Physiological Concentrations of Blueberry-Derived Phenolic Acids Reduce Monocyte Adhesion to Human Endothelial Cells
Abstract
Scope
Blueberry polyphenols are thought to confer cardiovascular health benefits, but have limited bioavailability. They undergo extensive metabolism and their phenolic acid metabolites are likely to be the mediators of bioactivity. The effect of blueberry-derived phenolic acids on one aspect of inflammation, monocyte adhesion to vascular endothelial cells, is investigated.
Methods and results
The major blueberry-derived phenolic acids in human plasma are identified and quantified. Three test mixtures representing compounds present at 0–4 h (Early), 4–24 h (Late), or 0–24 h (Whole) are used to investigate the effect on adhesion of monocytes to tumor necrosis factor alpha (TNFα)–activated endothelial cells. The Late mixture reduces monocyte adhesion, but there is no effect of the Early or Whole mixtures. Exclusion of syringic acid from each mixture results in inhibition of monocyte adhesion. Exposure to the phenolic acid mixtures has no effect on the endothelial surface expression of adhesion molecules intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), or E-selectin, suggesting that other molecular mechanisms are responsible for the observed effect.
Conclusion
This study shows that physiological concentrations of blueberry polyphenol metabolites can help maintain cardiovascular health by regulating monocyte adhesion to the vascular endothelium.
1 Introduction
Low-grade chronic inflammation is a major contributing factor to the pathology of many diseases such as cardiovascular disease, type 2 diabetes, and neurodegenerative diseases.1-4 This process is characterized by endothelial dysfunction with the increased expression of adhesion molecules,5 such as intercellular cell adhesion molecule-1 (ICAM-1),6 vascular cell adhesion molecule-1 (VCAM-1),7 and E-selectin.8 and results in the increased recruitment of circulating white blood cells including monocytes.9 In atherosclerotic plaque development, adhesion of monocytes to the endothelium is an early event and minimizing this in chronic inflammatory conditions is important for maintaining vascular health.4, 9-11
Epidemiological, clinical, and animal studies all support a link between blueberry consumption and improved vascular health.12-14 While mixed findings have been reported in human studies,14-18 animal studies consistently indicate that blueberry consumption downregulate inflammatory processes associated with endothelial monocyte adhesion. Eight-week supplementation of wild blueberry powder to obese Zucker rats reduced plasma levels of pro-inflammatory markers, including tumor necrosis factor alpha (TNFα), interleukin-6 and C-reactive protein, that are known to promote endothelial monocyte adhesion.19 Blueberry consumption reduced serum TNFα and Interleukin-6, and downregulated aortic TNFα expression in apolipoprotein E-deficient mice20 and reduced high-fat diet–induced chronic inflammation in rats.21 In these animal studies, the anti-inflammatory effects were tentatively attributed to the presence of blueberry polyphenols such as anthocyanins and/or proanthocyanidins.19-21 However, recent human studies have consistently shown that blueberry polyphenols, including anthocyanins, flavan-3-ols, proanthocyanidins and flavonols, have limited bioavailability.22-28 In contrast, their metabolites, mainly the phenolic acids, can be detected in plasma at higher concentrations relative to the parent polyphenols. These findings suggest that phenolic acid metabolites play an important part in conferring the vascular benefits of blueberry polyphenols.22, 26, 29-34
The aim of this study was to determine which blueberry-derived phenolic acid metabolites are consistently increased in human plasma following the consumption of blueberries, and to monitor the effect of different mixtures of these compounds on the adhesion of monocytes to vascular endothelial cells. We developed three mixtures, modeling the detectable phenolic acid aglycones found in plasma at <4 h, 4–24 h, and 0–24 h (Early, Late, and Whole mixtures, respectively) and determined their ability to influence monocyte adhesion. We hypothesized that circulating concentrations of blueberry-derived phenolic acids may differ in their ability to affect monocyte adhesion to TNFα-activated endothelial cells. The mixtures were also used to determine whether any observed effect on TNFα-driven monocyte adhesion could be linked to changes in the surface expression level of ICAM-1, VCAM-1, or E-selectin.
2 Experimental Section
2.1 Materials
The purity of all phenolic acids used was ≥95%: protocatechuic, syringic, homovanillic, trans-ferulic, vanillic and p-coumaric acids were purchased from Sigma-Aldrich, dihydrocaffeic and dihydro-m-coumaric acids were from Alfa Aesar, gentisic acid was obtained from BDH Chemicals, 4-hydroxyhippuric and 2-hydroxyhippuric acids were from CHEMOS and dihydroferulic acid was from Fluorochem.
Medium 199 (M199), RPMI and tissue culture reagents were from Gibco Life Technologies. Monoclonal mouse anti-human CD14 phycoerythrin-conjugated M5E2 antibody was from BD Biosciences. Monoclonal mouse anti-human CD54 (ICAM-1), CD106 (VCAM-1), and CD62E (E-selectin) phycoerythrin-conjugated antibodies were from eBioscience. Ficoll-Hypaque was from Global Science and Annexin-V fluorescein isothiocyanate (FITC) from Life Technologies. Fetal bovine serum, endotoxin-free PBS, propidium iodide (PI), and all other reagents were from Sigma-Aldrich.
2.2 Analysis of Phenolic Acid Metabolites in Plasma Following Blueberry Consumption
Plasma samples from three healthy individuals were probed over a 24 h period following a single serving of blueberry juice (5mL kg−1 bw) for the occurrence of 33 phenolic compounds (anthocyanins, flavan-3-ol monomers, flavonols, phenolic acids, and other aromatic compounds). Compounds were detected with an LC-MS/MS method operating in the multiple reaction monitoring mode as previously described22 and in Table S1, Supporting Information. Ethics approval for this study was from New Zealand Health and Disability Ethics Committees, ref: 15/NTA/46.
2.3 Preparation of the Phenolic Acid Mixtures
Each phenolic acid compound was dissolved in DMSO and combined into a 1400-fold stock solution of the final Early (<4 h), Late (4–24 h), and Whole (0–24 h) mixtures, with concentrations ranging from 0.06 to 726 mm. Single aliquots were stored at −18 °C then thawed and diluted to the desired concentrations in cell culture medium “complete M199” (see Table 2), with a final DMSO concentration of 0.071% (v/v).
2.4 Endothelial Cell Culture and Experimental Conditions
Human umbilical vein endothelial cells (HUVECs) were isolated by collagenase digestion of umbilical veins35 and were cultured on gelatin-coated dishes in M199 containing 10% penicillin and streptomycin, 75 mg L−1 endothelial cell growth supplement, 100 µg mL−1 heparin and 13% heat-inactivated fetal bovine serum (“complete M199”). The cells were used at the fourth passage following primary extraction and when 80–90% confluent. They were incubated with syringic acid alone or mixtures of the phenolic acids.
2.5 Cell Viability Assays
The effects of the three blueberry-derived phenolic acid mixtures on HUVEC viability was evaluated by flow cytometry, monitoring apoptosis and necrosis using dual labelling with Annexin-V FITC and PI.36 Briefly, following treatment, medium was collected and adherent cells were harvested using 200 µL of TrpLE Express. The detached and then adherent cell fractions were combined and suspended in 50 µL Annexin-V binding buffer (10 mm HEPES pH 7.4, 140 mm NaCl, and 2.5 mm CaCl2,) and 1:50 (v/v) of Annexin-V FITC antibody and incubated in the dark for 10 min at room temperature. PI (5 µg) was added and cell fluorescence monitored on a Beckman Coulter FC500 MPL flow cytometer (5000–10 000 cells analyzed per sample).
Cellular metabolic activity was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. After treatment, cell medium was replaced with 0.5 mL of phenol red free complete M199 and 1.2 mm MTT and incubated in the dark for 3 h at 37 °C. Medium was removed, the purple formazan crystals were dissolved in 0.5 mL of 4 mm HCl with 0.1% NP-40 in isopropanol and absorbance recorded at 570 nm.
2.6 Isolation of Peripheral Blood Mononuclear Cells from Human Blood
Whole blood from healthy donors was collected by venipuncture (ethics approval New Zealand Health and Disability Ethics Committees, ref: URA/06/12/083/AM05) into heparin-containing tubes. Blood was diluted 1:3 (v/v) with endotoxin-free PBS and 10 mL of dextran (5% w/v with 0.9% NaCl) was added to every 40 mL diluted blood. After allowing ≈20 min for sedimentation of erythrocytes, the leukocyte-enriched upper layer was collected and the white cells separated by centrifugation through Ficoll-Hypaque.37 Peripheral blood mononuclear cells (PBMCs) were collected from the Ficoll-Hypaque/plasma interface, washed once with PBS, twice with RPMI containing 10% fetal bovine serum and 10% penicillin and streptomycin (“complete RPMI”), suspended in complete RPMI and maintained at 37 °C (5% CO2, humidified) until use (within 4 h of isolation). Immediately before addition to the HUVECs, PBMCs were resuspended in fresh complete M199 medium and added to the HUVECs at a ratio of 17:1 (PBMC:HUVEC).
2.7 Monocyte Adhesion Flow Cytometry Assay
HUVECs cultured in 24-well plates were pre-treated with the Whole, Early, or Late phenolic acid mixtures, or syringic acid alone, for 4, or 18 h. The complete M199 was then replaced, TNFα (1 ng mL−1) added and the cells incubated for 2 h. PBMCs were then added and incubation continued for a further 30 min. The medium was removed and each well washed three times with PBS to remove non-adherent cells. The adherent cells, both HUVECs, and PBMCs, were detached using TrpLE Express and the well washed with complete M199 which was also collected. Cells were centrifuged at 1000 relative centrifugal force (RCF) for 5 min at room temperature, resuspended in 50 µL PBS and transferred to a round-bottomed 96-well plate. Mouse anti-human CD14 phycoerythrin-conjugated antibody (0.001 µg) was added to each well and incubated in the dark for 15 min at room temperature, washed once with 140 µL PBS prior to pelleting the cells at 300 RCF for 5 min at room temperature. The supernatant was removed and cells resuspended in 200 µL PBS. Fluorescence was measured by flow cytometry using a Beckman Coulter FC500 MPL flow cytometer, analyzing 5000 cells per sample. Data were analyzed using the MXP software (Beckman Coulter).
2.8 Cellular Adhesion Molecule Expression Assay
HUVECs were pre-incubated with the Early, Late, or Whole (0.1, 1, 10, or 100×) phenolic acid mixtures for 4 h. The medium was then replaced, 1 ng mL−1 TNFα added and incubation was continued for 2 h. HUVECs were harvested at 37 °C using TrpLE Express and then combined with the culture medium containing any detached cells. The cells were pelleted at 1000 RCF for 5 min resuspended in 50 µL PBS, transferred to a round-bottomed 96-well plate and 2.5 µL of an antibody stock (0.1 µg mL−1) containing mouse anti-human ICAM-1, VCAM-1, or E-selectin phycoerythrin-conjugated antibody was added. The plate was incubated in the dark for 20 min at room temperature and the cells washed once with 150 µL PBS. The HUVECs were finally resuspended in 200 µL PBS and fluorescence was measured using a Beckman Coulter Cytomics FC500 MPL, analyzing 5000 cells per sample. Data were analyzed using the MXP software (Beckman Coulter).
2.9 Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). For cell viability data, results were analyzed either by one-way analysis of variance (ANOVA) followed by Dunnet's multiple comparisons test, or by two-tailed one-sample t-test (against the hypothetical value of 100%). All monocyte adhesion and adhesion molecule expression (ICAM-1, VCAM-1, or E-selectin) data were analyzed by one-sample t-test, or by two-way ANOVA followed by Sidak's multiple comparisons test, as defined in figure legends. Linear regression was conducted to determine the relationship between the Late mixture dose and the extent of reduction in TNFα-mediated adhesion of monocytes to HUVECs. In all cases, a value of p < 0.05 was considered statistically significant.
3 Results and Discussion
3.1 Blueberry-Derived Phenolic Acid Aglycone Metabolites Detected in Plasma
We screened for the occurrence of 33 phenolic compounds (Table 1) consisting mainly of phenolic acids that are known to appear in β-glucuronidase/aryl-sulfatase treated plasma following blueberry consumption,23-25 or that are common phenolic metabolites of anthocyanins,38 flavan-3-ol monomers,39 or quercetin-based flavonols.40 A total of 18 of the 33 compounds were detected in plasma between 0 and 24 h in all subjects. Of these, 12 phenolic acid metabolites, including derivatives of benzoic, hippuric, cinnamic, and phenylacetic acids, were found at consistently increased levels relative to baseline (Table 1; Figure S1, Supporting Information). The remaining 21 compounds (Figure S2, Supporting Information) were either not detected, did not increase relative to baseline, or were detected at extremely low levels, below our LC-MS/MS assay's lower limit of quantitation. Therefore, we used the 12 phenolic acid metabolites, along with their 0–24 h plasma concentration-time profiles22 to represent the bioavailable polyphenol metabolites present in the circulation following ingestion of blueberries and to inform the design of the three test mixtures (Table 2) investigated in the present study.
| Compound | Retention time [min] | LLOQa [nm] | Detected in plasma? | Increased from baseline? |
|---|---|---|---|---|
| I. Flavan-3-ol monomers | ||||
| (−)-Epicatechin | 6.90 | 100 | × | N/A |
| (+)-Catechin | 6.48 | 100b | × | N/A |
| (−)-Epicatechin gallate (IS1) | 7.35 | 100b | × | N/A |
| II. Flavonols | ||||
| Quercetin-3-O-glucoside | 7.61 | 10 | × | N/A |
| Quercetin-3-O-rutinoside (IS2) | 7.35 | 10 | × | N/A |
| III. Benzoic acid derivatives (phenolic acid derivatives) | ||||
| Benzoic acid | 8.26 | 100 | √ | × |
| γ-Resorcylic acid | 10.58 | 100 | √ | × |
| 2-Hydroxybenzoic | 8.77 | 100 | √ | × |
| 4-Hydroxybenzoic | 6.24 | 100 | √ | × |
| Gallic acid | 2.67 | 10b | × | N/A |
| Protocatechuic acid | 4.29 | 15 | √ | √ |
| Gentisic acid | 6.60 | 25 | √ | √ |
| Syringic acid | 6.79 | 62.5 | √ | √ |
| Vanillic acid | 6.66 | 62.5 | √ | √ |
| IV. Hippuric acid derivatives (phenolic acid derivatives) | ||||
| Hippuric acid | 6.31 | 188 | √ | × |
| 2-Hydroxyhippuric acid | 7.39 | 25 | √ | √ |
| 4-Hydroxyhippuric acid | 4.12 | 25 | √ | √ |
| V. Cinnamic acid derivatives (phenolic acid derivatives) | ||||
| trans-Ferulic acid | 7.73 | 12.5 | √ | √ |
| Dihydroferulic acid | 7.39 | 62.5 | √ | √ |
| Dihydrocaffeic acid | 6.32 | 25 | √ | √ |
| Dihydro-m-coumaric acid | 7.56 | 100 | √ | √ |
| p-Coumaric acid | 7.54 | 10 | √ | √ |
| m-Coumaric acid | 8.03 | 10 | × | N/A |
| Caffeic acid | 6.88 | 100 | × | N/A |
| Sinapic acid (IS3) | 7.70 | 100 | × | N/A |
| Chlorogenic acid | 6.40 | 100 | √ | √ (Trace) |
| VI. Phenylacetic acid derivatives (phenolic acid derivatives) | ||||
| Homovanillic acid | 6.73 | 62.5 | √ | √ |
| 3-Hydroxyphenylacetic acid (IS4) | 6.79 | 100 | × | N/A |
| VII. Other aromatic compounds | ||||
| Pyrogallol | 3.01 | 100b | × | N/A |
| Phloroglucinol | 2.24 | 100 | × | N/A |
| VIII. Anthocyanins | ||||
| Cyanidin-3-O-glucoside | 6.08 | 100 | × | N/A |
| Malvidin-3-O-glucoside | 6.44 | 100 | × | N/A |
| Pelargonidin-3-O-glucoside (IS5) | 6.34 | 100 | × | N/A |
- √, present; × , not detected; N/A, not applicable; IS1/2/3/4/5, internal standard 1, 2, 3, 4, or 5
- a Lower limit of quantitation (LLOQ), defined as the lowest concentration screened with a quantifier multiple reaction monitoring chromatographic peak with signal:noise ratio >10
- b Concentration at which the quantifier peak was detected with signal:noise between 5 and 10.
| Treatment mixture | |||
|---|---|---|---|
| Compound | Early [nm] | Late [nm] | Whole [nm] |
| 2-Hydroxyhippuric acid | 50 | – | 50 |
| 4-Hydroxyhippuric acid | 60 | – | 70 |
| Syringic acida | 450 | – | 450 |
| Protocatechuic acid | 15 | 30 | 25 |
| Gentisic acid | – | 100 | 100 |
| Vanillic acid | 600 | 1000 | 1000 |
| trans-Ferulic acid | 60 | 60 | 60 |
| p-Coumaric acid | 40 | 50 | 50 |
| Dihydroferulic acid | – | 2000 | 2000 |
| Dihydrocaffeic acid | – | 200 | 200 |
| Dihydro-m-coumaric acid | – | 850 | 850 |
| Homovanillic acid | – | 300 | 300 |
| Total [nm] | 1275 | 4590 | 5155 |
The 12 compounds included in our test mixtures reflect a considerable proportion (39%) of all individual phenolic acid metabolites currently confirmed to occur in postprandial human plasma at consistently higher levels relative to their baseline concentrations following blueberry consumption (Table S2, Supporting Information). While available published blueberry polyphenol studies22-27 have screened for the possible occurrence of many individual aglycone and phase II phenolic acid metabolites, in total, only 31 phenolic acid metabolites have been definitively identified with plasma concentrations accurately measured against their respective authentic standards, by targeted LC-MS/MS (Table S2, Supporting Information). Twenty-seven (87%) of these metabolites are phenolic acid aglycones, found at increased levels in postprandial plasma, with maximal (Cmax)22, 27 or 2 h plasma concentrations in the nm–µm range.26 In contrast to the aglycones, only four individual phase II glucuronidated/sulfated phenolic acid metabolites (dihydrocaffeic acid-3-O-sulfate, isoferulic acid-3-O-glucuronide and 4-O-methylgallic acid-3-O-sulfate, catechol-O-sulfate), have been found at significantly higher concentrations in postprandial plasma relative to their levels at baseline, following blueberry consumption.26
Following consumption, polyphenols can undergo extensive metabolism by the combined action of the gut microbiota and phase II metabolic processes (glucuronidation, sulfation, and/or methylation).12-14 Therefore, the complete plasma profile of blueberry-derived phenolic acid metabolites will likely include many compounds existing as a combination of aglycone and phase II metabolites. At present, comprehensive characterization of the underlying chemical identities and accurate circulating concentrations of many phase II blueberry phenolic metabolites is lacking. This is evident from recent studies on the bioavailability and metabolism of blueberry polyphenols in humans,22-27 where only four individual phase II glucuronidated/sulfated phenolic acid metabolites have been reported in plasma at consistently higher levels relative to baseline (Table S2, Supporting Information). Our data have added significant new information to the investigation of the blueberry phenolic acid metabolites currently known to be increased relative to baseline levels following blueberry consumption. However, still more effort is needed to definitively profile the specific identities and circulating concentrations of the majority of uncharacterized phase II phenolic metabolites present in plasma following blueberry consumption.
3.2 Specificity of the Flow Cytometry-Based Assay for Monocyte–Endothelial Adhesion
As reported in our previous study,22 none of the three blueberry phenolic acid mixtures (Whole, Early, or Late) compromised the viability of HUVECs following 24-h treatment at 100× physiological concentrations.
As PBMCs isolated from whole blood consist primarily (≥98%) of a mixture of lymphocytes and monocytes,41 and both could potentially bind to the endothelial cells, it was important to distinguish between the two cell types in flow cytometry analysis. Monocytes are larger than lymphocytes and also express CD14 and can be distinguished in a mixed population of lymphocytes, monocytes and HUVECs (Figure 1). The flow-cytometry gating strategy allowed us to focus on analysis of HUVECs and monocytes only. Mature human peripheral blood monocytes were distinguished from HUVECs according to size and anti-CD14 antibody labelling (Figure 1).42
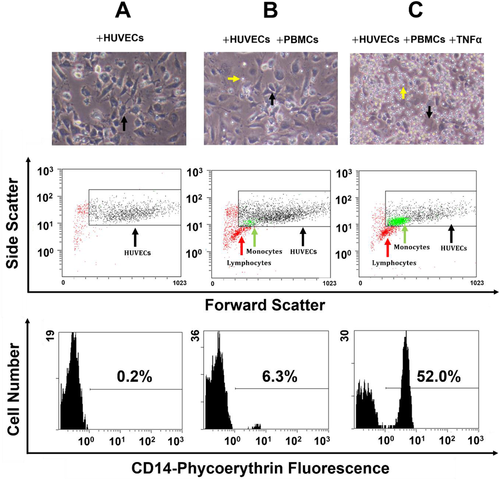
There was a marked increase in the number of monocytes adherent to HUVECs stimulated with TNFα compared with those associated with unstimulated HUVECs (Figure 1). There was no difference in the number of adherent lymphocytes regardless of activation with TNFα (Figure 1), indicating that TNFα-challenged HUVECs specifically recruit and bind monocytes.
3.3 The Effect of Blueberry-Derived Phenolic Acid Mixtures on Monocyte–Endothelial Adhesion
We investigated the efficacy of different mixtures of blueberry-derived phenolic acid metabolites that closely reflect their plasma concentration-time profiles to determine whether consumption of blueberries could affect monocyte adhesion to endothelial cells under stimulated inflammatory conditions. Our approach differs from others,29, 33, 34 who either tested the effect of individual compounds,34 or a limited number of metabolites,29, 33 on monocyte–endothelial adhesion and hence our data better represent the situation found in plasma after blueberry consumption.22, 30, 31 There is no information regarding a threshold concentration required to demonstrate an effect on monocyte–endothelial cell adhesion, nor on the effect of varying the duration of exposure. It is important to characterize the concentrations where reduction in monocyte adhesion is optimal as there is interest in developing novel delivery systems to improve the oral bioavailability of polyphenols, based on the hypothesis that increased bioavailability can lead to greater bioactivity.43 A prominent case in point is the recent study by Riva and co-workers (2018),44 wherein a new delivery system based on food-grade lecithin was used to increase the plasma concentration of quercetin by up to 20 times its normal Cmax.
Monocyte adhesion to HUVECs was investigated using three different blueberry-derived phenolic acid mixtures representing metabolites detected in plasma at 0–4 h (Early), 4–24 h (Late), or 0–24 h (Whole) time points at concentrations derived from the measured Cmax range (Table 2, 1× dose). HUVECs were pre-treated with the mixtures at four doses (0.1, 1, 10, and 100× of Cmax) for either 4 h (Figure 2) or 18 h (Figure 3) prior to activation with TNFα and subsequent co-incubation with PBMCs.

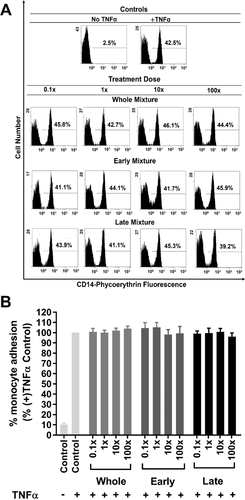
There was no significant effect on monocyte adhesion when HUVECs were pre-treated for 4 h with the phenolic acids present in the Early or Whole mixture at any dose (Figure 2A,B). In contrast, monocyte adhesion to HUVECs treated with the Late mixture was significantly reduced (12–17%), relative to the untreated control, at doses between 0.1 and 100×. The threshold concentration required for the Late mixture to mediate a consistent, significant reduction in monocyte adhesion relative to untreated control was 0.1×, as lower concentrations (0.01–10−5×) did not show a significant difference from control (p = 0.1587–0.7026) (Figure 2B). Importantly, a significant (R2 = 0.191, p = 0.0006) dose-dependent effect of the Late mixture on monocyte adhesion to TNFα-activated HUVECs was observed (Figure 2C), with ≈2.6 ± 0.7% reduction in monocyte adhesion for every tenfold increase in treatment dose of the Late mixture. The small (≈12%) but consistent effect observed at the 0.1× dose reduced proportionately with decreasing doses (0.01–10−5×). This suggests that the bioactivity of phenolic acids from blueberry juice is not limited to the Cmax, and that an anti-inflammatory effect could occur below Cmax. Conversely, increasing the treatment dose to 10 or 100-fold of Cmax resulted in only a marginally greater reduction in monocyte adhesion (Figure 2B,C). These findings are consistent with a recent study31 showing that, at concentrations as low as 0.1× Cmax, mixtures of phenolic acid metabolites derived from the anthocyanin cyanidin-3-O-glucoside could consistently inhibit the TNFα-stimulated expression of soluble VCAM-1 (sVCAM-1) by HUVECs and human aortic endothelial cells.
In contrast to the effects with the Late mixture, the Whole or Early mixtures had no effect on monocyte adhesion at any dose (Figure 2A,B). Our findings therefore provide new evidence in support of the hypothesis that although polyphenols are extensively metabolized, only some of their phenolic acid metabolites may be responsible for specific biological effects,13, 29 and that increasing the concentrations beyond Cmax may not substantially amplify their anti-inflammatory activity. Our findings agree with previous work by others29, 31, 45, 46 which have indicated that some compounds when tested in isolation, or as part of a limited mixture exhibited a modest protection against monocyte adhesion to endothelial cells.
Previous investigations on the effects of diet-derived phenolic acid metabolites on monocyte adhesion and/or markers of vascular inflammation typically report the effects of a single treatment duration only.29-31, 33, 34, 45, 46 We have demonstrated that treatment duration affects monocyte adhesion. Specifically, 4 h pre-treatment with the Late mixture decreased monocyte adhesion but no significant effect was observed when HUVECs were pre-treated for 18 h (Figure 3). These findings suggest that effects mediated by an active mixture of blueberry-derived phenolic acids are transient, and that prolonged exposure may underestimate the true physiological effects. Alternatively, the loss of efficacy of the Late mixture with 18 h pre-treatment may reflect the possibility that the phenolic acids are metabolized into less active compounds by the endothelial cells during this incubation period.
3.4 Syringic Acid Mitigates the Phenolic Acid–Mediated Reduction on Monocyte-Endothelial Adhesion
We investigated why the Late mixture, but not the Early or Whole mixtures, significantly reduced TNFα-mediated monocyte adhesion to HUVECs. Compounds absent in the Late mixture, but present in both the Whole and Early mixtures included 2-hydroxyhippuric, 4-hydroxyhippuric and syringic acids. α-Hydroxyhippuric acid, a compound related to 2- and 4- hydroxyhippuric acids, has been shown to attenuate monocyte adhesion to human aortic endothelial cells challenged with palmitate as a model of lipotoxicity,29 or in cells isolated from patients with diabetes,33 whereas syringic acid has been reported to increase monocyte adhesion to TNFα-treated HUVECs.34 We therefore determined the effect of syringic acid on the effect of Whole and Early mixtures on monocyte adhesion.
Pre-treatment of HUVECs for 4 h with syringic acid alone did not alter monocyte adhesion (Figure 4A). In contrast, monocyte adhesion to TNFα-treated HUVECs was significantly reduced by pre-treatment for 4 h with the Whole and Early mixtures lacking syringic acid (9%, p = 0.0227 or 6%, p = 0.0234, respectively). (Figure 4B). These findings are supported by two-way ANOVA analysis with Sidak's test, demonstrating that excluding syringic acid improved the ability of the Early mixture (p = 0.032), and showed a trend in the Whole mixture (p = 0.065), to reduce TNFα-induced monocyte adhesion (Figure 4B). This observation provides the first evidence for a direct effect of a single phenolic acid on an anti-inflammatory effect of physiologically relevant mixtures of blueberry-derived phenolic acids. In previous work, disagreement in findings between the anti-inflammatory ability of specific phenolic acid metabolites has been observed only when the compound is tested in isolation,34, 45 but never when investigated as part of a mixture. In one study, 0.2–2 µm protocatechuic acid was reported to reduce monocyte adhesion to TNFα-activated HUVECs,45 whereas in another, 650 nm was found to increase adhesion to the same cell line.34 These apparently conflicting findings prompted our hypothesis that different combinations of blueberry-derived phenolic acids, mimicking circulating concentrations at different time intervals after ingestion may elicit different effects on monocyte adhesion to endothelial cells.
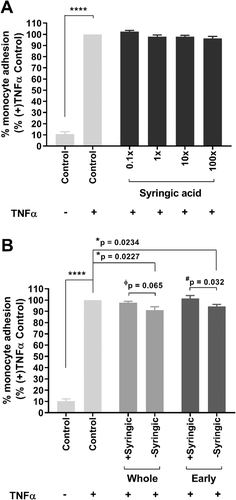
In agreement with our findings, Bharat and co-workers29 have recently shown significantly reduced monocyte adhesion to palmitate-challenged endothelial cells by the phenolic acids, benzoic acid-4-O-sulfate, vanillic acid-4-O-sulfate, isovanillic acid-3-O-sulfate, hippuric or α-hydroxyhippuric tested as a mixture. In contrast, there was no significant effect of any of these compounds tested in isolation.29
3.5 Effects of Blueberry-Derived Phenolic Acid Mixtures on Surface Expression of Endothelial Cell Adhesion Molecules
TNFα increases the endothelial cell surface expression of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin,11 thereby facilitating increased adhesion of monocytes.9, 11, 47 We therefore investigated the effect of 4 h pre-treatment with the Whole, Early or Late mixture on TNFα-induced HUVEC surface expression of ICAM-1, VCAM-1, or E-selectin. The results in Figure 5 show that there was no effect of any of the polyphenol metabolite mixtures on TNFα-induced cell surface expression.
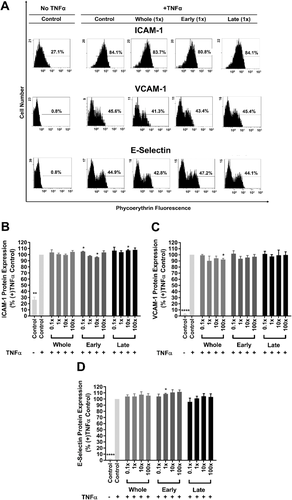
Under similar experimental conditions, other studies45, 48 have investigated the effects of phenolic acids, at physiologically relevant concentrations (≤2 µm), on the mRNA expression levels of the three adhesion molecules by HUVECs. One found that a mixture of protocatechuic, vanillic, ferulic and hippuric acids did not affect TNFα-induced mRNA expression levels of adhesion molecules,45 while the other reported no significant effect of 100 nm protocatechuic acid on mRNA and surface protein levels of adhesion molecules in TNFα-challenged HUVECs.48 We did not analyze mRNA levels or protein synthesis, but have concentrated on the translocation to the cell surface following activation. Our findings therefore complement these studies.45, 48 We have used a TNFα concentration (1 ng mL−1) which is close to the median plasma level (1.6-2.2 ng mL−1) reported for healthy middle-aged men having previously suffered myocardial infarction.49 This allowed us to model low-grade chronic inflammation in an in vitro system. Interestingly, recent studies have shown that phase II sulfated phenolic acids could downregulate ICAM-1 and/or VCAM-1 mRNA expression in human aortic endothelial cells challenged with palmitate as a model of lipotoxicity,29 or in cells isolated from patients with diabetes.33 However, the effects on ICAM-1 and VCAM-1 protein expression by the same test mixtures were dependent on the type of pro-inflammatory stimulus. Clearly, more work is needed to characterize the mechanisms regulating the effects of blueberry-derived metabolites on the mRNA and surface protein expression of these cell adhesion molecules.
Adhesion molecules other than those investigated in our study may be differently affected. Activated endothelial cells release sVCAM-1, which is not membrane-bound and does not directly bind monocytes,30, 44 and some studies have shown that phenolic acid metabolites can reduce sVCAM-1 protein expression in HUVECs challenged with supra-physiological doses of TNFα (10 ng mL−1)30, 31 or with cluster of differentiation 40 ligand.50
Recent studies29, 33, 41 have offered new insights into the molecular mechanisms regulating the ability of phenolic acid metabolites to influence inflammation-driven monocyte adhesion to the endothelium. These mechanisms include the reduced mRNA expression of inflammatory chemokines such as Interleukin-8 and monocyte chemoattractant protein-1,29, 33 the restoration of cell surface glycosaminoglycans,33 or the inhibition of NF-kB-p65 phosphorylation,46 each of which represents an important process in monocyte–endothelial adhesion. Further studies are needed to fully elucidate the molecular mechanisms behind the anti-inflammatory effects of blueberry-derived phenolic metabolites.
4 Concluding Remarks
The variable bioavailability and metabolism of bioactive compounds present in the circulation following consumption of blueberries22 likely result in the vasculature being exposed to a complex mixture of compounds and not to single compounds.22, 31 Our study has shown that a subset of bioavailable blueberry-derived phenolic metabolites demonstrated a differential ability to protect endothelial cells from TNFα-mediated monocyte adhesion. The presence of a specific phenolic acid, syringic acid, negatively influenced the anti-inflammatory efficacy. Overall, this study suggests that combinations of physiologically-relevant phenolic acid aglycones established in the circulation following a single serve of blueberry juice could contribute to reducing monocyte adhesion to the vascular endothelium and this may be a mechanism by which the intake of blueberries may help maintain vascular health.
Acknowledgements
M.C.M.V., L.D.M., and R.F.A., conceived and managed the project. J.S.T., M.C.M.V., S.M.B., L.D.M., and R.F.A. designed the experiments. J.S.T. and S.M.B. conducted all experiments, with M.C.M.V., J.L.M., L.D.M., and R.F.A. contributing to data analysis and interpretation. J.S.T., M.C.M.V., S.M.B., L.D.M., and R.F.A. drafted the initial manuscript. All authors reviewed and approved the final content. The authors thank Callaghan Innovation Project Grant (MVBB1501), Riddet Institute Centre of Research Excellence for Food Research, University of Auckland and University of Otago for financial support (JST), John Pearson for advice on statistical analyses, Christine C. Winterbourn and Mark B. Hampton for insightful discussions.
Conflict of Interest
The authors declare no conflict of interest.




