; Young et al., 1998; Farin et al., 2006). They include a high rate of early embryonic death and abortion, production of large-size fetuses, musculoskeletal deformities, disproportionate fetal growth, abnormal organ growth, failures in the development of the allantois, and placental vascular abnormalities and development, including hydrallantois (Farin et al., 2006). Thus, LOS represents a wide range of developmental abnormalities that may arise from different mechanisms (Farin et al., 2006). Although problems generated by a variety of SCNT and IVP procedures have been reported to be associated with LOS, the occurrence of these problems is not predictable or reproducible (Young et al., 1998). Moreover, phenotypic instabilities in SCNT-cloned dogs have not been reported. Therefore, in the present study, we examined phenotypic abnormalities, resulting from SCNT, including increased muscle mass and macroglossia, in stillborn or deceased neonatal SCNT-cloned dogs.
RESULTS
There Are Morphological Abnormalities in SCNT-Cloned Dogs
We necropsied 12 SCNT-cloned dogs, and examined their morphologies relative to normal dogs. Dogs that failed to survive from previous studies were used. Dogs No. 1 and 2 were from Kim et al. (2009), dogs No. 3 and 4 from Hossein et al. (2009a), and dogs No. 5–12 were from Hossein et al. (2009b). The SCNT methods used in these studies were slightly different, but the major characteristics of abnormal cloned dogs were similar. These dogs were stillborn just prior to delivery or died with dyspnea shortly after birth. Dog No. 2 had incurred severe postmortem autolysis so it was not possible to render necropsy and examination. It had been diagnosed as stillborn in its mother's uterus 1 day prior to delivery and was excluded from examination. Ten of the 12 dogs exhibited identical morphological lesions. Specifically, they showed macroglossia (Fig. 1B), marked increases in skeletal muscle mass (Fig. 1D), and anterior abdominal wall defects (Fig. 1E). Mild to moderate edema or atelectasis lesions were observed in their lungs (Fig. 1F). Their tongues were very thick and big, protruded out of the mouth, and obstructed most of the throat area. This anatomical abnormality may induce dyspnea, and subsequent death. Increased heart and liver size were also observed. The normal cardiac silhouette in dogs should be 3–3.5 times the width of the intercostals space on the lateral view (Love and Berry, 2002); the size of the hearts in the SCNT-cloned dogs was more than four times the width of the intercostals space (Fig. 1G). The caudal margin of the normal liver is enclosed within the rib cage or is very close to the costal margin (Love and Berry, 2002); the livers of SCNT-cloned dogs were large enough to occupy most of the abdominal cavity region (Fig. 1H). Dog No. 10 exhibited a normal sized skeletal muscle mass and tongue (Fig. 1A,C). However, its heart and liver size were also increased, and other lesions were similar to those of the other dogs, with the exception of failed medulla development in the cerebrum (inset of Fig. 1C).
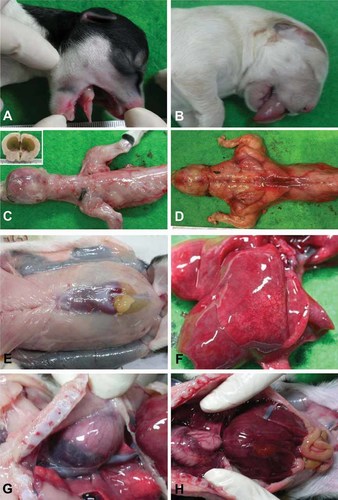
Morphological abnormalities in SCNT-cloned dogs. A–D: The No. 10 SCNT-cloned dog had a normal sized tongue (A) and normal skeletal muscle mass (C). The other ten SCNT-cloned dogs showed marked macroglossia (B) and an increase of the skeletal muscle mass (D) compared to dog No. 10. Dog No. 10 showed failed development of the cerebral medulla (inset of C) without increase of the skeletal muscle mass and macroglossia. E: There was an anterior abdominal wall defect. F: In the lungs, there was mild to moderate edema or atelectasis lesions in SCNT-cloned dogs. G: The heart size of SCNT-cloned dogs was large, and over four times the width of the intercostals space. H: The livers of SCNT-cloned dogs were very large, occupying most of the abdominal cavity region.
Skeletal Muscle and Tongue Hypertrophy in SCNT-Cloned Dogs
The most remarkable morphological changes observed in SCNT-cloned dogs compared with dog No. 10 were large skeletal muscles and tongues. Therefore, we microscopically examined their tongues and skeletal muscles. The tongue sizes of ten SCNT-cloned dogs (No. 1, 3–9, 11, 12) were larger than that of dog No. 10, which had normal-sized tongue and muscles (Fig. 2A,B). The normal-sized tongue of dog No. 10 contained relatively uniform-sized myofibers (Fig. 2C), whereas the tongues of the ten other SCNT-cloned dogs (No. 1, 3–9, 11, and 12) exhibited irregularly sized myofibers, with small and large myofibers mixed in a muscle bundle (Fig. 2D). The mean lesser fiber diameter (MFD) in the tongues of the ten other SCNT-cloned dogs was significantly larger than that of myofibers in dog No. 10 (Fig. 2E, Table 1). The number of myofibers containing internal nuclei did not show significant changes in the tongues (Fig. 2F, Table 1). Skeletal muscles of the other ten SCNT-cloned dogs also displayed significant hypertrophy compared with skeletal muscle of dog No. 10 (Fig. 3A–D, Table 1). Normal skeletal muscle cells of newborn-to-1-week-old dogs contain relatively uniform populations of myofibers, and larger cells are occasionally observed having internal nuclei (Braund and Lincoln, 1981). MFD of most their fibers was reported as 7–9 µm (Braund and Lincoln, 1981), and also MFD of dog No. 10 was 7.44 ± 0.58 µm. However, the skeletal muscle cells of the ten other SCNT-cloned dogs exhibited irregularly sized myofibers, and their MFD was 13.15 ± 1.97 µm. Moreover, many internal nuclei were observed in their myofibers compared to those of dog No. 10 (Fig. 3D). Myofibers containing internal nuclei were usually larger than any other myofibers in the ten other SCNT-cloned dogs. Thus, the MFD and number of internal nuclei in skeletal muscle were significantly greater in the ten other SCNT-cloned dogs compared with dog No. 10 (Fig. 3E,F).
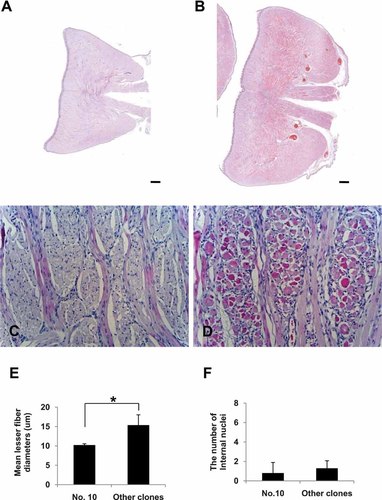
Macroglossia of SCNT-cloned dogs. A,B: The cross-sectioned tongue size of the other ten SCNT-cloned dogs (B) was larger and more bulging than that of the No. 10 SCNT-cloned dog, with a normal sized tongue (A). C: The tongue of the No. 10 SCNT-cloned dog contained uniform-sized myofibers. D: The tongue of the other ten SCNT-cloned dogs contained irregular sized myofibers, in which small and large myofibers were mixed in a muscle bundle. E,F: The MFD and the number of internal nuclei were measured on five randomly selected microscopic fields (400×). H&E (A–D), original magnification; scale bar = 1 mm (A,B), 200× (C,D).
| ID of cloned dog | MFD ± SD | Internal nuclei (mean ± SD) | ||
|---|---|---|---|---|
| Tongue | Skeletal muscle | Tongue | Skeletal muscle | |
| 1 | 16.99 ± 1.28 | 12.28 ± 0.34 | 0.4 ± 0.5 | 8.0 ± 5.2 |
| 2 | NA | NA | NA | NA |
| 3 | 12.46 ± 0.52 | 9.23 ± 0.53 | 0.6 ± 0.5 | 2.8 ± 1.8 |
| 4 | 12.49 ± 0.47 | NA | 0.4 ± 0.5 | NA |
| 5 | 16.72 ± 2.10 | 16.64 ± 3.23 | 1.4 ± 1.5 | 7.2 ± 1.6 |
| 6 | 14.86 ± 2.68 | 12.81 ± 2.12 | 1.8 ± 1.6 | 6.6 ±2.1 |
| 7 | 21.04 ± 3.44 | 14.14 ± 1.29 | 1.2 ± 0.4 | 7.6 ± 3.9 |
| 8 | 13.62 ± 1.95 | 13.22 ± 1.67 | 2.8 ± 1.3 | 2.8 ± 0.4 |
| 9 | NA | 14.24 ± 1.34 | NA | 5.0 ± 2.3 |
| 10 | 10.21 ± 0.34 | 7.44 ± 0.58 | 0.8 ± 1.1 | 2.0 ± 1.2 |
| 11 | 14.57 ± 1.51 | 12.46 ± 1.44 | 1.8 ± 0.4 | 5.6 ± 1.9 |
| 12 | 15.47 ± 2.23 | 13.33 ± 0.77 | 1.2 ± 1.3 | 7.0 ± 5.6 |
- NA, not applicable.
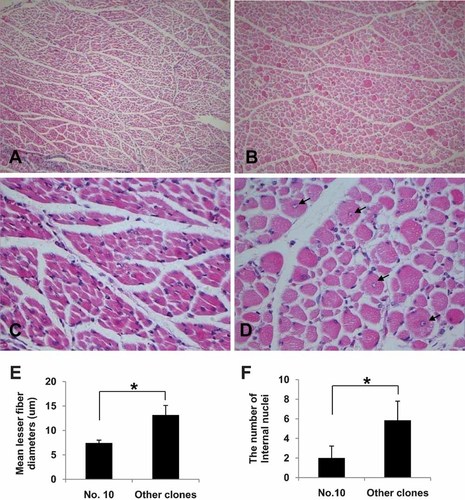
The increased skeletal muscle mass of SCNT-cloned dogs. A,C: The No. 10 SCNT-cloned dog contained uniform-sized myofibers. B,D: The other ten SCNT-cloned dogs contained various sized myofibers that were filled with muscle bundles. Internal nuclei (arrow) in myofibers were observed occasionally in very large myofibers. E,F: The size of myofibers and the number of internal nuclei were measured on five randomly selected microscopic fields (400×). H&E (A–D), original magnification; 100× (A,B), 400× (C,D). [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com]
Expression of Myogenic Regulatory Factors and Myostatin Genes Is Altered in SCNT-Cloned Dogs
We speculated that abnormal myogenesis may possibly occur in SCNT dogs. To test this hypothesis, we examined the mRNA expression level and DNA content of myogenic-related genes using RT-PCR and PCR analyses. Embryogenic myogenesis in vertebrates is controlled by a series of complex myogenic transcriptional regulatory factor (MRF) networks including myogenic factor 5 (Myf-5), myogenic differentiation 1 (MyoD), myogenin and myogenic factor 6 (MRF4), and myogenin (Bryson-Richardson and Currie, 2008). In addition, myostatin has been reported to be a negative regulator of muscle mass in skeletal muscle cells (McPherron et al., 1997). Therefore, we examined mRNA expression and DNA content of MRFs and myostatin in the tongues and skeletal muscles of four representative SCNT-cloned dogs (Fig. 4). The No. 10 SCNT-cloned dog was used as a normal control, and the biopsied triceps brachial muscle of a normal adult (4 years old) mixed breed dog was used as a standard control. There were no significant differences in the DNA content level of MRFs and myostatin among the animals. At the mRNA level, all factors were highly expressed in skeletal muscle of the adult dog. Dog No. 10 also showed a high level of mRNA expression of MRFs and myostatin, but not myogenin. However, expression of the MRFs and myostatin was down-regulated or absent in the tongue and skeletal muscle of the other SCNT-cloned dogs compared with dog No. 10 and the adult dog.
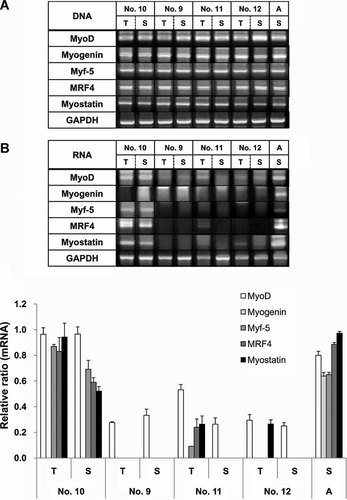
The expression of myogenic regulatory factors and myostatin decrease in SCNT-cloned dogs. A: Amplification of specific factors from genomic DNA. B: The transcription of factors, as measured by reverse-transcription PCR from mRNA. C: Relative ratio of specific mRNA/GAPDH based on intensity of amplified bands. (T) tongue, (S) skeletal muscle, (A) the skeletal muscle of a normal adult dog.
DISCUSSION
Since the first report of a clone produced by SCNT (Wilmut et al., 1997), several other species have been cloned. However, SCNT currently has many limitations, including a low success rate. The limited success of SCNT is often related to incorrect nuclear reprogramming of donor cells. The underlying potential mechanisms that may affect reprogramming efficiency include epigenetic modifications such as DNA methylation and histone remodeling (Young and Fairburn, 2000; Dean et al., 2001; Farin et al., 2004). Different cells and tissue types acquire different programs of gene expression and epigenetic modification during development (Bird, 2002; Li, 2002). It has been thought that the epigenetic dysregulation caused by SCNT or in vitro production may lead to inappropriate expression of key developmental genes that may contribute to lethality of cloned embryos (Farin et al., 2006).
Large Offspring Syndrome (LOS), characterized by excess fetal size, enlarged organs, fetal hydrops, respiratory distress, etc., has been observed during late gestation and has been shown to cause perinatal deaths in cloned cattle, sheep, and mice (Wilson et al., 1995; Wakayama et al., 1998; Young et al., 1998; Eggan et al., 2001; Chavatte-Palmer et al., 2002; Constant et al., 2006; Farin et al., 2006; Everts et al., 2008). Offspring with LOS are frequently born following SCNT or from embryos exposed to in vitro culture environments (Wilson et al., 1995; Farin et al., 2004; Young et al., 1998; Eggan et al., 2001). An enlarged and edematous placenta is an important abnormal morphology that is present in all cases of LOS (Constant et al., 2006; Everts et al., 2008). Altered expression of mRNA or protein expression of genes such as vascular endothelial growth factor (VEGF), peroxisome proliferators-activated receptor γ (PPARγ), leptin, bovine placental lactogen, transforming growth factor (TGF)-β, and TGF-β receptors have been found in the placentas from IVP or SCNT pregnancies (Farin et al., 2006). However, there are few reports regarding altered candidate genes in cloned animals caused by reprogramming errors.
LOS is clinically correlated in humans to Beckwith–Wiedemann syndrome (BWS), which is associated with imprinting defects (DeBaun et al., 2003). BWS is a rare congenital overgrowth syndrome with considerable phenotypic heterogeneity. The critical, causative region for BWS is located on chromosome 11p15, and the major features are anterior abdominal defects, macroglossia, and pre-or postnatal overgrowth (Elliott et al., 1994; Whisson et al., 1994). However, patients with BWS are not phenotypically identical to SCNT-cloned dogs. BWS patients display other complex features such as a cleft palate, ear abnormalities, and embryonal tumors, which was not evident in SCNT dogs (Elliott et al., 1994; Whisson et al., 1994).
Myostatin-deficient animals exhibit similar disorders to LOS animals (Lee, 2004; Mosher et al., 2007). Animals with mutations in myostatin are characterized by increased muscle mass resulting from a combination of an increased number of muscle fibers and increased fiber size (Lee, 2004). The myostatin sequence is highly conserved across species, and mutations in the myostatin gene have been observed in mice, cattle, sheep, dogs, and humans. These mutations frequently result in a large and widespread increase in skeletal muscle mass (Lee, 2004; Schuelke et al., 2004; Mosher et al., 2007). Interestingly, the increased muscle phenotype in myostatin-mutated animals appears to be similar to the phenotype we observed in our SCNT-cloned dogs except for macroglossia.
The MRFs including MyoD, Myf-5, myogenin and MRF4 coordinate differentiation of skeletal muscle fibers during development (Gerrard and Grant, 1994). MyoD and Myf-5 are expressed in proliferating myoblasts before the onset of muscle differentiation (Emerson, 1990; Cornelison et al., 2000). Myogenin and MRF4 regulate terminal differentiation of myoblasts after the establishment of myotubes (Cornelison et al., 2000; Bryson-Richardson and Currie, 2008). Although myostatin is not a MRF, it regulates the final number of muscle fibers that are formed during embryogenesis (Lee, 2004). Manceau et al. (2008) revealed that myostatin acts in vivo to regulate the balance between proliferation and differentiation of embryonic muscle progenitors by promoting terminal differentiation. Moreover, Crosier et al. (2002) reported that there were no differences in expression of MRFs, although the expression of myostatin mRNA was down-regulated in skeletal muscles of IVP-derived fetuses compared with fetuses from embryos produced in vivo. In our case, we assumed that expression of MRFs might be increased in cloned dogs because we observed an increase in muscle fibers with internal nuclei, which is a characteristic of muscle regeneration. However, we observed undetectable or low mRNA expression levels of MRFs.
Embryogenesis and development are regulated by various genes. Hyperplasia and hypertrophy of the muscle are regulated by various growth factors, including hepatocyte growth factor, fibroblast growth factor 2, TGF-β, and insulin like growth factor (IGF) (Allen and Goll, 2003). IGF is a growth hormone that has been shown to play an important role in the development of mammals (Cingel-Ristic et al., 2004; Walenkamp and Wit, 2006). IGF-I has been reported to play a major role in the development, growth, differentiation, and maintenance of skeletal muscles (Florini et al., 1996). IGF-II is essential for normal embryonic and early fetal growth, and it has been suggested to play a role in the pathogenesis of BWS and in myostatin-mutated cattle (Gerrard and Grant, 1994; Best et al., 2006). Interestingly, myostatin is a member of the TGF-β family, and IGFs may regulate the expression and secretion of TGF-β during cell proliferation and initiation of differentiation (Florini et al., 1996). Therefore, we hypothesized that a disruption in myostatin gene expression may contribute to abnormalities such as increased muscle mass and macroglossia in SCNT-cloned dogs. However, this mechanism is not clear in our SCNT-cloned dogs. Therefore, a further investigation is required to determine the relationship between reduced myostatin gene expression and epigenetic modification during cloning. In addition, it is possible that other genes, which were not investigated in this study, may be responsible for the morphological abnormalities observed in SCNT-cloned dogs.
In summary, we observed the macroglossia, increased muscle mass, increased heart and liver sizes, and anterior abdominal wall defects in ten SCNT-cloned dogs. The reduced expression of myostatin, which is a negative regulator of skeletal muscle mass, was particularly observed in their large muscles and tongues. The underlying mechanisms of this decrease in myostatin expression and epigenetic dysregulation in SCNT-cloned dogs are not clear. However, the present study demonstrates that myostatin is a candidate gene that contributes to phenotypic developmental abnormalities in SCNT-cloned dogs.
MATERIALS AND METHODS
Animals
Twelve dead dogs were submitted to find pathologic changes and cause of death. All dogs were cloned by the transfer of a nucleus from a somatic cell of donors into an enucleated oocyte of different surrogates. These cloned dogs died during experiments presented in previous work (Hossein et al., 2009a,b; Kim et al., 2009). They were either stillborn just prior to delivery or died early from dyspnea after birth. The history of each dog was presented in Tables 2 and 3. Tissue samples were fixed in 10% neutral buffered formalin or frozen in liquid nitrogen, and then stored at −70°C until required for analysis.
| Dog breed | Donor cell type | No. of cloned dog produced | No. of abnormal dog produced (ID of cloned dog) | Refs.a |
|---|---|---|---|---|
| Golden retriever | Skin fibroblast | 5 | 2 (1, 2) |
Kim et al. (2009) |
| Beagle | Skin fibroblast | 6 | 2 (3, 4) |
Hossein et al. (2009a) |
| Mixed | Skin fibroblast | 16 | 8 (5–12) |
Hossein et al. (2009b) |
- a Submitted cloned dogs were nonsurvival ones during previous works.
| ID of cloned dog | Gestation period (day) | Delivery method | Birth weight (g) | Post-natal day | No. of littermate/status | Large muscle and macroglossia |
|---|---|---|---|---|---|---|
| 1 | 57 | NB | 620 | 1 | 1/live | Yes |
| 2 | 53 | C-sec | NA | SB | 0 | ? |
| 3 | 60 | C-sec | 370 | 16 | 0 | Yes |
| 4 | 58 | NB | NA | SB | 2/live | Yes |
| 5 | 60 | C-sec | 500 | SB | 0 | Yes |
| 6 | 58 | C-sec | 570 | 1 | 0 | Yes |
| 7 | 60 | NB | NA | 1 | 0 | Yes |
| 8 | 58 | C-sec | NA | SB | 0 | Yes |
| 9 | 58 | C-sec | NA | 1 | 0 | Yes |
| 10 | 60 | C-sec | 200 | 1 | 0 | No |
| 11 | 59 | NB | 400 | 2 | 0 | Yes |
| 12 | 59 | C-sec | 550 | 1 | 0 | Yes |
- NB, natural birth; SB, stillbirth; C-sec, cesarean section; NA, not applicable.
Histology
For histological finding, the central tongue belly was biopsied, and skeletal muscles were biopsied from sternocephalicus, longus capitis, or biceps femoris muscles. Formalin-fixed skeletal muscles and tongues of cloned dogs were processed routinely and embedded in paraffin wax. Sections were cut to 4-µm thickness. The sections were stained with hematoxylin and eosin (H&E). For quantitative studies, five microscopic fields (400×) of muscle transverse sections were randomly selected, and MFD were measured using computer-assisted image analysis (Image J, National Institute of Health) from a random count of between 300 and 400 fibers of tongues, 400 and 600 fibers of skeletal muscles. The number of internal nuclei was counted on five randomly selected microscopic fields (400×). The surface of transverse sectioned tongue was scanned (ScanScope, Aperio, Vista, CA) and computerized with a digital image to show the difference of their size (ImageScope, Aperio).
PCR and RT-PCR Analysis
DNA and total RNA were extracted from the tongues and skeletal muscles of SCNT-cloned dogs by using the proteinase K/SDS method and Trizol methods, respectively. The biopsied triceps brachial muscle of a normal adult (4 years old) mixed breed dog was used as a standard control. DNA amplification, cDNA synthesis, and mRNA isolation were performed by using AccuPower™ PCR premix (Bioneer, Daejeon, Korea) and AccuPower® RT/PCR premix (Bioneer) respectively, according to the manufacturer's instructions. The primer sets used in the PCR are listed in Table 4. These products were detected by agarose gel electrophoresis and visualized with ethidium bromide on a transilluminator.
| Primer | Sequence (5′ → 3′) | Fragment size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| MyoD | S: GTCAACGAGGCTTTCGAGAC | 218 | 49 |
| A: GAGTCGCCGCTGTAGTGTTC | |||
| Myogenin | S: GGGCGTGCAAGGTGTGTA | 223 | 50 |
| A: GCCGCTGGTTGGGGTTGA | |||
| MRF4 | S: GTGTTTCGGATCATTCCAGG | 252 | 49 |
| A: GAGGAAATGCTGTCCACGAT | |||
| Myf5 | S: CCATTTTGCATGGTTAACCCCACG | 218 | 60 |
| A: GCAAGTGTGTTTGCAGGGCTTGGC | |||
| Myostatin | S: AAACCCATGAAAGACGGTACA | 219 | 48 |
| A: ATTCAGCCCATCTTCTCCTG | |||
| GAPDH | S: ACCACAGTCCATGCCATCAC | 498 | 60 |
| A: CCACCACCCTGTTGCTGTA |
Statistical Analysis
All values are presented as mean ± standard deviation. Statistical analyses were performed using the Student's t-test. Statistical significance was assumed when *P < 0.05.
Acknowledgements
This research was supported by a grant from H Bion, Republic of Korea.