Metabolites and tissue distribution of resveratrol in the pig
Abstract
Scope: trans-Resveratrol (RES) and/(or) its metabolites exert many effects in vivo. Our aim was to study the metabolism and tissue distribution of RES using the pig, a mammal physiologically close to humans.
Methods and results: Forty-seven tissues, organs and fluids were analyzed 6 h after intragastric RES administration (5.9 mg/kg body weight) using HPLC-MS/MS. Twelve RES and seven dihydroresveratrol (DH-RES) metabolites were detected. DH-RES was the main metabolite in cecum, colon and rectum, whereas RES-3-O-glucuronide was the most abundant one in fluids and organs. Approximately 74.5% of the total RES administered was recovered in the form of RES, DH-RES and derived metabolites (65.1% along the gastrointestinal tract, 7.7% in urine, 1.2% in bile and 0.5% in organs). We report here, for the first time, the occurrence of RES ribosyl-sulfate derivative, DH-RES diglucuronide, DH-RES sulfoglucuronide and DH-RES disulfate as well as the metabolic profile of RES and DH-RES in the aorta, lymph, lymph node, ovaries, uterus, cerebellum, pancreas, urinary bladder tissue, fat and muscle.
Conclusion: This study contributes to the clarification of the metabolism and tissue distribution of RES and could help to further understand the mechanisms underlying its effects.
1 Introduction
The naturally occurring polyphenol resveratrol (3,5,4′-trihydroxy-trans-stilbene, RES) has been reported to exert many different health-promoting effects including antioxidant, anti-inflammatory, antitumor, anti-platelet aggregation, cardioprotective and aging-delay effects 1. However, despite the reported beneficial effects, the bioavailability of RES is poor as it is rapidly absorbed, metabolized and excreted in humans and different animal models 2-7. Therefore, this apparent paradox (high activity but low bioavailability) implies that the real bioactive RES metabolites as well as the mechanisms underlying RES properties are not yet fully understood 1, 8.
Most studies regarding the RES metabolism have focused on the analysis of RES and derived metabolites in plasma, urine (U) or feces. However, there is a general lack of studies looking at the distribution of RES in organs, fluids or tissues upon oral or intravenous administration 9, 10. RES tissue distribution has only been reported so far in rodents 11-16 amongothers and one study in rabbits 17.
It is known that the pig gastrointestinal (GIT) system and the associated gut microbiota are closer with the human digestive system than that of rodents 18, 19. Despite the fact that research on pigs requires large and rather expensive facilities, its use has been reported to be a valuable tool when investigating the metabolism of nutrients as well as phenolic compounds such as phenolic acids 20, flavan-3-ols 21, anthocyanins 22, 23, ellagitannins 24 and quercetin 25, 26. Recently, we have reported the pharmacokinetics of RES in pigs 7. In the pig, the metabolic profile of RES in plasma revealed the presence of RES diglucuronide, two isomers of RES sulfoglucuronide, two isomers of RES glucuronide, RES sulfate, and RES, being RES-3-O-glucuronide, the most abundant plasma metabolite.
Taking into account the lack of studies regarding the tissue distribution of RES in big mammals, physiologically closer to humans than rodents, the aim of this study was to explore the metabolism and tissue distribution of RES extensively in the pig.
The results presented here could shed some light on the apparent ‘RES paradox’; (high activity but low bioavailability) since we show that RES and its derived metabolites are widely distributed in different pig tissues and biological fluids, many of them, not previously reported. In addition, the metabolic profile of RES in different organs, tissues and fluids, including some new RES and αβ-dihydroresveratrol (DH-RES) metabolites, are reported here for the first time.
2 Materials and methods
2.1 Chemicals
trans-Resveratrol (resveratrol, RES, 3,5,4′-trihydroxy-trans-stilbene, >99% purity) and the internal standard quercetin (>98%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Organic solvents such as methanol (MeOH), acetone and acetonitrile (ACN) were obtained from Merck (Darmstadt, Germany). Milli-Q system (Millipore, USA) ultrapure water was used throughout this experiment. Tiletamine–zolazepam (Zoletil 50) was purchased from Virbac España S.A. (Esplugues de Llobregat, Spain) and sodium pentobarbital (Dolethal) from Vétoquinol (Alcobendas, Madrid, Spain).
2.2 Synthesis of resveratrol metabolites
trans-Resveratrol-3-O-β-D-glucuronide (RES glucuronide, >95% purity) was prepared from RES following a reported synthesis 27. DH-RES (>95% purity) was synthesized as described earlier by catalytic hydrogenation of RES 28). Finally, the metabolite αβ-dihydroresveratrol-3-O-β-D-glucuronide (DH-RES glucuronide) was prepared by hydrogenation of the same protected resveratrol 3-O-β-D-glucurunopyranosyl intermediate used in the preparation of resveratrol-3-O-β-D-glucuronide (3,4′-O-tert-butyl-5-O-(2,3,4-tri-O-acetyl-β-D-glucurunopyranosyl)resveratrol). Final deprotection to obtain DH-RES glucuronide was carried out in two steps, silyl deprotection with tetrabutylammonium fluoride in tetrahydrofuran and acetyl cleavage with sodium carbonate in a MeOH–water mixture. Purity was >95%. δH (300 MHz, D2O) 6.96 (d, J=8.0 Hz, 2H, Harom), 6.68 (d, J=8.1 Hz, 2H, Harom), 6.44, 6.40, 6.31 (3s, 3H, Harom), 4.80 (d, J=7.0 Hz, 1H, H-1), 3.74 (m, 1H, H-5), 3.52 (m, 3H, H-3, H-4, H-2), 2.75 (s, 4H, 2× CH2Ar); δ13C (75 MHz, D2O) 176.6 (C=O), 160.2, 159.2, 156.4, 145.5, 134.0, 130.5, 116.1, 110.9, 109.5, 102.9, 102.5 (C-1), 77.7, 76.5, 74.6, 73.5 (C-2, C-3, C-4, C-5), 39.5, 37.9 (CH2Ar). ESI-MS (ES−): Calcd. for C20H22O9 (M−H) 405.1, found: 405.0.
All reactions were monitored by TLC on precoated Silica gel 60 plates F254 (Merck), and detected by heating with Mostain (500 mL of 10% H2SO4, 25 g of (NH4)6Mo7O24 ·4H2O, 1 g Ce(SO4)2·4H2O). Products were purified by flash chromatography with Merck Silica gel 60 (200–400 mesh). High-resolution FAB (+) mass spectral analyses were obtained on a Micromass AutoSpec-Q spectrometer (Micromass, Manchester, UK). NMR spectra were recorded on either a Bruker Avance 300 or ARX 400 or Bruker Avance DRX 500 MHz [300 or 400 MHz (1H), 75 or 100 (13C) (Bruker Biospin GmbH, Rheinstetten, Germany)], at room temperature for solutions in CDCl3, D2O or CD3OD. Chemical shifts are referred to the solvent signal. Further, NMR experiments (COSY, TOCSY, ROESY and HMQC) were carried out when necessary to assign the compound. Data were processed using the manufacturer software, raw data were multiplied by shifted exponential window function prior to the Fourier transform, and the baseline was corrected using polynomial fitting.
2.3 Animals and study design
All experiments were in accordance with the recommendations of the European Union regarding animal experimentation (Directive of the European Council 86/609/EC). Experimental design, included in the Spanish National Research Project BFU2007-60576, was approved by the Ethics Committee of the University of Murcia (Murcia, Spain) and by the Bioethics Committee-CSIC (Madrid, Spain). Five female pigs (cross-breed 25% Landrace×25% Large White×50% Duroc) were provided by the Veterinary Teaching Farm of the University of Murcia (sanitary registry number 302315). Housing and animal interventions were carried out in the same Teaching Farm. The pigs (80±8 kg and 7 months old) were fasted overnight with free access to water. Animals were tranquilized with tiletamine–zolazepam (3.125 mg/kg body weight) to allow animal handling during intragastric RES administration. The dose of RES was prepared as previously described and adjusted to 6.25 mg/kg body weight (500 mg for an 80 kg animal). To determine the actual final dose, the amount of RES remaining in the probe after intragastric administration was evaluated [7]. One animal was used as control and received only water. All animal interventions were carried out at the same hour in the morning.
Six hours after RES administration, the pigs were sacrificed using an overdose of sodium pentobarbital and complete necropsies were carried out. All organs, the content of hollow organs as well as different body fluids, were weighed and measured. The following samples were obtained: tissue and content from stomach (St), duodenum, jejunum (three segments), ileum (I), cecum (Ce), proximal colon (PC), distal colon (DC) and rectum (R), plasma from jugular (JP) and portal veins (PP), U, lymph (L), bile (B), cerebrospinal fluid (CSF), superficial inguinal lymphatic node (LYN), brain (Br), cerebellum (Cr), heart, liver (Lv), kidney (K), lung, spleen, pancreas (Pn), uterus (Ut), urinary bladder (UBl), ovary, three adipose (perirenal, mesenteric and subcutaneous) and two skeletal muscle tissue types (Longissimus dorsi (LgD) and Semimembranosus (SM)), erythrocytes, white blood cells (WBC) and serum low-density lipoprotein (LDL) particles. The organs were thoroughly rinsed with PBS to avoid external blood contamination.
Blood samples were collected just before euthanasia from the jugular vein and immediately after the sacrifice from the portal vein in BD Vacutainer lithium heparin tubes (BD, Franklin Lakes, NJ). To separate the plasma, the collected blood samples were immediately centrifuged at 3000×g for 10 min at 4°C in a Sigma 1–13 microcentrifuge (Braun Biotech., Melsungen, Germany). The plasma samples were immediately frozen at −80°C for further analyses.
Red blood cells (RBC) were isolated from pig blood (4 mL) according to Nakamura et al. 29. WBC were isolated from pig heparinized blood (3 mL). Blood was diluted 1:1 with PBS and processed by density-gradient centrifugation with Histopaque-1077 and Histopaque-1119 (Sigma-Aldrich, Madrid, Spain) according to the manufacturer instructions.
U, B, L and CSF were collected by puncture, with sterile syringes and needles, from the UBl, gallbladder, the cisterna chyli and the subarachnoid space through the atlanto-occipital articulation, respectively. After collection, all fluid samples were placed into sterile containers and frozen at −80°C for further analyses.
LDL isolation from 4 mL of pig serum was carried out according to the protocol of Vieira et al. 30 but using 68 000 rpm for 90 min at 4°C in a Beckman 70.1 Ti rotor.
2.4 Sample preparation
Plasma (300 μL), L (150 μL), CSF (200 μL) and B (100 μL) samples were extracted using ACN and processed according to Azorín-Ortuño et al. 7. The supernatant was evaporated under vacuum in a SpeedVac Concentrator Savant SPD121P (Thermo Scientific, Alcobendas, Spain). The pellet was re-dissolved in 150 μL MeOH. Methanolic samples were diluted 1:1 (v:v) with acidified (0.2% formic acid) ultrapure Milli-Q water (Millipore), filtered through a 0.45-μm membrane filter Millex-HV13 (Millipore) and an aliquot was analyzed by HPLC.
-
Whole blood (300 μL) was processed according to Biasutto et al. 31. U samples were diluted five-fold with ultrapure water, filtered through the Millex filter and injected into the HPLC equipments. The content (1 g) of the St, duodenum, jejunum (three segments), I, Ce, PC, DC and R were processed according to Espín et al. 24. The skeletal muscle tissues (LgD and SM), RBC, WBC as well as the organs (tissues from the above parts of the GIT, LYN, Br, Cr, H, Lv, K, lungs, spleen, Pn, Ut, UBl, aorta and ovaries (Ov)) were processed (0.5 g) according to Azorín-Ortuño et al. 32. Adipose (perirenal, mesenteric and subcutaneous) tissues (1 g) were processed following the protocol of González et al. 33. LDL particles (700 μL) with a mean protein content of 0.9 mg/mL protein were extracted according to de la Torre-Carbot et al. 34.
In all the cases, sample manipulation was performed avoiding the direct light exposure to prevent the possible photochemical isomerization of trans-resveratrol to the cis-form. The internal standard quercetin (20 μM) was routinely used in all the samples.
2.5 LC-MS/MS analyses
The identification of RES and derived metabolites was achieved by HPLC-DAD-ESI-MS/MS, UHPLC-triple quadrupole (QqQ) MS detection and HPLC-Q-TOF.
The LC-DAD-ESI ion-trap system was a 1100 series HPLC-DAD device (Agilent Technologies, Waldbronn, Germany) equipped with an ion-trap mass spectrometer (Agilent). The heated capillary and voltage were maintained at 350°C and 4 kV, respectively. Mass scan (MS) and MS/MS daughter spectra were measured from m/z 100 up to 800 m/z. Collision-induced fragmentation experiments were performed in the ion trap using helium as the collision gas, and the collision energy was set at 50%. Mass spectrometry data were acquired in the negative ionization mode. Chromatographic separations were achieved on a 250×4 mm id, 5 μm, C18 Mediterranean Sea column (Teknokroma, Barcelona, Spain) using water:formic acid (99:1, v:v) (A) and ACN (B) as the mobile phases at a flow rate of 1 mL/min. The gradient started with 5% B in A to reach 55% B at 30 min, 90% B at 31 min for 5 min and returning to the initial conditions (5% B). For quantifying DH-RES and derived metabolites in UV, the conditions were the same but using a 250×4.6 mm id, 3 μm, C18 100 Å Phenomenex column (Phenomenex®, Torrance, CA, USA). The samples were diluted in water (1:1) and different sample volumes (from 50 to 80 μL, depending on the sample) were injected.
The UHPLC-MS QqQ consisted of a 1290 Infinity HPLC series (Agilent) equipped with a 6460 series triple quadrupole mass spectrometer (Agilent). Chromatographic separations were carried out at room temperature on a 100×3 mm id, 2.7 μm, C18 Poroshell 120 column (Agilent) using water:formic acid (99.9:0.1, v:v) (A) and ACN:formic acid (99.9:0.1, v:v) (B) as the mobile phases at a flow rate of 0.6 mL/min. The gradient started with 15% B in A to reach 50% at 15 min, 90% at 18 min for 5 min and returning to the initial conditions (15% B). The injected sample volume was 10 μL. The optimum mass spectrometer parameters for detection of the available metabolites (DH-RES, RES and their corresponding glucuronides) were optimized connecting directly the column inlet to the Jet Stream source. The source parameters were the following: capillary voltage −3500 V, charging potential −500 V, nebulizer pressure 40 (psi), auxiliary gas heated to 275°C at a flow rate of 9000 cm3/min. MS data were collected in multiple reaction monitoring (MRM) mode by monitoring specific transitions of parent and product ions for each metabolite: RES triglucuronide 755/227, RES diglucuronide 579/227, RES glucuronide 403/227, RES sulfoglucuronide 483/227, RES trisulfate 467/227, RES disulfate 387/227, RES sulfate 307/227, RES 227/185, DH-RES triglucuronide 757/229, DH-RES diglucuronide 581/229, DH-RES glucuronide 405/229, DH-RES sulfoglucuronide 485/229, DH-RES trisulfate 469/229, DH-RES disulfate 389/229, DH-RES sulfate 309/229 and DH-RES 229/187.
The Q-TOF equipment consisted of an HPLC-DAD-MS system (1200 series, Agilent) equipped with a UHD Accurate-Mass Q-TOF (6540A series, Agilent). The column, flow rate, injected volume and gradient conditions were the same as those described above for the UHPLC-QqQ equipment. The optimum mass spectrometer parameters for detection of both RES and DHR-RES and their corresponding 3-O-glucuronides (at 80 μM) were optimized, connecting directly the column inlet to the Jet Stream source. The source parameters were the following: capillary voltage −3500 V, charging potential −500 V, nebulizer pressure 40 (psi), auxiliary gas heated to 275°C and a flow rate of 9000 cm3/min. The parameters of the mass spectrometer were similar to those optimized in the UHPLC-QqQ equipment. The acquiring spectra were in the range from 100 to 800 m/z in the negative mode and spectra time in MS mode 0.5 s. The acquisition mode was in auto MS2, the MS1 ranged from 100 to 900 Da and the MS2 ranged from 70 to 700 Da, while the scan rate was set at 0.3 s. Finally, the collision energy slope was 7 V and the collision energy offset was 5 V. The Mass Hunter tool (Agilent) was used for the calculation of elemental composition of compounds. This tool lists and rates possible molecular formulas consistent with the accurate mass measurement and the true isotopic pattern (TIP).
Different RES and DH-RES metabolites were identified by their UV spectra, molecular mass, daughter ions, fragmentation pattern and specific transitions in the HPLC-DAD-ESI-MS/MS, UHPLC-MS-QqQ and HPLC-Q-TOF equipments. Different organs and fluids from the control pig were spiked with the four available standards (RES, RES 3-O-glucuronide, DH-RES and DH-RES 3-O-glucuronide) at six different concentrations (from 0.05 to 10 μM). The curves were characterized by regression coefficients of R2=0.999 or above. The compounds RES and RES 3-O-glucuronide as well as DH-RES and DH-RES 3-O-glucuronide were quantified in the 1100 series equipment using UV detection at 320 and 276 nm, respectively, and using their corresponding available standards. The rest of RES-derived and DH-RES-derived metabolites were quantified using UV detection and the commercial RES and DH-RES as external standards, respectively. The mean recovery efficiency of the standards from the different samples was 85±4%, ranging from 80±5% (in the case of RES 3-O-glucuronide in colon content) to 99±3% (in the case of RES 3-O-glucuronide in plasma). The intra-day and inter-day precision (coefficient of variation; CV) was calculated by triplicate for each available standard and concentration assayed (1, 5 and 10 μM) in three representative samples (colon content, Lv and plasma). The intra-day precision ranged from 0.35% (10 μM RES 3-O-glucuronide) to 7.03% (10 μM DH-RES 3-O-glucuronide) in colon content samples; from 0.51% (10 μM RES) to 4.44% (10 μM DH-RES 3-O-glucuronide) in plasma and finally, from 0.53% (10 μM RES) to 4.39% (10 μM RES 3-O-glucuronide) in Lv samples. The inter-day (assessed in three different days) ranged from 0.14% (10 μM RES) to 6.18% (10 μM DH-RES 3-O-glucuronide) in colon content samples; from 0.92% (10 μM RES) to 4.07% (10 μM DH-RES) in plasma samples and finally, from 0.42% (10 μM DH-RES) to 6.65% (10 μM RES 3-O-glucuronide) in Lv samples. The accuracy was measured as the mean percentage of error of the measured concentration to the theoretical concentration of each standard. The best accuracy was obtained with the plasma extraction protocol (% bias) that ranged from −2.26% (10 M RES 3-O-glucuronide) to 2.35% (10 μM DH-RES 3-O-glucuronide). In the case of both colon content and Lv samples, the accuracy was lower than in plasma, ranging from 18.77% (10 μM RES 3-O-glucuronide) to 21.41% (10 μM RES) in colon content samples and finally, from 12.87% (10 μM DH-RES 3-O-glucuronide) to 14.97% (10 μM RES 3-O-glucuronide) in Lv samples. The limits of detection in UV ranged from 0.025 μM (RES) to 1.5 μM (DH-RES 3-O-glucuronide). The limits of quantification in UV ranged from 0.1 μM (RES) to 3 μM (DH-RES 3-O-glucuronide). The CV was always lower than 10%.
When the low amount of metabolites prevented UV quantification, the compounds RES, RES 3-O-glucuronide, DH-RES and DH-RES 3-O-glucuronide were quantified in the QqQ equipment using the corresponding available standards. The limits of detection in QqQ (μM) ranged from 0.025 (RES) to 0.05 (DH-RES). The limits of quantification in QqQ (μM) ranged from 0.05 (RES) to 0.25 (DH-RES). The coefficient of variation was always lower than 10%. In the case of other non-available standards, the relative abundance for these metabolites was scored in the QqQ system as high (+++), medium (++) and low (+). ‘Low values’ were those that permitted proper compound identification according to both ion fragmentation and transitions. ‘Medium’ and ‘high’ exceeded the ion intensity of ‘low’ by around 10- and 100-fold (or higher), respectively. These values were used to assess the relative abundance of the same metabolite in different organs and fluids but not to compare abundance among different metabolites. For ion intensity comparison, the ionization of the mass spectrometers was daily checked using RES, DH-RES, RES 3-O-glucuronide and DH-RES 3-O-glucuronide standards.
3 Results
3.1 RES and DH-RES metabolites identification
The metabolites were characterized by their UV spectra and MS analyses using ion trap, UHPLC-QqQ and HPLC-Q-TOF. Nineteen metabolites were detected in different pig organs, tissues or fluids after intragastric RES administration (Table 1). The combination of different analytical approaches was important to support the identification of metabolites. For example, sulfoglucuronide and diglucuronide isomers that coeluted using the QqQ equipment (Table 1) were separated and characterized using the 1100 Agilent ion-trap equipment (results not shown). In addition, the use of control samples (organs and fluids) from a pig without RES administration was crucial to discard confounding ions and specific transitions not related to RES-derived metabolites that were found in different samples analyzed from the control pig (results not shown).
| Metabolite | ♯ | Rt (min) | MS (M−H) | MS–MS | Occurrencea) |
|---|---|---|---|---|---|
| RES diglucuronide-1 | 1 | 1.4 | 579 | 403, 227 | DC, J1C, J2C, J3C, IC, U, B |
| RES diglucuronide-2 | 2 | 1.4 | 579 | 403, 227 | U, B, JP |
| DH-RES diglucuronide | 3 | 1.4 | 581 | 405, 229 | U |
| DH-RES sulfoglucuronide | 4 | 1.8 | 485 | 405, 309, 229 | J3C, Lv, Pn, U, B |
| RES sulfoglucuronide-1 | 5 | 1.9 | 483 | 403, 307, 227 | StC, DC, J1C, J2C, J3C, IC, J1T, J2T, J3T, IT, Cr, K, Lv, Ov, Pn, U, B, JP |
| RES sulfoglucuronide-2 | 6 | 1.9 | 483 | 403, 307, 227 | DC, J1C, J1T, J2C, J2T, J3C, IC, DT, J3T, IT, Cr, K, Lv, Ov, Pn, U, B |
| RES trisulfate | 7 | 2.3 | 467 | 307, 227 | StC, DC, J1C, J2C, J3C, IC, StT, DT, J1T, J2T, J3T, IT, A, Cr, H, Lv, Ln, Ov, U, B, PP, JP |
| DH-RES trisulfate | 8 | 2.4 | 469 | 309, 229 | J3C, IC, IT, U, B |
| RES ribosyl sulfate | 9 | 3.3 | 439 | 359, 307, 227 | IC, U, B |
| RES 3-O-glucuronide | 10 | 4.4 | 403 | 227 | StC, DC, J1C, J2C, J3C, IC, StT, DT, J1T, J2T, J3T, IT, CeT, PCT, DCT, A, UBl, Br, Cr, H, K, Lv, Ln, Ov, Pn, Sp, Ut, LYN, PF, MF, SF, LgD, U, B, PP, JP, L, LDL |
| RES 4′-O-glucuronide | 11 | 4.5 | 403 | 227 | DC, J1C, J2C, J3C, IC |
| DH-RES 3-O-glucuronide | 12 | 5.1 | 405 | 229 | StC, DC, J1C, J2C, J3C, IC, StT, DT, J1T, J2T, J3T, IT, CeT, PCT, DCT, A, Cr, H, K, Lv, Ln, Ov, Sp, U, B, PP, JP, L, LYN, PF, MF, SF, LgD |
| RES sulfate | 13 | 5.5 | 307 | 227 | StC, DC, J1C, J2C, J3C, IC, CeC, PCC, DCC, RC, StT, DT, J1T, J2T, J3T, IT, CeT, PCT, DCT, RT, A, UBl, Br, Cr, H, K, Lv, Ln, Ov, Pn, Sp, Ut, LYN, PF, MF, SF, LgD, SM, U, B, PP, JP, L, CSF, RBC, WBC |
| DH-RES sulfate | 14 | 5.6 | 309 | 229 | StC, DC, J1C, J2C, J3C, IC, CeC, PCC, DCC, RC, StT, DT, J1T, J2T, J3T, IT, CeT, PCT, DCT, RT, A, UBl, Cr, H, K, Lv, Ln, Ov, Sp, LYN, PF, MF, LgD, SM, U, B, PP, JP, L, RBC |
| RES disulfate | 15 | 6.5 | 387 | 307, 227 | U, B |
| DH-RES disulfate | 16 | 6.5 | 389 | 309, 229 | U, B |
| RES | 17 | 7.1 | 227 | 185 | StC, DC, J1C, J2C, J3C, IC, CeC, PCC, DCC, RC, StT, DT, J1T, J2T, J3T, IT, CeT, PCT, DCT, RT, A, UBl, Br, Cr, H, K, Lv, Ln, Ov, Pn, Sp, Ut, LYN, PF, MF, SF, LgD, U, B, PP, JP, L, CSF, LDL, RBC, WBC |
| DH-RES | 18 | 7.5 | 229 | 187 | IC, CeC, PCC, DCC, RC, IT, CeT, PCT, DCT, RT, U |
| cis-RES | 19 | 8.9 | 227 | 185 | StC, DC, J1C, J2C, J3C, IC, CeC, PCC, DCC, RC, StT, DT, J1T, J2T, J3T, IT, CeT, PCT, DCT, Cr, Lv, PF, MF, LgD, U, JP, L, CSF, LDL, RBC, WBC |
- a) a) A, aorta tissue; B, bile; Br, brain; CeC and CeT, cecum content and tissue, respectively; Cr, cerebellum; CSF, cerebrospinal fluid; DC and DT, duodenum content and tissue; DCC and DCT, distal colon content and tissue; H, heart; IC and IT, ileum content and tissue; J1C and J1T, jejunum-1 content and tissue; J2C and J2T, Jejunum-2 content and tissue; J3C and J3T, jejunum-3 content and tissue; JP, jugular vein plasma; K, kidneys; LgD, Longissimus dorsi muscle; Ln, lungs; Lv, liver; L, lymph; LDL, low density lipoprotein; LYN, superficial inguinal lymphatic node; MF, mesenteric fat; Ov, ovaries; PCC and PCT, proximal colon content and tissue; PF, perirenal fat; Pn, pancreas; PP, portal vein plasma; RBC, red blood cells; RC and RT, rectum content and tissue; SF, subcutaneous fat; SM, Semimembranosus muscle; Sp, spleen; StC and StT, stomach content and tissue; U, urine; UBl, urinary bladder tissue; Ut, uterus; WBC, white blood cells. Rt are referred to the QqQ assays.
The metabolites detected in this study were RES, cis-RES, nine RES-conjugates (glucuronide, sulfate and sulfoglucuronide derivatives), the microbiota-derived metabolite DH-RES and six DH-RES conjugates (also glucuronide, sulfate and sulfoglucuronide derivatives) (Table 1). One additional metabolite with RES UV-like spectrum was also detected. This metabolite, compound 9, was tentatively identified as an RES ribosyl-sulfate derivative according to its ion mass (439 m/z-) and daughter ions (359, 307, 227) (Table 1).
Compounds 10 (RES 3-O-glucuronide), 12 (DH-RES 3-O-glucuronide), 17 (RES) and 18 (DH-RES) were fully identified by direct comparison with the available standards. The rest of metabolites were tentatively identified according to their UV spectra, ion mass and daughter ions. The UV spectra were very useful to distinguish between RES-like metabolites (maximum at 305 and 320 nm) and DH-RES-like metabolites (bell-shaped spectrum with maximum at 276 nm). Compound 11, another glucuronide conjugate, was indirectly identified as the other possible more common glucuronide isomer, RES 4′-O-glucuronide. In the case of the rest of metabolites, the tentative identification was assessed by a combination of analyses using ion trap and specific transitions in the QqQ. The lack of available standards for many metabolites as well as their small amount prevented their isolation and full characterization by other spectroscopic means. To the best of our knowledge, metabolites 3 (DH-RES diglucuronide), 4 (DH-RES sulfoglucuronide), 9 (RES ribosyl-sulfate) and 16 (DH-RES disulfate) are reported here for the first time (Table 1).
Table 1 is also useful to observe the relative distribution of each metabolite in all the pig organs, tissues and fluids analyzed. Some metabolites such as compounds 10 (RES 3-O-glucuronide), 13 (RES sulfate), 14 (DH-RES sulfate) and 17 (RES) were widely distributed in different tissues and fluids. On the contrary, other metabolites such as compounds 15 (RES disulfate) and 16 (DH-RES disulfate) were detected only in U and B and metabolite 3 (DH-RES diglucuronide) was only detected in U using QqQ (Table 1).
3.2 Distribution of RES-derived metabolites in the pig GIT tract
RES was intragastrically administered followed by various washes of the probe. This was important to ensure a maximum delivery of RES into the St due to its low solubility in water. Otherwise, upon oral administration, even if using hydroalcoholic or cyclodextrins solutions, a significant part of RES would remain in the esophagus (results not shown) and this would prevent correct quantification of the mass balance and calculation of RES recovery. The actual RES dose administered in the present study was calculated by subtracting the RES quantity that remained in the probe after intragastric administration from the initial amount prepared and delivered (500 mg for a 80 kg animal). The final total intragastric RES quantity administered was 472 mg RES (5.9 mg/kg body weight; equivalent to a similar human dose) 35.
Table 2 shows the different RES-derived metabolites detected in both the lumen and the gastrointestinal tissues of the pig GIT from St to I, 6 h after the intragastric RES administration. An increasing amount of RES conjugates was found from St to I, with maximun levels of compunds detected in the I. The most abundant metabolite was the RES sulfate derivative (13), although other RES conjugates such as 1 (diglucuronide), 5 and 6 (sulfoglucuronides) and 10 and 11 (glucuronides) were also very abundant. Other minor RES conjugates such as metabolites 7 and 9 were also detected using QqQ. It should be noted that approximately 42 mg RES (17) (8.9% of the initial RES amount administered) was still present in the pig GIT after 6 h of its intragastric administration (Table 2). RES was also found in the St (14.2 mg; 3% of the initial RES amount given) and decreased along the GIT until the I, where the amount rose again to 21 mg (4.25% of the initial RES amount given) (Table 2). A small amount of cis-RES (19) was also detected along the GIT. Although exposure to direct light was carefully avoided during the sample processing, a small isomerization from the trans- to the cis-form cannot be discarded. The microbiota-derived metabolite DH-RES and some DH-RES conjugates, compounds 4 (DH-RES sulfoglucuronide), 8 (DH-RES trisulfate), 12 (DH-RES 3-O-glucuronide), 14 (DH-RES sulfate) and 18 (DH-RES) were also detected at trace levels in the GIT from St to I (Table 2).
| GIT part (g) | Lumen content | Tissue | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 6 | 10 | 11 | 13 | 17 | QqQ | 5 | 6 | 10 | 13 | 17 | QqQ | ||||||
| Stomach (St) | ND | 1.34±0.21 | ND | 2.43±0.77 | ND | 1.06±0.41 | 13.40±2.06 | 7 (++) | ND | ND | 0.11±0.06 | 0.11±0.06 | 0.79±0.50 | 7 (+) | |||||
| Wc*: 193±38 | 12 (+++) | 12 (0.005)a | |||||||||||||||||
| WT: 440±40 | 14 (+) | 14 (+) | |||||||||||||||||
| 19 (+++) | 19 (+) | ||||||||||||||||||
| Duodenum (D) | 0.07a | 0.22±0.14b | 0.88±0.69b | 0.51±0.22 | 0.79±0.74b | 2.95±2.73 | 2.41±1.36 | 7 (++) | ND | 0.06±0.01b | 0.09±0.22 | 0.08±0.03 | 0.10±0.05 | 7 (++) | |||||
| WC: 61±19 | 12 (+) | 12 (++) | |||||||||||||||||
| WT: 255±95 | 14 (++) | 14 (+) | |||||||||||||||||
| 19 (++) | 19 (+) | ||||||||||||||||||
| Jejunum-1 (J1) | 0.31±0.15 | 1.27±0.41 | 0.40±0.17 | 2.40±0.69 | 0.30±0.15b | 1.60±0.52 | 0.53±0.16 | 7 (++) | ND | ND | 0.04±0.03 | 0.09a | 0.08±0.05 | 5+6 (++) | |||||
| WC: 165±96 | 12 (0.024)a | 7 (++) | |||||||||||||||||
| WT: 270±15 | 14 (++) | 12 (+) | |||||||||||||||||
| 19 (+) | 14 (++) | ||||||||||||||||||
| 19 (+) | |||||||||||||||||||
| Jejunum-2 (J2) | 0.49±0.27b | 1.51±0.95 | 0.24±0.04 | 2.19±0.79 | 0.47±0.26b | 1.98±1.08 | 0.55±0.14 | 7 (++) | ND | ND | 0.06±0.03 | 0.12a | 0.03a | 5+6 (++) | |||||
| WC: 118±23 | 12 (+) | 7 (++) | |||||||||||||||||
| WT: 263±16 | 14 (++) | 12 (++) | |||||||||||||||||
| 19 (+) | 14 (++) | ||||||||||||||||||
| 19 (+) | |||||||||||||||||||
| Jejunum-3 (J3) | 1.76±1.27 | 6.40±4.56 | 3.28±1.94 | 10.90±5.92 | 1.52±1.11a | 7.67±5.18 | 2.87±1.00 | 4 (+) | 0.18a | 0.08±0.01c | 0.19±0.06 | 0.45±0.30 | 0.21±0.14 | 7 (++) | |||||
| WC: 116±25 | 7 (+++) | 12 (++) | |||||||||||||||||
| WT: 281±20 | 8 (++) | 14 (++) | |||||||||||||||||
| 12 (0.043)a | 19 (+) | ||||||||||||||||||
| 14 (+++) | |||||||||||||||||||
| 19 (++) | |||||||||||||||||||
| Ileum (I) | 6.28±2.96c | 23.93±13.38c | 16.88±8.99c | 19.28±12.72 | 11.93±4.21b | 55.31±30.47 | 19.99±10.79 | 7 (+++) | 0.47±0.38b | 0.55±0.23b | 0.67±0.26 | 2.59±1.41 | 1.04±0.42 | 7 (+++) | |||||
| WC: 177±41 | 8 (++) | 8 (+) | |||||||||||||||||
| WT: 525±125 | 9 (+) | 12 (+++) | |||||||||||||||||
| 12 (+++) | 14 (+++) | ||||||||||||||||||
| 14 (+++) | 18 (++) | ||||||||||||||||||
| 18 (++) | 19 (++) | ||||||||||||||||||
| 19 (++) | |||||||||||||||||||
| Total metabolites (GIT content)=228.4±119.82 | Total metabolites (GIT tissues)=8.19±4.08 | ||||||||||||||||||
- a Values (quantified using UV detection) are expressed as total mg (mean±SE) taking into account the total weight of the lumen content and of tissue from the different GIT parts. *WC, weight content; WT, weight tissue; In QqQ, when not quantified, the relative abundance of each metabolite was scored as high (+++); medium (++) and low (+) (these values compare the abundance for the same metabolite in the different GIT parts but not among different metabolites); ND, not detected in UV. 1, RES diglucuronide-1; 2, RES diglucuronide-2; 4, DH-RES sulfoglucuronide; 5, RES sulfoglucuronide-1; 6, RES sulfoglucuronide-2; 7, RES trisulfate; 8, DH-RES trisulfate; 9, RES ribosyl sulfate; 10, RES 3-O-glucuronide; 11, RES 4′-O-glucuronide; 12, DH-RES glucuronide; 13, RES sulfate; 14, DH-RES sulfate; 17, RES; 18, DH-RES; 19, cis-RES; n=4 except in an=1, bn=2 and cn=3.
Overall, it is noticeable that a total amount of 228.40±119.82 mg (lumen content) and 8.2±4.1 mg (tissues) RES-derived metabolites (including DH-RES metabolites) was recovered from St to I. This meant that approximately 50% the initial RES amount administered was still present in the GIT, mainly in the I (33.7%), in the form of RES and derived metabolites (Table 2 and Fig. 1).
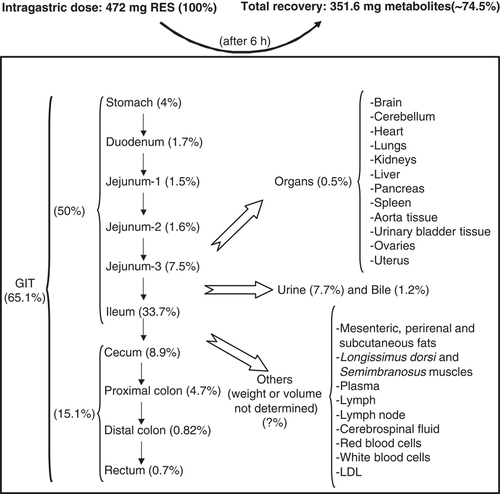
Recovery of RES-derived metabolites in pig organs, fluids and tissues 6 h after intragastric RES administration.
At this time point, 6 h after RES administration, the Ce was also an important reservoir for RES and its metabolites (∼42 mg) (Table 3). However, in this case, the most abundant compounds were the microbiota-derived metabolite DH-RES (18) (27.04±11.87 mg) and RES (17) (14.74±5.06 mg) (Table 3). Other minor RES and DH-RES conjugates (12–14) as well as cis-RES (19) were also detected (Table 3). The amount of metabolites decreased in the PC to ∼22 mg and was substantially lower in the DC and R (Table 3). Therefore, approximately 15% of the initial RES administered was recovered in the pig large intestine, mostly in the Ce (Table 3 and Fig. 1). The transit time from St to I was quite similar in all the animals studied (results not shown), however, the transit time from Ce to R was very different for each pig, yielding a variable distribution of RES and DH-RES along the large intestine of the animals. This variability is illustrated in Fig. 2. For example, in pig number 1 (P1 in Fig. 2), RES and DH-RES were detected only in the Ce, whereas in pig number 4 (P4 in Fig. 2) these metabolites were mainly detected in the DC and R (Fig. 2).
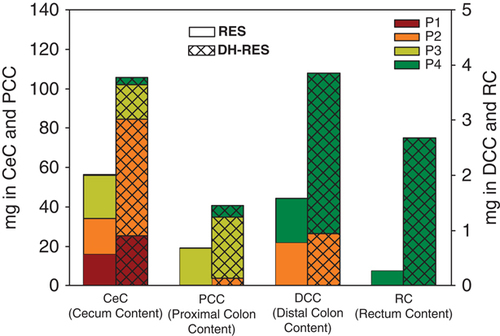
trans-Resveratrol (RES) and dihydroresveratrol (DH-RES) distribution in the large intestine of pig from Ce to R. This figure complements Table 2 and illustrates the different transit time of metabolites along the large intestine 6 h after intragastric RES administration. Open bars (RES); cross-hatched bars (DH-RES). P1–P4 designates different pigs (n=4).
| GIT part (continued) (g) | Lumen content | Tissue | ||||||
|---|---|---|---|---|---|---|---|---|
| 17 | 18 | QqQ | 10 | 13 | 17 | 18 | QqQ | |
| Cecum (Ce) | 14.06±4.75 | 26.40±11.81 | 13 (+) | 0.02±0.01 | 0.05±0.02 | 0.68±0.31 | 0.64±0.06 | 12 (0.007)a |
| WC: 342.5±102.8 | 14 (++) | 14 (+) | ||||||
| WT: 175±25 | 19 (+) | 19 (+) | ||||||
| Proximal colon (PC) | 6.42±5.96c | 13.55±8.87c | 13 (++) | ND | 0.063±0.008 | 0.54±0.27 | 1.52±0.34 | 10 (0.02)a |
| WC: 445±105 | 14 (++) | 12 (0.008)a | ||||||
| WT: 330±10 | 19 (++) | 14 (++) | ||||||
| 19 (+) | ||||||||
| Distal colon (DC) | 0.79±0.02b | 1.92±0.97b | 13 (+) | ND | ND | 0.06±0.01 | 1.12a | 10 (0.015)a |
| WC: 165±65 | 14 (+) | 12 (++) | ||||||
| WT: 310±90 | 19 (+) | 13 (++) | ||||||
| 14 (+) | ||||||||
| 19 (+) | ||||||||
| Rectum (R) | 0.26a | 2.67a | 13 (+) | ND | ND | 0.01±0.004 | 0.52a | 13 (+) |
| WC: 40±20 | 14 (+) | 14 (+) | ||||||
| WT: 85±15 | 19 (+) | |||||||
| Total metabolites (lumen): 66.07±32.4 | Total metabolites (tissues): 5.29±1.05 | |||||||
- a Values (quantified using UV detection) are expressed as total mg (mean±SE) considering the total weight (g) of each lumen content and tissue. In QqQ, when not quantified, the relative abundance of each metabolite was scored as high (+++); medium (++) and low (+) (these values compare the abundance for the same metabolite in the different tissues but not among different metabolites); ND, not detected in UV. 10, RES-3-O-glucuronide; 12, DH-RES glucuronide; 13, RES sulfate; 14, DH-RES sulfate; 17, RES; 18, DH-RES; 19, cis-RES; n=4 except in an=1, bn=2 and cn=3.
3.3 Distribution of RES-derived metabolites in pig fluids, LDL and blood cells
U and B were the reservoirs in which the highest diversity of metabolites was found, with 18 and 15 different metabolites detected, respectively (Table 4). The volume of both U and B was determined and the total amount of metabolites was quantified, reaching a total amount of 36.3 mg in U (7.7% of the initial RES amount administered) and 5.6 mg in B (1.2% recovery) (Fig. 1). The most abundant metabolite found in U was compound 10 (RES 3-O-glucuronide), whereas, in B, the most abundant one was compound 6 (RES sulfoglucuronide isomer-2) followed by compounds 10 and 13 (Table 4).
| Fluids | 1 | 2 | 5 | 6 | 7 | 9 | 10 | 13 | 17 | QqQ |
|---|---|---|---|---|---|---|---|---|---|---|
| Urine (U) | Det | 2.36±0.12 | 0.41±0.02 | 3.06±0.53 | Det | Det | 21.38±3.62 | 2.99±1.25 | Det | 3 (+); 4 (+); 8 (++); |
| (1172±262) mL | (2.79±0.53) | (0.49±0.09) | (3.72±1.01) | (24.11±0.97) | (3.83±1.59) | 12 (0.85)a; 14 (+++) | ||||
| 15 (++); 16 (++) | ||||||||||
| 18 (0.52)a; 19 (++) | ||||||||||
| Bile (B) | Det | 9.60±2.60 | 9.21±4.00 | 33.75±13.81 | 13.40±2.92 | 7.55±2.44 | 28.63±1.08 | 26.70±9.53 | 2.22±0.22 | 4 (+); 8 (++) |
| (42.0±9.5) mL | (0.40±0.11) | (0.39±0.17) | (1.42±0.58) | (0.56±0.12) | (0.32±0.10) | (1.20±0.05) | (1.12±0.40) | (0.10±0.01) | 12 (0.10)a; 14 (+++); 15 (++); 16 (++) | |
| Portal vein plasma (PP) | ND | ND | ND | ND | ND | ND | 0.67±0.27 | 0.17±0.04 | 0.11±0.03 | 7 (++); 12 (0.44; 1.1 μM)a; 14 (++) |
| 1.67±0.67 μM | 0.56±0.13 μM | 0.47±0.12 μM | ||||||||
| Jugular vein plasma (JP) | ND | Detb | Detb | ND | ND | ND | 0.28±0.05 | 0.08±0.02 | ND | 7 (+); 12 (0.09; 0.22 μM)a; 14 (+) |
| 0.69±0.13 μM | 0.24±0.05 μM | 17 (0.012; 0.05 μM) | ||||||||
| 19 (+) | ||||||||||
| Lymph (L) | ND | ND | ND | ND | ND | ND | 0.10±0.02 | 0.05±0.002b, Deta | 0.024, Deta | 12 (0.21)a; 14 (+) |
| 0.25±0.05 μM | 0.17±0.01 μMb | 0.1 μM | 19 (+) | |||||||
| Cerebrospinal fluid (CSF) | ND | ND | ND | ND | ND | ND | ND | ND | ND | 13 (+); 17 (+) |
| 19 (+) | ||||||||||
| LDL (ng/mL) | ND | ND | ND | ND | ND | ND | Det | ND | Det | 10 (+)a; 12 (+) |
| 17 (++); 19 (+) | ||||||||||
| Red blood cells (RBC) (ng/mL) | ND | ND | ND | ND | ND | ND | ND | ND | ND | 13 (+); 14 (+) |
| 17 (+); 19 (+) | ||||||||||
| White blood cells (WBC) (ng/mL) | ND | ND | ND | ND | ND | ND | ND | ND | Det | 13 (+); 17 (++) |
| 19 (+) | ||||||||||
| Total metabolites (mg): 36.33±5.94 (urine) and 5.60±1.54 (bile) | ||||||||||
- a Values (quantified using UV detection) are expressed as the mean±SE μg/mL. In PP, JP and L, the values are also expressed as μM. LDL, RBC and WBC values are expressed as ng/mL. In the case of urine and bile, the total volume of the fluid was known and the values expressed between parenthesis are total mg (mean±SE). In QqQ, when not quantified, the relative abundance of each metabolite was scored as high (+++); medium (++) and low (+) (these values compare the abundance for the same metabolite in the different fluids but not among different metabolites); Det, detected but not quantified; ND, not detected in UV. 1, RES diglucuronide-1; 2, RES diglucuronide-2; 5, RES sulfoglucuronide-1; 6, RES sulfoglucuronide-2; 7, RES trisulfate; 8, DH-RES trisulfate; 9, RES ribosyl sulfate; 10, RES 3-O-glucuronide; 12 DH-RES 3-O-glucuronide; 13, RES sulfate; 14, DH-RES sulfate; 15, RES disulfate; 16, DH-RES disulfate; 17, RES; 18, DH-RES; 19, cis-RES; n=4 except in an=1, bn=2 and cn=3.
Regarding the presence of metabolites in plasma, the concentration was higher in the PP (3.8±0.92 μM total metabolites) than in jugular vein plasma (JP; 1.19±0.36 μM). The most abundant metabolites were 10, 12 and 13 in both plasma from PP and JP. Compound 17 (RES) was also quantified in the case of PP (Table 4).
Regarding the rest of the analyzed samples, L (1.1±0.12 μM total metabolites) and CSF as well as LDL, RBC and WBC, the concentration of metabolites detected at this time point (6 h) was low (Table 4). To the best of our knowledge, this is the first report on the metabolic profile of RES and its metabolites in L, CSF and PP.
3.4 Distribution of RES-derived metabolites in systemic pig organs and tissues
Ten different RES-derived metabolites (including those derived from DH-RES) were detected in the 18 organs and tissues analyzed (Table 5). Six hours after intragastric administration of RES, approximately 0.5% of the initial quantity was recovered in these reservoirs in the form of metabolites (Fig. 1). For those organs and tissues in which we were able to quantify the total amount of the metabolites, the highest values were found in the K and Lv followed by the rest of organs and tissues in which much lower quantities were detected. Regarding those reservoirs with unknown total weight, such as lymph node (LYN), muscle and fat, the amount of metabolites detected was very low and expressed as μg/g (Table 5).
| Organ and tissue | Weight (g) | 10 | 13 | 17 | QqQ |
|---|---|---|---|---|---|
| Kidneys (K) | 293±26 | 0.610±0.207 | 0.167±0.095 | 0.020±0.014c | 5+6 (+); 12 (0.014)a; 14 (+) |
| Liver (Lv) | 1603±26 | 0.516±0.215 | 0.365±0.145c | 0.185±0.057c | 4 (+); 5+6 (++); 7 (++) |
| 12 (0.020)a; 14 (++), 19 (+) | |||||
| Lungs (Ln) | 483±17 | 0.038±0.006 | 0.026±0.004b, Deta | 0.042±0.014b, Deta | 7 (+); 12 (0.008)a; 14 (+) |
| 19 (+) | |||||
| Cerebellum (Cr) | 12±1 | 0.053 | ND | 0.001 | 5+6 (+); 7 (+); 12 (0.006)a |
| 13 (++); 14 (+++); 19 (+) | |||||
| Heart (H) | 420±37 | 0.020±0.004b, Detb | 0.011 | Detb | 7 (+); 12 (0.006)a; 14 (+) |
| Brain (Br) | 118±5 | ND | ND | 0.022, Deta | 10 (+); 13 (+) |
| Aorta tissue (A) | 38±4 | 0.019±0.003 | 0.006, Detb | 0.002, Detb | 7 (+); 12 (0.005)a; 14 (+) |
| Ovaries (Ov) | 40±5 | 0.008±0.001 | 0.004±0.0003 | 0.001±0.0002c, Deta | 5+6 (+); 7 (+); 12 (0.002)a |
| 14 (+) | |||||
| Uterus (Ut) | 88±4 | 0.009±0.001c | 0.005, Deta | 0.001, Deta | − |
| Urinary bladder tissue (UBl) | 60±10 | 0.012 | Deta | Deta | 14 (++) |
| Pancreas (Pn) | 103±8 | Deta | Deta | 0.005 | 4 (+); 5+6 (+); 10 (0.001)a |
| Spleen (Sp) | 122±14 | Detc | Detb | Deta | 10 (0.005)a; 12 (0.001)a |
| 14 (+) | |||||
| Total metabolites (mg)=2.21±0.77 | |||||
| Lymph node (LYN)* | – | 0.057, Deta | Detb | Detb | 12 (0.07)a; 14 (++) |
| Perirenal fat (PF)* | – | Deta | ND | 0.089±0.022 | 10 (0.017)a; 12 (0.023)a |
| 13 (++); 14 (++); 19 (+) | |||||
| Mesenteric fat (MF)* | – | 0.025, Deta | ND | 0.015, Deta | 12 (0.120)a; 13 (+); 14 (++) |
| 19 (+) | |||||
| Subcutaneous fat (SF)* | – | Detb | ND | 0.019, Deta | 10 (0.020)a; 12 (0.030)a |
| 13 (+) | |||||
| Longissimus dorsi (LgD)* | – | ND | ND | 0.02±0.003b | 10 (0.01)a; 12 (+) |
| 13 (++); 14 (++); 19 (++) | |||||
| Semimembranosus muscle (SM)* | – | ND | ND | ND | 13 (+); 14 (+) |
- a Results (quantified using UV detection) are expressed as total mg taking into account the weight of the organ or tissue (mean±SE). *Results are expressed as μg/g (the total weight was not determined). Det., detected but not quantified. ND, not detected in UV. In QqQ, when not quantified, the relative abundance of each metabolite was scored as high (+++); medium (++) and low (+) (these values only compare the abundance for the same metabolite in the different organs or tissues but not among different metabolites). 4, DH-RES sulfoglucuronide; 5, RES sulfoglucuronide-1; 6, RES sulfoglucuronide-2; 7, RES trisulfate; 10, RES 3-O-glucuronide; 12, DH-RES 3-O-glucuronide; 13, RES sulfate; 14, DH-RES sulfate; 17, RES; 19, cis-RES; n=4 except in an=1, bn=2, cn=3.
Cr, Lv, Ov and Pn were the organs in which a wider variety of metabolites were detected. It is also noteworthy the detection of six different metabolites in both perirenal fat (PF) and Longissimus dorsi muscle (LgD) (Table 5).
The most abundant metabolites in systemic pig organs and tissues were compounds 10 (RES 3-O-glucuronide), 13 (RES sulfate) and 17 (RES). RES (17) and compound 10 were the most abundant metabolites detected in pig Br and Cr, respectively (Table 5).
4 Discussion
A number of studies dealing with the bioavailabiliy and metabolism of the molecule trans-resveratrol (17, RES) in humans and in rodents suggest that RES has a low bioavailability as it is rapidly metabolized and excreted, mainly via U 5, 9, 10, 36. These studies report both inter- and intra-species differences together with a high variability in the metabolic profiles which may be caused by different variables such as gender, age, polymorphisms (phase I and II enzymes, transporters, etc.), different administration procedures, sample processing, analytical protocols and type of drugs and protocols used in anesthesia as recently discussed 7. The main RES-derived metabolites identified so far in humans and rodents are the sulfates, disulfates, trisulfates, glucuronides, diglucuronides and sulfoglucuronides derivatives as well as the microbiota-derived DH-RES and its corresponding sulfate and glucuronide conjugates 2, 6, 9, 10, 14, 16, 36-38. The recovery of RES metabolites in the previous studies, after oral RES administration, was very variable 36. This depended on the dose and route of administration, the analytical approach, the time of analysis post-administration as well as the organs and fluids finally analyzed. The highest recoveries reported (higher than 95%) were attained by measuring total radioactivity in both U and feces 10, 36.
Besides this amount of information, there is a lack of studies looking at RES and RES metabolites presence and specific distribution in most organs and tissues and thus the aim of the present study was to explore more in depth the metabolism of intragastrically administered RES in the pig. A complete study regarding the tissue distribution of RES metabolites, at different time-points, after RES administration is not common although achievable in rodents. Recently, a detailed study on the gastrointestinal or transit kinetics of RES in the GIT at different time-points after RES administration, has been reported in mice 39. However, given the size and characteristics of the pig as an animal model and the high-cost facilities required for the experimentation with this animal, it is reasonable to choose only one post-ingestion time. Our approach included a systematic analysis at a single time-point post-ingestion using different equipments. We finally chose 6 h as post-ingestion time analysis to increase the chances of finding microbiota-derived metabolites (DH-RES), based on the previous studies carried out in the pig with other polyphenols 20 as well as on the data obtained for RES in our previous mice study 39. Under this approach, we focused on the identification of possible new in vivo reservoirs for RES or derived metabolites. This could cover, at least partially, the gap regarding the lack of studies of metabolism and tissue distribution of RES in animals (such as the pig) physiologically much closer to humans than rodents. Bearing in mind all the above, we admit that this is a specific picture of RES and derived metabolites distribution in the pig 6 h after a single intragastric RES administration and therefore, the distribution as well as the type and amount of metabolites could differ at other time-points or upon chronic consumption.
Most of the metabolites identified in the present study are in agreement with those previously published in the literature. However, we have identified a number of new RES and DH-RES-derived metabolites, i.e. metabolites 3 (DH-RES diglucuronide), 4 (DH-RES sulfoglucuronide), 9 (RES ribosyl-sulfate) and 16 (DH-RES disulfate) (Table 1). Regarding compound 9, the occurrence of ribosyl conjugates, as another mechanism of xenobiotic metabolism in mammals, has been reported in several occasions on N-containing xenobiotics, and the N-ribosyl derivatives were tentatively described. In some cases, combination of this ribosyl substitution with the addition of hydroxyl-sulfate has also been reported 40. Our data suggest that this minor metabolite is a ribosyl derivative of RES by conjugation in one of the phenolic hydroxyls, and in addition, there is a sulfate conjugation on another phenolic hydroxyl. Therefore, this is the first time that pentosyl (most probably ribosyl) conjugates of phenolics, RES in this case, are reported in nature.
The RES-microbiota-derived metabolite dihydroresveratrol (18, DH-RES) and its conjugates have been largely underestimated. In 2004, Walle et al. 4 already highlighted the possible importance of these microbial compounds in the RES metabolism. This underestimation has been due to the use of UV detection at 305 or 320 nm (maxima of absorbance for RES spectrum), whereas the maximum UV for DH-RES is 276 nm, a bell-shaped spectrum with very low absorption at either 305 or 320 nm 10, 41. In addition, the molar absorptivity of DH-RES-like metabolites is significantly lower than that of the RES-like metabolites which hamper their detection.
In our study, the levels of DH-RES and its conjugates in the pig GIT from St to I were very low although a number of DH-RES metabolites (8, 12, 14, 18) were detected (Table 2). In contrast, RES metabolites were very abundant, specially in the I (Table 2). It is also noticeable the presence of a relatively high concentration of RES (17) in the St content 6 h after RES administation as well as the occurrence of cis-RES (19) and conjugates from both RES (5, 7, 13) and, to a lesser extent, from DH-RES (12, 14). The presence of RES conjugates in the St could be related to the previously reported ability of gastric cells to metabolize phenolics, including RES 42. However, the presence of a minor amount of microbial DH-RES metabolites in the St content cannot be easily explained. A tentative explanation could be the presence of a small amount of microbial groups from St to I involved in the formation of DH-RES from RES. The abundance of RES conjugates in the pig I could be due to the biliar secretion of the enterohepatic circulation together with an active efflux of conjugates to the lumen content (Table 2), which has been reported in the previous studies 4, 43. However, the RES profile changed from the I (with abundant conjugates such as sulfoglucuronides, glucuronides and sulfates) to Ce (mainly aglycones and higher amount of DH-RES than RES) (Table 3, Fig. 2). Although the distribution of the microbiota along the GIT involved in the conversion from RES (17) to DH-RES (18) is not known yet, the above changes could be explained by the significant increase in the Ce of the microbial groups involved in the production of DH-RES. Moreover, the lower presence of conjugates in the Ce and colon could be due to a decrease in the efflux of metabolites to the lumen caused by a lower amount of the RES transporters BCRP and MRP2, as previously reported in the rat 44. The expression of these transporters has been reported to be maximal in the I 45. Finally, another reason to explain the change in the metabolic profile from I to Ce could be the substantial increase in the glucuronidase and sulfatase activities associated with the large intestine microbiota 45. The predominant presence of DH-RES in the pig large intestine is in agreement with Alfaras et al. 46 who identified DH-RES as the most abundant metabolite in the colon rat upon oral RES administration. This is specially relevant considering the colon cancer chemopreventive activities attributed to RES. Recently, the distribution of RES and some of its conjugates has been reported in colorectal tissue of patients that consumed eight daily doses of resveratrol 47. Interestingly, RES and some conjugates (glucuronides, sulfates and sulfoglucuronide) were detected in that study. However, the possible occurrence of DH-RES and derived conjugates was not explored. Therefore, the presence of DH-RES and derived metabolites cannot be discarded in the colorectal tissue of the patients enrolled in the above study, which could add to the controverted issue of the identification of the actual molecules responsible for the biological activity attributed to RES.
In the present pig study, the occurrence of metabolites in the bloodstream was low at this post-ingestion time (Table 4), in accordance with the previous pharmacokinetic studies in both humans 6 and pigs 7. The PP mainly drains blood from the GIT to capillary beds in the Lv. Accordingly, the amount of RES metabolites in plasma from the PP approximately doubled the amount of metabolites present in jugular vein plasma (JP). In addition, the relative contribution of RES (17) to the metabolic profile was also more relevant in the case of PP than in JP (Table 4).
A rich metabolic profile with high concentration of RES metabolites was found in the pig B which reinforced the active enterohepatic circulation of RES (Table 4). In contrast, the presence of DH-RES metabolites in B was much lower (Table 4) which could be due either to a low enterohepatic circulation or to the post-ingestion time analysis (6 h), probably not enough for producing DH-RES metabolites. Both possibilities could explain the low amount of DH-RES metabolites detected in the content of the GIT from St to I after 6 h of intragastric RES administration (Table 2).
Interestingly, we also report here for the first time the metabolic profile of RES in L which approximately matched that of plasma (Table 4). Although the lymphatic system plays an important role in the absorption of dietary lipophilic compounds, it is not usually investigated in metabolic studies with polyphenols, in which many of them are amphiphilic molecules. Our results are in agreement with those reported by Terao and Murota 48, who detected quercetin metabolites mostly in the rat lymph after intragastric administration of quercetin. The authors also supported their results by the ability of the St to metabolize quercetin 42, 48, which was in accordance with our study in the presence of RES metabolites in the St. On the contrary, Chen et al. 49 mainly detected unmetabolized quercetin and rutin in the L upon intraduodenal administration of these flavonoids. The conclusions drawn by these authors have been previously questioned due to the methodological approach followed in that study 50.
As expected, the presence of RES and DH-RES metabolites in systemic organs and tissues was very small and amounted up to 0.5% of the initial dose of RES administered (Table 5 and Fig. 1). RES and derived metabolites were already extensively distributed in the pig body 6 h after RES administation with the highest amounts detected in the K and the Lv. The main compound detected in pig Br was RES (17) in accordance with the previous studies on rats 15. This is of interest in relation to the modulation of several blood flow variables in humans 45 min after RES (500 mg) intake 51 and indicates that RES itself could be the bioactive molecule in Br. Although the RES amount found in the pig Br was very low (0.18 μg/g; 22 μg in the entire Br), the detection of higher amounts cannot be discarded at shorter post-ingestion times.
The occurrence of RES and derived metabolites (including DH-RES and its conjugates) in perirenal, mesenteric and subcutaneous fats (PF, MF and SF, respectively) as well as in skeletal muscle such as LgD and SM muscles (Table 5) is important due to the increasing evidence of the link between RES intake and fat mobilization 52 as well as the attenuation of sarcopenia 53.
The most abundant ciculating RES metabolites have been reported to be RES 3-O-sulfate in humans 6 and RES 3-O-glucuronide in pigs 7. This is noticeable taking into account the physiological similarities between both species. In the present study, RES 3-O-glucuronide (compound 10) was the most abundant metabolite in plasma from jugular and portal veins (Table 4) which confirmed the previous report 7. In addition, compound 10 was also the main metabolite in systemic organs and U (Tables 4 and 5). In contrast, RES sulfate (compound 13) was the most abundant metabolite in the lumen of the pig GIT, especially in the I, followed by RES sulfoglucuronide isomer-2 (compound 6). A possible explanation could lie in the pig ATP-binding cassette transporters, involved in the disposal of resveratrol 54, which could be especiallly efficient in the efflux of sulfate conjugates from the enterocyte to the GIT lumen. This would decrease the absorption and systemic distribution of RES sulfates as well as increase their accumulation in the pig intestine, in agreement with the results found in our study.
In summary, we report here a comprehensive study regarding the metabolism and tissue distribution of RES in the pig, an animal physiologically close to humans. This is the first report regarding the occurrence of RES and DH-RES metabolites in aorta tissue, UBl, Cr, Ov, Ut, Pn, fat (PF, MF and SF) and muscle (LgD and SM). In addition, new RES and DH-RES metabolites are also reported here for the first time. It should be noted that the specific RES and DH-RES metabolites distribution described here could be different at other post-ingestion time-points. For example, shorter post-ingestion time analyses such as 1 h (approximately coincident with plasma Tmax) could yield lower DH-RES metabolites (if any), but possibly a higher RES metabolites detection in systemic organs. The research for reaching this challenging goal is warranted.
The information included in this study may be very useful to advance the knowledge about trans-resveratrol bioactivity and beneficial health effects as well as in the understanding of the mechanisms underlying its properties.
Acknowledgements
This work has been supported by the Projects CICYT-BFU2007-60576, Fundación Seneca de la Region de Murcia (grupo de excelencia GERM 06, 04486) and Consolider Ingenio 2010, CSD2007-00063 (Fun-C-Food). The authors are grateful to Dr. Alberto Quiles and Dr. Cándido Gutiérrez from the Veterinary School (Univ. Murcia, Spain) and José Luis Pineda from Proquimur S. L. (Murcia, Spain) for their assistance in some parts of the study. M. A. O. is a holder of a predoctoral JAE grant from CSIC and M. L. and R. L. of a JAE-DOC contract from CSIC (Spain).
The authors have declared no conflict of interest.




