Ethanol Extract of Citrus grandis ‘Tomentosa’ Exerts Anticancer Effects by Targeting Skp2/p27 Pathway in Non-Small Cell Lung Cancer
Abstract
Scope
This study aims to investigate the anticancer properties of Citrus grandis ‘Tomentosa’ (CGT) in non-small cell lung cancer (NSCLC).
Methods and results
The ethanol extract of CGT (CGTE) is prepared by using anhydrous ethanol and analyzed by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), revealing that the main chemical components in CGTE are flavonoids and coumarins, such as naringin, rhoifolin, apigenin, bergaptol, and osthole. CGTE at concentrations without inducing cell death significantly inhibits cell proliferation via inducing cell cycle G1 phase arrest by MTT, colony formation, and flow cytometry assays, implying that CGT has anticancer potential. CGTE markedly inhibits the activity of Skp2-SCF E3 ubiquitin ligase, decreases the protein level of Skp2, and promotes the accumulation of p27 by co-immunoprecipitation (co-IP) and in vivo ubiquitination assay; whereas Skp2 overexpression rescues the effects of CGTE in NSCLC cells. In subcutaneous LLC allograft and A549 xenograft mouse models, CGTE, without causing obvious side effects in mice, significantly inhibits lung tumor growth by targeting the Skp2/p27 signaling pathway.
Conclusion
These findings demonstrate that CGTE efficiently inhibits NSCLC proliferation both in vitro and in vivo by targeting the Skp2/p27 signaling pathway, suggesting that CGTE may serve as a therapeutic candidate for NSCLC treatment.
1 Introduction
Lung cancer is the second most frequently diagnosed cancer and the leading cause of cancer deaths worldwide.[1, 2] Approximately, 85% of patients with lung cancer are diagnosed as having a histological subtype of non-small cell lung cancer (NSCLC).[3] Targeted therapy has shown great success in the treatment of NSCLC. However, intolerable adverse effects and drug resistance remain a major barrier to NSCLC targeted therapy.[4-6] The SCF E3 ubiquitin ligase, which consists of the scaffold protein Cul1, RING-finger protein Rbx1, the adaptor protein Skp1, and substrate recognition protein F-box family, has emerged as a promising therapeutic target for NSCLC.[7-10] Skp2 as an oncogenic F-box protein contributes to neoplastic transformation via the specific recognition and ubiquitination of p27, a CDK4 inhibitor, in the cellular transition from G1 to S phase.[11-14] Therefore, therapeutic drugs targeting Skp2 can efficiently induce p27 accumulation and inhibit tumor growth, resulting in promising therapeutic potential.
Several compounds, such as compound #25 (SZL P1-41), compound A, betulinic acid, and silybin, have been demonstrated to target Skp2 and prevent Skp2–Skp1 interaction, leading to the inhibition of Skp2-SCF E3 ubiquitin ligase activity and stabilization of p27.[12, 15-18] Previous reports have also demonstrated that the overexpression of Skp2 in human NSCLC tumor tissues is positively associated with poor clinical prognoses.[19-21] Therefore, targeting the Skp2-SCF complex to restore p27 levels is a promising strategy in NSCLC treatment.
Traditional Chinese medicine (TCM) has accumulated considerable evidence to support the medicinal properties of numerous foods beyond their nutritional function, which includes anti-inflammatory, antioxidant, immunomodulatory, and anticancer properties.[22] The Citrus grandis fruit is a rich source of food and medicine. Crude extracts or phytochemicals obtained from the whole fruit or peel of C. grandis exhibit antitumor, antioxidant, anti-inflammatory, hypoglycemic, and hypolipidemic properties. The main bioactive components of Citrus fruits include flavonoids, coumarins, polysaccharides, volatile oils, terpenoids, polyphenols, limonoids, acridone alkaloids, and vitamins. Notably, naringin, naringenin, and limonin are crucial bioactive components of C. grandis and have been widely investigated as adjuvant remedy in the prevention and treatment of various human malignancies, such as prostate and breast cancer, lymphoma, and colorectal carcinoma.[23-26]
Citrus grandis ‘Tomentosa’ (CGT), also referred to as C. grandis Exocarpium in the Chinese Pharmacopoeia, is the immature fruit of C. grandis (L.) Osbeck originated from Huazhou city, Guangdong province. CGT is a well-known herb in TCM, commonly used to treat cough, phlegm, and pulmonary inflammation.[27, 28] With the identification of its abundant bioactive components, such as flavonoids, coumarins, polysaccharides, and essential oils, CGT has drawn significant pharmacological interest.[29, 30] However, its potential as an anticancer agent and the underlying mechanisms remain unclear. Illustrating the anticancer potential of CGT may provide valuable evidence to further explore its novel clinical applications.
In this study, the aqueous and the ethanol extract of CGT (CGTA and CGTE) were prepared and evaluated for their anticancer activity. These results indicate that CGTE demonstrates a more promising anticancer activity than CGTA. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis helped identify the major phytochemical compounds in CGTE, such as naringin, naringenin, rhoifolin, apigenin, neoeriocitrin, meranzin, bergaptol, osthole, aurapten, obacunone, and limonin. Subsequently, we demonstrated that CGTE inhibited the Skp2/p27 signaling pathway and induced G0/G1 phase arrest, thereby suppressing NSCLC cell proliferation both in vitro and in vivo.
2 Results
2.1 CGTE Inhibits the Proliferation of NSCLC by Inducing G0/G1 Phase Arrest
To explore the anticancer potential of CGT, we prepared both CGTA and CGTE and tested their cytotoxicity against A549, H1299, and LLC cells. CGTE inhibited NSCLC cell proliferation in a dose-dependent manner, obtaining much lower IC50 values than CGTA (Figure 1A). Therefore, we used the ethanol extract to test the anti-NSCLC activity of CGT. As expected, CGTE treatment obviously suppressed cell colony formation in NSCLC cells (Figure 1B). Furthermore, CGTE significantly induced G0/G1 phase arrest in NSCLC cells (Figure 1C), without inducing apoptosis (Figure S1, Supporting Information). These results revealed that CGTE exerted anticancer effects by inducing G0/G1 phase arrest.
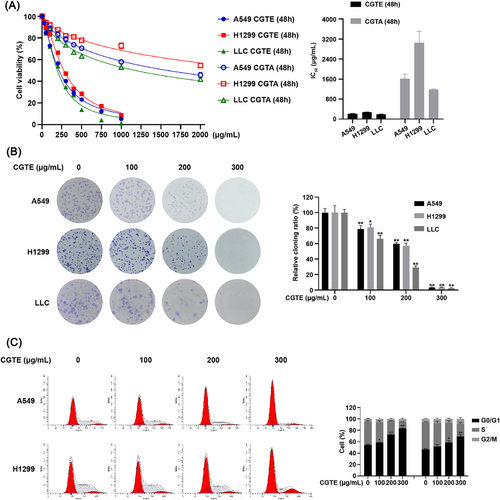
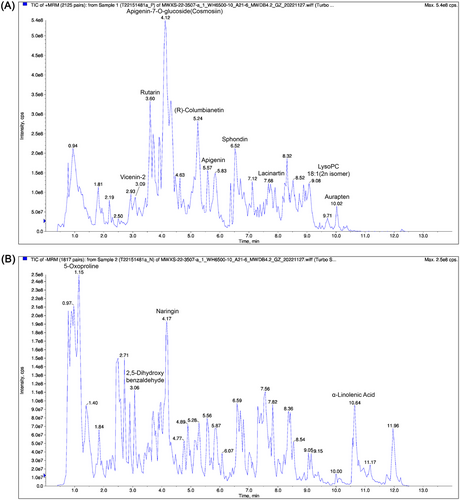
Then, we analyzed the main phytochemical constituents of CGTE by using UPLC-MS/MS. As a result, 390 compounds, mainly including flavonoids and coumarins, were identified using the self-built Metware database (Figure 2 and Table S1, Supporting Information). In line with a previous report,[30] the compounds such as naringin, naringenin, rhoifolin, apigenin, neoeriocitrin, meranzin, bergaptol, osthole, aurapten, obacunone, and limonin were found to be relatively abundant in CGTE, indicating that these compounds can be considered as chemical markers of CGTE.
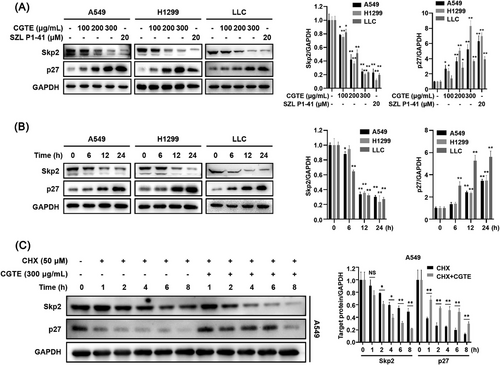
2.2 CGTE Shows Inhibitory Effect on Skp2/p27 Signaling Axis in NSCLC
Ample evidence has shown that the Skp2-SCF complex promotes cell cycle progression from G1 phase to S phase via ubiquitin-dependent degradation of its substrate p27.[31, 32] We tested whether CGTE inhibits the Skp2/p27 axis. The results revealed that CGTE treatment significantly decreased Skp2 while increased p27 protein levels in NSCLC cells in a dose- and time-dependent manner (Figure 3A,B). Notably, the inhibitory effect on Skp2/p27 signaling observed at high CGTE concentration was comparable with that of the Skp2 inhibitor SZL P1-41. Because Skp2 inhibitor can decrease the protein stability of Skp2, we examined the effect of CGTE on Skp2 and p27 protein stability using the cycloheximide (CHX) assay. Compared with the protein synthesis inhibitor CHX treatment alone, combination treatment with CHX and CGTE significantly decreased Skp2 protein levels within 2 h. As expected, the combination treatment maintained p27 protein levels for 8 h, compared with 1 h in the CHX treatment group, indicating that CGTE induced p27 protein accumulation by decreasing Skp2 protein stability (Figure 3C).
2.3 CGTE Inhibits Skp2-SCF E3 Ubiquitin Ligase Activity
The interaction between Skp2 and Skp1 is crucial for maintaining the integrity of Skp2-SCF complex and the stability of Skp2 protein. As expected, our results revealed that CGTE at 300 µg mL−1 dramatically inhibited Skp2–Skp1 interaction in A549 cells, compared with SZL P1-41 (Figure 4A). CGTE induced the proteasome-dependent degradation of Skp2, evidenced by the fact that the proteasome inhibitor MG-132 dramatically reversed the reducing effect of CGTE on Skp2 protein levels (Figure 4B). These results suggest that the chemicals in CGTE may directly target the Skp2-SCF complex. Notably, CGTE significantly increased the thermal stability of Skp2 protein under heat challenge by the cellular thermal shift assay (CETSA), demonstrating that the chemicals in CGTE are capable of directly binding to Skp2 (Figure 4C), thereby disrupting the Skp2–Skp1 interaction. Accordingly, CGTE inhibited the activity of Skp2-SCF E3 ubiquitin ligase, as evidenced by the decreased ubiquitination level of p27 upon CGTE treatment (Figure 4D,E).

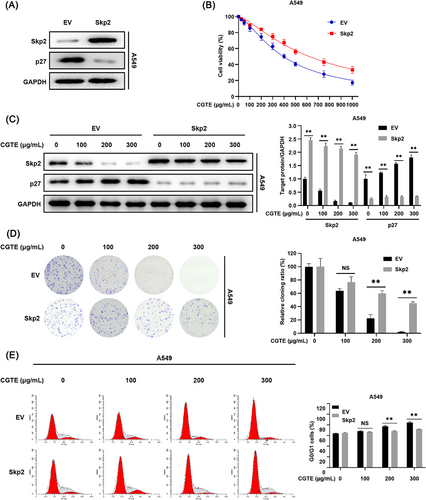
2.4 Skp2 Overexpression Reverses the Anticancer Properties of CGTE
To further verify whether CGTE exerts anticancer effects by targeting Skp2/p27 signaling axis, Skp2 overexpressing A549 cells were established using lentivirus infection. It was found that Skp2 overexpression decreased p27 protein levels in A549 cells (Figure 5A). Consistently, Skp2 overexpression markedly attenuated the effects of CGTE on Skp2 downregulation and p27 accumulation (Figure 5C). Meanwhile, Skp2 overexpression partially reversed the inhibitory effects of CGTE on cell viability, colony formation, and cell cycle progression in A549 cells (Figure 5B,D,E). Therefore, these findings strongly confirm that CGTE primarily targets Skp2/p27 signaling axis to exert anticancer activity in NSCLC.
2.5 CGTE Suppresses Tumor Growth In Vivo
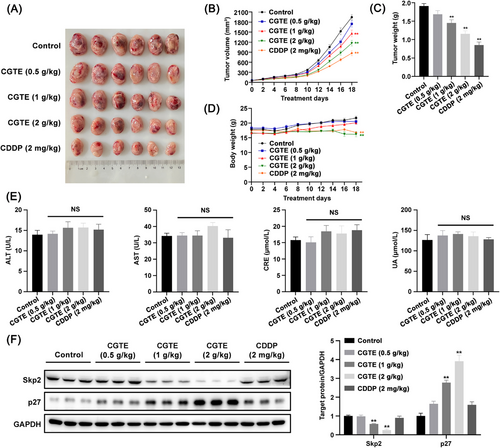
To examine the in vivo therapeutic efficacy of CGTE against NSCLC, we established subcutaneous LLC allografts in C57BL/6 mice and A549 xenografts in BALB/c nude mice. Our results showed that CGTE inhibited tumor growth at medium and high doses in subcutaneous LLC allografts without causing obvious side effects in C57BL/6 mice (Figure 6A–D). CGTE at a high dose causes moderate body weight loss but did not increase alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CRE), and uric acid (UA) serum levels (Figure 6D,E). Moreover, the mice remained active, ate normally, exhibited healthy skin and hair with no irregular secretions or excretions, and none of the mice perished during the experiment. Consistent with the in vitro results, CGTE administration significantly decreased Skp2 while increasing p27 expression in LLC allograft tumor tissues (Figure 6F).
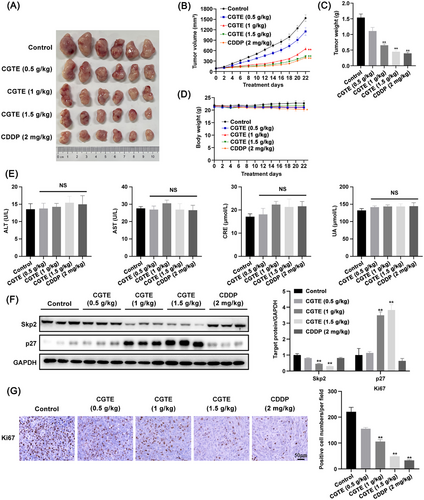
Consistently, CGTE administration also markedly inhibited tumor growth in subcutaneous A549 xenografts, and CGTE at a high dose resulted in an antitumor effect comparable to that of the chemotherapeutic drug cisplatin (CDDP) (Figure 7A–C). Of note, CGTE did not cause any physiological abnormalities, body weight loss, or hepatorenal toxicity, as evidenced by the normal serum levels of ALT, AST, CRE, and UA in nude mice (Figure 7D,E). There were no observed mortalities among nude mice during the experiments. Moreover, H&E staining also showed that CGTE did not cause any obvious side effects toward these organs (Figure S2, Supporting Information). Consistently, immunohistochemistry (IHC) analysis showed that CGTE administration significantly induced Skp2 downregulation while promoting p27 accumulation in tumor tissues (Figure 7F), leading to decreased expression of the proliferation biomarker Ki67 (Figure 7G). Therefore, the results revealed that oral administration of CGTE efficiently inhibited tumor growth in vivo by targeting Skp2/p27 axis.
3 Discussion
A variety of foods have been used in TCM to treat human diseases. CGT, a well-known TCM and dietary supplement, has been commonly used to treat respiratory diseases owning to its expectorant, antitussive, and anti-inflammatory activities.[27] In this study, we explored that CGT has therapeutic potential in the treatment of NSCLC. CGTE treatment led to a significant downregulation of Skp2 and an accumulation of p27, resulting in cell cycle arrest at the G0/G1 phase and tumor growth inhibition.
CGT is typically harvested at its immature stage and then processed in a drying oven; the whole dried fruits with a diameter of less than 6 cm are characterized by the densely covered tomentum. Despite its bitter taste, the decoction of the whole dried fruit pieces are commonly used in China as a dietary supplement or medication; because CGT at the immature stage is rich in its main active components, such as naringin, rhoifolin, and naringienin, which decreases during the ripening process.[33] Additionally, the whole fruit of CGT contains a higher level of flavonoids than that in epicar.[30] According to the results determined by UPLC–MS/MS (Figure 2 and Table S1, Supporting Information), flavonoids and coumarins were the major chemical components of CGTE. These chemicals in Citrus fruits have been demonstrated to exhibit therapeutic potential in the prevention of cancer and chronic diseases.[24-26, 34] In this study, our results revealed that CGTE plays promising anti-lung cancer activity, indicating that the bioactive components in CGT can be further explored for cancer treatment in humans. The C. grandis that produced in different regions or countries may have different chemical constituents, leading to differences in therapeutic efficacy. However, C. grandis produced in different regions may share common chemical features. Therefore, whether the C. grandis products from other regions also have anticancer properties must be verified.
Both aqueous and ethanol extracts are commonly used to explore the therapeutic applications of medicinal herbs. It has been demonstrated that the ethanolic extract of CGT may has more favorable pharmacological activities including antitussive, expectorant, and anti-inflammatory effects, than that of the aqueous extract.[27] In our study, we also found that the ethanol extract exerted much stronger cytotoxicity toward NSCLC cells than the aqueous extract, indicating that the ethanol extract may contain bioactive anticancer compounds that warrant further exploration. The main components in the aqueous extract of CGT are flavonoids, polysaccharides, and polyphenols, including naringin, naringenin, narirutin, vicenin-2, rhoifolin, apigenin, melitidin, neodiosmin, eriocitrin, luteolin, neoeriocitrin, veronicastroside, hesperidin, poncirin, and kaempferol. Among these, naringin, naringenin, and narirutin were the most abundant compounds.[35] However, CGTE mainly contains flavonoids, coumarins, terpenoids, and limonoids, such as naringin, rhoifolin, neoeriocitrin, meranzin hydrate, and isoimperatorin. Furthermore, CGTE also contains naringenin, narirutin, apigenin, bergaptol, bergapten, osthole, aurapten, imperatorin, isoimperatorin, obacunone, limonin, nomilin, protocatechuic acid, and caffeic acid, which is consistent with our results.[27, 30] These compounds, especially coumarins, terpenoids, and limonoids in CGTE, may account for their antitumor effects against NSCLC.
Sustaining proliferative signaling is an important hallmark in cancer development.[36] SCF complex is a major class of E3 ubiquitin ligases and plays a critical role in cell cycle regulation through the ubiquitin-dependent degradation of cyclins and CDK inhibitors. Targeting SCF E3 ubiquitin ligases have been identified as a promising strategy for developing anticancer drugs.[37, 38] Skp2, an F-box protein in the SCF complex, specifically recognizes p27,[39] and Skp2 expression is inversely correlated with p27 expression in many types of cancers.[37] Accumulative evidence has demonstrated that Skp2-targeted drugs can directly induce p27 accumulation and G1 phase cell cycle arrest. We also found that CGTE significantly decreased Skp2 protein level and increased p27 expression both in vitro and in vivo. The CETSA experiment suggested that the compounds in CGTE may directly target Skp2, thus leading to G1 phase arrest. Therefore, our findings suggest that CGTE may contain potential Skp2 inhibitors that could be further investigated for the development of anticancer drugs.
Because CGTE is commonly used as food and medicine, it did not cause obvious body weight loss in mice and exhibited no clear hepatotoxicity or nephrotoxicity. H&E staining also demonstrated that CGTE was not toxic to any major organs of mice. In summary, our results provide evidence that CGTE serve as a safe drug candidate for NSCLC treatment.
4 Experimental Section
Reagents and Antibodies
Methanol and acetonitrile suitable for UPLC were purchased from Merck (Darmstadt, Germany). Formate suitable for UPLC was purchased from Aladdin (Shanghai, China). SZL P1-41, Cycloheximide (CHX), MG-132, and cisplatin (CDDP) were purchased from MedChemExpress (NJ, USA). Antibodies against Skp2, p27, Skp1, and HA tag were purchased from Proteintech (Wuhan, China). Antibodies against GAPDH were purchased from Bioworlde (Nanjing, China). Antibodies against Myc-Tag were purchased from Abbkine (Wuhan, China). Antibodies against Ki67 were purchased from BD Biosciences (NJ, USA). Normal Rabbit IgG was purchased from Beyotime (Shanghai, China). All secondary antibodies (HRP-conjugated anti-rabbit and anti-mouse IgG) were procured from Affinity Biosciences (Liyang, China). Kits used for mice hepatorenal toxicity assay were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Analysis of Phytochemicals in CGTE
The whole immature fruit of CGT, a genuine regional herbal, was obtained from Dahe Planting Base in Huazhou town, Guangdong, China. The materials were identified by professor Ruoting Zhan. For the preparation of aqueous and ethanol extracts of CGT, the dried powders of CGT were extracted with 10-fold pure water by using reflux or anhydrous ethanol by using ultrasound (three times for 1 h each), respectively. The two extracting solutions were then concentrated under reduced pressure at 40 °C using a rotary evaporator (EYELA, Tokyo, Japan) and then lyophilized under vacuum at −80 °C for 36 h using a freezer dryer (Martin Christ, Osterode, Germany), yielding about 30% w/w aqueous extracts and 10% w/w ethanol extracts, respectively.[27] The CGTA and CGTE were stored at 4 °C until use. For the cell experiments, the CGTA was dissolved in pure water to make a stock solution of 200 mg mL−1 and the CGTE was dissolved in DMSO to make a stock solution of 300 mg mL−1. For the animal experiments, the CGTE was dissolved in a solution of 30% ethanol in corn oil to the doses of 50, 100, 150, and 200 mg mL−1.
To perform phytochemical analysis of CGTE, the drug was dissolved in methanol with a concentration of 50 mg mL−1, then the CGTE solution was then subjected to filtration using a 0.22 µm microporous membrane prior to UPLC-MS/MS analysis. The phytochemical composition of CGTE was qualitatively characterized using an UPLC-ESI-MS/MS system (UPLC, ExionLC AD and MS, Applied Biosystems 6500 Q TRAP. SCIEX, MA, USA).
The analytical conditions were as follows: the UPLC analysis was carried out on an Agilent SB-C18 column (1.8 µm, 2.1 mm × 100 mm). The mobile phase consisted of solvent A (pure water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid) v/v. Sample measurements were performed with a gradient program that employed the starting conditions of 95% A, 5% B. Within 9 min; a linear gradient to 5% A, 95% B was programmed, and a composition of 5% A, 95% B was kept for 1 min. Subsequently, a composition of 95% A, 5.0% B was adjusted within 1.1 min and kept for 2.9 min. The flow velocity was set as 0.35 mL per minute. The column oven temperate was set at 40 °C. The injection volume was 2 µL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.
The electrospray ionization (ESI) source operation parameters were as follows: source temperature 500 °C, ion spray voltage (IS) 5500 V (positive ion mode)/−4500 V (negative ion mode), ion source gas I (GSI), gas II (GSII), curtain gas (CUR) were set at 50, 60, and 25 psi, respectively. The collision-activated dissociation (CAD) was high. QQQ scans were acquired as MRM experiments with collision gas (nitrogen) set to medium. DP (declustering potential) and CE (collision energy) for individual MRM transitions was done with further DP and CE optimization. A specific set of MRM transitions were monitored for each period according to the metabolites eluted within this period.
Cell Culture and Treatment
The human NSCLC cell lines A549 and H1299, the mouse Lewis lung carcinoma (LLC) cell line, and human kidney cell line 293T within four passages were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The A549, LLC, and 293T cells were cultured in DMEM and H1299 cells were cultured in RPMI-1640 medium (Procell, Wuhan, China), supplemented with 10% FBS (Procell, Wuhan, China), penicillin (100 IU mL−1), and streptomycin (100 µg mL−1) (Gibco, CA, USA) in a 5% CO2 humidified incubator at 37 °C.
Cell Viability Assay
The A549, H1299, and LLC cells (5 × 103 cells per well) were plated in 96-well plates. After overnight incubation, the cells were treated with varying concentrations of CGTA (0, 50, 100, 200, 300, 400, 500, 1000, 2000 µg mL−1) or CGTE (0, 25, 50, 100, 200, 300, 400, 500, 750, 1000 µg mL−1) for 48 h. After treatment, the cells were incubated with MTT working solution (0.5 mg mL−1) and incubated for an additional 4 h, then the absorbance was measured at the wavelength of 490 nm using a microplate reader (BioTek, CA, USA) after MTT formazan crystals were dissolved in DMSO (100 µL per well). The relative cell viability was calculated by the mean percentage of absorbance in the treatment group versus the control group.
Colony Formation Assay
The A549, H1299, and LLC cells (1 × 103 cells per well) were plated in 6-well plates and cultured overnight. Then the cells were treated with CGTE until visible colonies formed (about 7 days). Then the cell colonies were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet and counted.
Cell Cycle Assay
The A549 and H1299 cells (3 × 105 cells per well) were plated in 6-well plates. After overnight incubation, the cells were treated with CGTE at indicated concentrations for 24 h. Then the cells were collected and fixed with 70% cold ethanol overnight at 4 °C. Subsequently, the cells were incubated with propidium iodide (PI) and RNase A at 37 °C for 30 min using the Cell Cycle Detection Kit (Meilunbio, Dalian, China). The cell cycle distribution was determined by flow cytometry (BD, CA, USA) and analyzed by ModFit LT version 5.0 (Verity Software House, CA, USA).
Western Blotting
In brief, the cells were seeded into 6-well plates (3 × 105 cells per well) and cultured overnight. After the treatment with CGTE at indicated concentrations for 24 h, the cells were collected and washed with cold PBS (Procell, Wuhan, China), lysed in RIPA lysis buffer (NCM Biotech, Suzhou, China) containing 1% protease inhibitor cocktail (Solarbio, Beijing, China), and centrifuged to harvest total cellular proteins. For harvesting total protein from the tumor tissues, the dissected tumor tissue specimens were rapidly ground in RIPA lysis buffer by using an automatic sample grinding instrument (Jingxin, Shanghai, China), sonicated, and centrifuged to harvest total tissue proteins. The BCA Protein Assay Kit (Biosharp Life Sciences, Hefei, China) was used to quantify the protein concentration in cells and tissue specimens. Equal amounts of samples were subjected to SDS-PAGE (12%) and transferred to PVDF membranes (Pall Life Sciences, NY, USA). These membranes were then blocked by 5% nonfat milk, incubated with indicated primary antibodies overnight at 4 °C and further incubated with the corresponding secondary antibody for 2 h at room temperature. The signal of protein bands with enhanced chemiluminescence HRP substrate reagent (Vazyme, Nanjing, China) was detected by Tanon 5200 image acquisition system (Tanon, Shanghai, China). The densitometry analysis of the immunoblots was analyzed using ImageJ software (NIH, USA).
Cellular Thermal Shift Assay
The cellular thermal shift assay (CETSA) was employed to assess whether a drug candidate directly binds to a protein target of interest within cells.[40] Briefly, the A549 cells were cultured in a 10 cm dishes overnight. Then the cells were harvested after treatment with DMSO or CGTE for 4 h. Then the cell suspensions in PBS were aliquoted into six PCR tubes and heated at 46, 50, 54, 58, and 62 °C for 3 min in a PCR thermocycler, followed by three repeated freeze-thaw cycles in liquid nitrogen. After centrifugation (12,000 rpm, 20 min, 4 °C), the supernatants were collected for Western blotting.
Co-Immunoprecipitation (co-IP) Assay
The A549 cells cultured in a 10-cm dish overnight. Afterwards, the cells were treated with or without CGTE for 4 h and then lysed with co-IP lysis buffer (Beyotime) at 4 °C for 30 min. Cell lysates were centrifuged and equal amounts of the supernatants (100 µL) were aliquoted as “input” samples. For the co-IP assay, the remaining supernatants were incubated with indicated antibodies overnight at 4 °C under gentle rotation, after which protein A + G agarose beads (Beyotime) was added and incubated at 4 °C for an additional 4 h under gentle rotation. The agarose beads were washed five times in IP lysis buffer and boiled with 1× SDS-PAGE loading buffer. The samples were analyzed by Western blotting analysis.
In Vivo Ubiquitination Assay
The assay was performed as previously described in 293T cells co-transfecting three genes (p27, Ubiquitin, and Skp2).[15, 41] In the study, both 293T cells and A549 cells were co-transfected with the indicated plasmids, including Myc-p27, HA-tagged Ubiquitin, and Skp2 by using Calcium Phosphate Cell Transfection Kit (Beyotime) or Lipofectamine 2000 reagent (Invitrogen), cells were then treated with or without CGTE for 18 h and cultured with MG-132 for an additional 6 h, followed by immunoprecipitation and Western blotting assays.
Skp2 Overexpressing Stable Cell Line
The Skp2 stably overexpressing cell lines were established as previously described.[16] The typical NSCLC cell line A549 cells were infected with lentivirus containing Skp2 gene for 24 h. Then the cells were incubated with medium containing puromycin (0.5 µg mL−1) to generate cells stably overexpressing Skp2. The cells were then harvested for MTT, colony formation, cell cycle, and Western blotting assays.
In Vivo Animal Experiments
All animal studies were performed according to the guidelines of the Animal Ethics Committee of Guangzhou University of Chinese Medicine. The authorization number was A202203012 (C57BL/6 mice) and ZYD-2022-062 (BALB/c nude mice). C57BL/6 mice (male and female, 4 weeks old) and BALB/c nude mice (male and female, 4 weeks old) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China) and acclimated for 1 week. Six mice were kept in one cage and housed in the specific pathogen free environment (23 ± 1 °C, 55 ± 5% humidity) under 12 h light/dark cycle with free access to water and food. The mice maintenance food was purchased from HFK Bioscience Co., Ltd. (Beijing, China, Cat# 1022). The main components of the diet include corn, flour, bran, bean pulp, fish meal, oil, calcium carbonate, calcium hydrogen phosphate, salt, multivitamins, and microelements.
For LLC tumor mouse model, LLC cells (1 × 106 100 µL−1 PBS) were injected subcutaneously into the right flank of C57BL/6 mice. When the tumor size was about 60 mm3 after 5 days, the mice were randomized into five groups (n = 6 per group): control group (solvent: 30% ethanol in corn oil, gavage, daily); CGTE treatment groups (0.5, 1, and 2 g kg−1, gavage, daily); and CDDP treatment group (2 mg kg−1, i.p., once every 3 days). C57BL/6 mice were administered with CGTE, CDDP, or equivalent amount of vehicle (30% ethanol in corn oil) for 18 days and sacrificed by carbon dioxide inhalation at 19 days since drug treatment.
For A549 tumor mouse model, A549 cells (5 × 106, cells suspended with 50 µL PBS and 50 µL matrigel) were injected subcutaneously into the right flank of the BALB/c mice. When the tumor size reached about 90 mm3 after 21 days, the mice were randomized into five groups (n = 6 per group): control group (solvent: 30% ethanol in corn oil, gavage, daily); CGTE treatment groups (0.5, 1, and 1.5 g kg−1, gavage, daily); and CDDP treatment group (2 mg kg−1, i.p., once every 3 days). BALB/c nude mice were treated with CGTE, CDDP, or equivalent amount of vehicle (30% ethanol in corn oil) for 22 days and sacrificed by carbon dioxide inhalation at 23 days since drug treatment.
The body weight and tumor volume were measured every other day. The tumor volume (mm3) was estimated by using the well-recognized formula: (length × width2)/2.[42, 43] The length and width measured with a vernier caliper represent the longest dimension parallel to the surface and the dimension perpendicular to the length, respectively. The tumors were dissected, weighed, and processed for Western blotting and immunohistochemistry.
Evaluation of the Side Effects of CGTE in Mice
The C57BL/6 and BALB/c nude mice were subjected to daily observations of the physical activity levels, food and water intake, as well as changes in hair, skin, secretions, and excretions, all mice survived till the end of the experiments. To detect the hepatorenal toxicity of CGTE, the mice serums were collected to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CRE), and uric acid (UA) levels, according to the manufacturer's instructions. Moreover, the liver, kidney, stomach, and lung were dissected and processed for hematoxylin and eosin (H&E) staining to evaluate the side effects toward these organs.
H&E Staining and Immunohistochemistry (IHC)
Tissues fixed in 4% paraformaldehyde were dehydrated with a graded ethanol series (70%, 85%, 95%, and 100%) and xylene, and embedded in paraffin. The tissues were sectioned for further study. For histological examination, the tissue sections (4 µm thickness) were stained with H&E according to the manufacture's instruction (Solarbio). For IHC, the tumor sections were soaked in boiling citrate buffer (0.01 M, pH 6.0) for antigen retrieval, followed by the incubation with 3% hydrogen peroxide for 15 min. Then the sections were blocked with normal goat serum for 20 min and incubated with primary antibody against Ki67 (1:200, BD) overnight at 4 °C. Sections were then incubated with corresponding secondary antibodies for 30 min at room temperature, stained with DAB, and counterstained with hematoxylin using a SP (mouse/rabbit IgG)-POD Kit (Solarbio). The percentage of Ki67 positive cells were quantified by counting brown-stained cells from five arbitrarily selected fields of each tumor section at 400× magnification.
Statistical Analysis
Data analysis and plotting were performed by using GraphPad Prism 9.0 (GraphPad Software, CA, USA) and SPSS 25.0 (IBM, NY, USA). All data were presented as mean ± standard error of the mean (SEM) from at least three independent experiments. Statistically significant differences between two groups were evaluated by unpaired t-test; differences between multiple groups were determined by using a one-way analysis of variance (ANOVA) or two-way ANOVA followed by Dunnett's T3 Post hoc tests. NS indicates non-significance. A value of p < 0.05 indicated statistically significant.
Acknowledgements
This study was supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515011881, 2021B1515140052), the Independent Research and Development Projects of Maoming Laboratory (2021ZZ004), the Project of South Medicine Innovation Team in Modern Agricultural Industry Technology System of Guangdong Province (2023KJ148), and the Rural Revitalization Plan from Guangdong Provincial Universities (2019KZDZX2017).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Y.Q.L. and H.X. designed the research. D.H., P.E.W., Z.J.C., Y.C.P., Z.W.X., J.T., Z.H.J., and B.B.Y. performed the experiments and collected data. D.H. and Y.Q.L. wrote the manuscript. D.H., P.E.W., Y.Q.L., and H.X. analyzed the data. Y.Q.L., H.X., and R.Z. revised the manuscript. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




