Characterization of Variants of Uncertain Significance in ACADVL Gene From a Very–Long-Chain Acyl-CoA Dehydrogenase Deficiency Patient
Funding: This work was supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515220126), Shenzhen Maternity and Child Healthcare Hospital Basic and Applied Basic Research Foundation (FYA2022016), and the Sanming Project of Medicine in Shenzhen (SZSM202311005).
ABSTRACT
Background
Very–long-chain acyl-CoA dehydrogenase deficiency (VLCADD) is a rare disorder of long-chain mitochondrial fatty acid oxidation (FAO) caused by biallelic mutations in the acyl-CoA dehydrogenase very–long-chain (ACADVL) gene with autosomal recessive (AR) inheritance. Currently, the ACADVL gene has over 350 VUSs in the ClinVar database that require characterization to determine potential pathogenicity.
Methods
In this study, we performed functional studies and three-dimensional protein structure analysis to identify the pathogenicity of two ACADVL VUSs in a Chinese VLCADD patient with severe clinical symptoms.
Results
Biallelic variants in ACADVL gene c.1055T>C (p.Met352Thr) and c.1269G>A (p.Ser423=) were identified by whole-genome sequencing (WGS) and confirmed using Sanger sequencing. Both variants were recorded in ClinVar database with “conflicting interpretation of its pathogenicity” and need appropriate evidence for reclassification to guide family reproductive planning. Synonymous variant p.Ser423= could result in skipping of exon 12 through mini-gene splicing experiment testing. Further functional studies reveal that both variants yield a mild-to-severe decrease in ACADVL mRNA and protein expression in vitro.
Conclusion
In this study, we determined the pathogenicity of ACADVL variants c.1055T>C (p.Met352Thr) and c.1269G>A (p.Ser423=) via experimental and in silico analysis. The findings contribute to expanding the variant spectrum in the ACADVL gene, and exploring the pathogenicity of VUS may provide us with further understanding of the disease.
1 Introduction
Very–long-chain acyl-CoA dehydrogenase deficiency (VLCADD, OMIM# 201475) is an autosomal recessive (AR) disorder with an estimated incidence of 1:30,000 to 1:100,000 births (Andresen et al. 1996; Andresen et al. 1999). The deficiency includes a variety of clinical symptoms and a spectrum of severity that ranges from severe life-threatening illness in the newborn period to relatively mild disease first developing late in childhood or early adulthood (Spiekerkoetter 2010). The disease is caused by defects in the ACADVL gene, of very–long-chain acyl-CoA dehydrogenase (VLCAD), which catalyzes the initial step of mitochondrial β-oxidation of long-chain fatty acids, and abnormality in this step leads to the accumulation of high plasma levels of long-chain acylcarnitine conjugates, especially the tetradecenoyl (C14:1) acylcarnitine (Spiekerkoetter and Mayatepek 2010; Wilcken 2010). Treatment of VLCADD is aimed at preventing catabolism by avoidance of fasting, involving a diet with medium-chain triglycerides (MCT) or triheptanoin supplementation (Solis and Singh 2002; Yamada and Taketani 2019). VLCAD patients are identified through newborn screening (NBS) by measuring acylcarnitines using tandem mass spectrometry (MS/MS) in many countries, and follow-up genetic or functional testing of the ACADVL gene is recommended. The crystal structure of VLCAD is a homodimer associated with the inner mitochondrial membrane, and it provides the opportunity to investigate variant effects in a three-dimensional framework.
The genetic basis of VLCADD is complex. A growing number of variations have been reported, while some variants are VUSs, and few of them have been functionally determined (Hesse et al. 2018). Genotype–phenotype correlations have been documented; however, this correlation is still imperfect (Andresen et al. 1999; Coughlin 2nd and Ficicioglu 2010; Diekman et al. 2015; Li et al. 2020; Schiff et al. 2013). Previous studies have shown that a nonsense mutation in the ACADVL gene could result in a severe and early presentation of cardiomyopathy with a complete loss of enzymatic function, while some missense mutations were associated with both severe and attenuated clinical phenotypes with residual enzyme activity, indicating VLCAD deficiency is a highly heterogeneous disease (Gregersen et al. 2001; Hoffmann et al. 2012; Mathur et al. 1999; Watanabe et al. 2018).
A growing percentage of the population receives diagnostic genetic testing, and the return of VUS results can pose challenges for patients and providers, which proves to be an emerging problem (Kemp et al. 2023). VUSs have an uncertain significance because there is insufficient functional or clinical evidence to determine their pathogenicity. A potentially large number of VUS results leads to uncertainty of diagnosis. VUSs deposited in ClinVar have increased by ~5-fold by 2020, and 36% of variants in ClinVar are VUSs, and 5% are conflicting, which cannot be acted upon clinically, and many, if not most VUSs, are waiting for classification (Fowler and Rehm 2024). Currently, there are over 350 VUSs in the ACADVL gene in the ClinVar database (D'Annibale et al. 2022).
In this study, we collected two ACADVL VUSs in a Chinese VLCADD patient, and functional experiments were conducted, combining with protein structure modeling to determine the pathogenicity of the two variants. Our results enrich the ACADVL variant spectrum and lead to clinically meaningful changes in comprehensive genetic counseling and understanding of the genetic basis of the disease.
2 Materials and Methods
2.1 Ethical Compliance
The present study was approved by the hospital's Institutional Review Board, and written informed consents for study participation and publication of their clinical details and/or clinical images were obtained from all subjects and their legal guardians. Written informed consents for publication of their clinical details and/or clinical images were obtained from the parents.
2.2 Patient
The parents of the proband were referred for genetic counselling at Shenzhen Maternity and Child Healthcare Hospital. The present study was approved by the Hospital's Institutional Review Board, and written informed consents for study participation and publication of their clinical details and/or clinical images were obtained from all subjects and their legal guardians. All experiments were approved and performed in accordance with the guidelines and regulations of the Hospital's Institutional Review Board.
2.3 DNA Variant Analysis
The genomic DNA was extracted from peripheral blood of the patient and her parents. Trio-based whole-genome sequencing (WGS) was performed by Aegicare Technology Co. Ltd. (Shenzhen, China). Sanger sequencing was performed to confirm the result of WGS (Sanger sequencing primers, c.1055-F, GCAACCAAGTCCAACACAAAA, c.1055-R, GGAGACCTAAGCTAGCAAGTC, c.1269-F, TGATGAACTGACCCAGAACAA c.1269-R, AGCCACAAACAGCCGAAGAAT, Ref Seq NM_000018.4, GRCh38/hg38).
2.4 Protein Structure Analysis
The single amino acid exchanges caused by the mutation were introduced into the crystal structure of human ACADVL (PDB Accession Code: 2uxw) using the model-building program Coot (Emsley et al. 2010). The figures showing the point mutation and environment of the ACADVL protein were generated using the graphic program PyMol (Schiffrin et al. 2020).
2.5 Construction of ACADVL Minigene
One minigene was constructed for the variant c.1269G>A (p.Ser423=) predicted to affect the splicing of the ACADVL gene. Fragments containing exon 11, exon 12, exon 13, and adjacent introns were amplified from genomic DNA samples of the wild-type control and patient using overlapping extension PCR. After restriction enzyme digestion of the plasmid used by KpnI and EcoRI, the PCR products were inserted into the pcDNA3.1 plasmid. The 5′ and 3′ flanking regions (743 bp) of the splicing mutation site c.1269G>A were amplified and the PCR products were electrophoresed on a 1.8% agarose gel and sequenced (pcDNA3.1-F, CTAGAGAACCCACTGCTTAC, pcDNA3.1-R, TAGAAGGCACAGTCGAGG).
2.6 Plasmid Construction and Cell Transient Transfection
The coding sequence (CDS) of the full-length human ACADVL gene was cloned into the Phage vector (phage-ACADVL-Sal1-F, TGACGTCGACcATGCAGGCGGCTCGGATGGC, phage-ACADVL-Not1-R, CGACGCGGCCGCgGAAGCCAAGTGGGTTGCTG, Bioeagle Biotech Company). Two ACADVL variants were constructed by site-directed mutagenesis using the megaprimer technique. All of the constructed plasmids were sequenced completely to verify the introduction of nucleotide changes. Hela and 293T cells were cultured in DMEM (Gibco, #C11995500BT) supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, and 250 μg/mL fungizon at 37°C in a 5% CO2 humidified incubator. Hela and 293T cells were transfected with Lipofectamine 2000 according to the manufacturer's instructions.
2.7 ACADVL Expression Analysis
Total RNA was extracted from 293T and HeLa cells after transfection and was reverse transcribed to cDNA using the GoScript Reverse Transcription System kit (Promega). QPCR was performed on the ABI QuantStudio5 system using the SYBR Green Realtime PCR Master Mix kit (ACADVL-qPCR-F, AGATTACGCTGGATCCGCTA, ACADVL-qPCR-R, AGGGGTGGGAATCTGACTTG, Thermo). The relative abundance of target mRNA was normalized to wild type, and mRNA expression levels in cells expressing phage-ACADVLwt were set to 100%. The mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Cell supernatants from 293T cells containing 20 μg of total protein were fractionated using RIPA lysis buffer. The antibody used was the anti-HA tag antibody (Dai An Biotech Co.).
3 Results
3.1 Patient and Genetic Analysis
The proband was a 4-month-old girl born to healthy nonconsanguineous Chinese parents. She was born at 39 weeks of gestation following an uncomplicated pregnancy. The birth weight and frontal occipital circumference were measured at 3100 g and 32.6 cm, respectively. The Apgar score was 10 at 1 and 5 min after birth, which was normal. She presented with feeding difficulty, hypomyotonia, and was unable to hold her head at 3 months old. No significant abnormalities were found in the emergency biochemical tests. At 4 months, she developed vomiting with low fever, lasting for 2 days, with a highest body temperature of 37.9°C. The baby had a moaning respiration and slight shortness of breath. She was admitted to the hospital with fever, vomiting, groan, drowsiness, and tachypnea, and soon she showed loss of consciousness, no limb movement, pale complexion, cyanotic lips, and went into coma with acute metabolic crisis and died suddenly. Emergency blood routine test, blood gas analysis, liver function tests, myocardial enzymes, and electrolyte tests revealed multiple abnormal results.
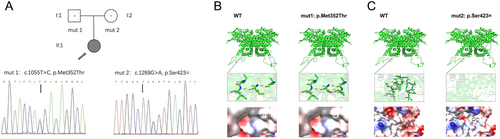
Peripheral blood samples were collected from the proband and her parents for genetic analysis with written informed consent. WGS used the MGISEQ-2000RS platform with an overall coverage depth of 31×, and in silico variant prediction analysis was performed. Compound heterozygous variants c.1055T>C (p.Met352Thr) (mut1) and c.1269G>A (p.Ser423=) (mut2) in ACADVL (NM_000018.4) were identified in the patient as VUSs. Sanger sequencing confirmed that the patient's father was a c.1055T>C (p.Met352Thr) heterozygous variant carrier and her mother was c.1269G>A (p.Ser423=) heterozygous variant carrier (Figure 1A). C.1055T>C (p.Met352Thr) and c.1269G>A (p.Ser423=) are conflicting classifications of pathogenicity recorded in the ClinVar database with no functional analysis. Characterization of variants is mentioned in Table 1.
| Clinical signs | ACADVL variants | Type of variants | Inheritance | Variant origin | ClinVar variant classification* | Pathogenicity in present study | 3-Dimensional analysis (PDB: 2uxw) | |
|---|---|---|---|---|---|---|---|---|
| Structural effects | Electrostatic potential change | |||||||
| 4-month-old girl, feeding difficulty, hypomyotonia, febrility, vomiting, tachypnea, and sudden death. | c.1055 T>C, p.Met352Thr (mut 1) | Missense | Compound heterozygous | Paternal inherited | Conflicting classifications of pathogenicity [likely pathogenic (1); uncertain significance (2)] | Pathogenic (PM2 + PM3 + PP3 + PP4 + PS3) | M352 is located at α-helical domain 2 region in a buried hydrophobic pocket, two hydrogen bonds were formed between Thr352 side chain and Leu348 (2.9 Å), Ala349 (3.0 Å), respectively, small bond distance change, variant may lead to less stable protein. | Slightly neutral trend but with very limited change. |
| c.1269G>A, p.Ser423= (mut2) | Synonymous | Maternal inherited | Conflicting classifications of pathogenicity [likely pathogenic (1); uncertain significance (1)] | Likely pathogenic (PM2 + PP4 + PS3) | S423 lies in the middle of the monomer as part of an α-helix, segment deleted from Ser395 to Ser423, which affected the highly conserved motif (Gly-Gly-x-Gly) close to the FAD binding pocket, may hinder binding and protein stability. | Changed from negative-charged to neutral-charged. | ||
- Note: The sequence variation position for ACADVL transcript is NM_000018.4. The amino acid position for ACADVL protein is NP_000009.1.GRCh38/hg38.
- * The bracket indicates the submitted data number in ClinVar.
3.2 Structure Effects and Electrostatic Potential Changes of Two Mutations in the Crystal Structure of Human ACADVL (PDB: 2uxw)
Two variants were further analyzed based on the recently determined crystal structure of VLCAD (PDB: 2uxw) (McAndrew et al. 2008). The three-dimensional analysis revealed that the variants could induce profound structural alterations or electrostatic potential changes. The conserved residue M352 is located at α-helical domain 2 region in a buried hydrophobic pocket. Two hydrogen bonds are formed between the Thr352 side chain and Leu348 (2.9 Å) and Ala349 (3.0 Å), respectively. The structure of the side chain is changed. Electrostatic potential has a slightly neutral trend but with very limited change (Figure 1B). S423 lies in the middle of the monomer as part of an α-helix, and the segment is deleted from Ser395 to Ser423. The change affects the highly conserved motif (Gly-Gly-x-Gly) close to the FAD binding pocket, which may hinder protein binding and stability. The electrostatic potential is changed from negatively charged to neutral (Figure 1C). Human ACADVL protein structure is predicted by the model-building program Coot. The figures showing the point mutation and environment of the ACADVL protein were generated using the graphics program PyMol. The analysis results are mentioned in Table 1.
3.3 Splicing Analysis of c.1269G>A (p.Ser423=) Synonymous Variant in the Minigene
The minigene splicing products were analyzed by PCR amplification with plasmid-specific primers and visualized with polyacrylamide gel electrophoresis. The RT-PCR products of total RNA, which was extracted from HeLa and 293T cells transfected with minigene constructs, produced two splicing products of approximately 440 bp for the wild-type construct and one splicing product of approximately 353 bp for the mutant construct, respectively. Sequence analysis of the PCR fragment confirmed that the 440 bp product corresponded to the expected normal splicing product containing exon 11, exon 12, and exon 13. The 353 bp product was generated by alternative splicing and corresponded to a shorter RNA transcript, lacking ACADVL exon 12 (Figure 2A,B).
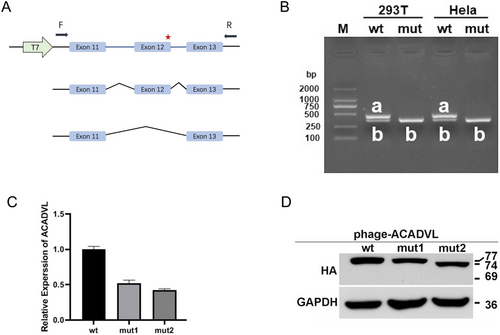
3.4 Expression of ACADVL mRNA and Protein in Transfected Cells of ACADVL Variants
To examine whether the variants affected the ACADVL transcription and translation, two variants were investigated in vitro, and subsequent analysis of the ACADVL protein expression was performed. ACADVL variants c.1055T>C (p.Met352Thr) (mut1) and c.1269G>A (p.Ser423=) (mut2) decreased mRNA expression to 0.52 and 0.43, respectively, compared to wild-type ACADVL (Figure 2C). Western blot analysis showed that mut1 and mut2 induced a mild reduction in ACADVL protein level compared with wild-type in phage-ACADVLwt (Figure 2D).
4 Discussion
In this study, we report the identification and evaluation of two ACADVL variants (c.1055T>C (p.Met352Thr) and c.1269G>A (p.Ser423=)) from a Chinese patient with VLCAD deficiency. VLCAD deficiency is an autosomal recessive inborn error of fatty acid oxidation, which is caused by variants in the ACADVL gene. The disorder could be screened by measuring characteristic acylcarnitine profiles using tandem mass spectrometry (MS/MS). Assays of VLCAD activity or enzyme testing can be performed in fibroblasts and lymphocytes. The patient in our study presented with an acute metabolic crisis with a neonatal early-onset form and died in infancy. We obtained the patient's blood sample other than fibroblasts, and WGS identified two VUSs in the ACADVL gene, which potentially provided a diagnosis of VLCADD. We performed functional and structural analyses to provide additional lines of evidence to reclassify these two VUSs as pathogenic and likely pathogenic, respectively.
The gene ACADVL (MIM 609575) is located on chromosome 17p13.1, containing 20 exons and 19 introns. The total length of the gene is 5.4 kb, encoding 655 amino acids. There are various variants, including missense mutations, nonsense mutations, splicing mutations, and frameshift mutations; among them, missense mutations account for the largest proportion. More than 500 pathogenic or likely pathogenic variants in the ACADVL gene have been recorded in the ClinVar database, and the number of newly identified mutations is constantly increasing. The genotype–phenotype correlations have been documented; however, this correlation is imperfect. Previous studies have shown that a nonsense mutation in the ACADVL gene could result in a severe and early presentation of cardiomyopathy with complete loss of enzymatic function, while some missense mutations were associated with both severe and attenuated clinical phenotypes with residual enzyme activity. Of note, the onset time and symptoms can differ even among siblings, indicating VLCAD deficiency is a highly heterogeneous disease, and environmental factors or genetic modifiers may contribute to individual clinical diversity (Diekman et al. 2015; Watanabe et al. 2018). Molecular sequencing alone may not be sufficient to confirm the newly identified mutations, and accurate interpretation of VUS is crucial for clinical genetic counseling and understanding the genetic basis of the disease.
Synonymous mutations have been considered to be silent mutations and not to affect amino acid sequence. However, increasing evidence indicates that many synonymous mutations in coding sequences have been shown to alter RNA processing and subsequently cause changes in protein expression, conformation, and function (Manning and Cooper 2017; Sauna and Kimchi-Sarfaty 2011). In addition to generating misfolded proteins that are less active, the location of the synonymous substitutions in the gene is important. Synonymous variant c.1269G>A (p.Ser423=) (mut2) falls at the last nucleotide of exon 12 in the ACADVL gene, which is part of the consensus splice site for this exon and has been classified as VUS with conflicting interpretation in the ClinVar database (Andresen et al. 1999; Hesse et al. 2018). Minigene analysis was used to accurately predict that exon 12 was skipped, and the structural analysis showed regions of helix H and helix I are partly dissolved and resulting in a different conformation of the VLCAD protein with partial helix H and I, which could negatively affect FAD binding. Minigene assays have proven to be a robust and valuable experimental approach to predict splicing outcomes of variants in the absence of patient RNA, which provided key data for the interpretation of VUSs in patients (Baralle et al. 2009; Bueno-Martinez et al. 2022).
Two VUS variants are further demonstrated to have pathogenicity according to the evidence from transcriptional and translational levels with plasmid construction phage-ACADVLwt/mut, and our results facilitated the parents of the proband for their further assisted reproductive program. Both variants lead to the degradation of ACADVL mRNA and a reduction in ACADVL protein expression level. Mut 1 changed the structure of the side chain and may lead to a less stable protein. Mut 2 lies in the highly conserved motif and affects FAD binding and electrostatic potential. This variant was recently reported in a Chinese VLCADD patient, which was classified as VUS, potentially inhibiting the binding of very–long-chain fatty acids in the substrate binding pocket (Lin et al. 2020). Structural changes in the mRNA can affect its stability and translation efficiency and ultimately affect phenotypic variation. Possible adverse effects of variants on the function of amino acid residues in three-dimensional modeling lead to insight into the molecular mechanism of the disease.
VUSs are increasingly reported with widespread use of next generation of sequencing (NGS), and the prevalence of a VUS finding in clinical genetic test reports can reach up to 53%, which poses challenges to diagnostic legitimacy and genetic counselling (Johnson et al. 2022; Rehm et al. 2023). Reclassifying VUSs not only provides greater clarity for a “patient in waiting” but also assists in genetic counselling and personalized clinical management, but also further our understanding of the disease (Horowitz et al. 2024). In ClinVar database, more than 150 conflicting classification variants in ACADVL gene need to appropriate follow-up assessment with further evidence. Plasmid construction phage-ACADVLwt/mut model can be used similarly for expression study without the presence of DNA or the need for an invasive procedure such as a skin biopsy. Based on our study, we propose that variants in CinVar, c.1055T>C, p.Met352Thr and c.1269G>A, p.Ser423=, be reclassified from VUSs to pathogenic and likely pathogenic, respectively.
In conclusion, we combined genetic, functional data, and available protein structural models to determine the pathogenicity of two VUSs in the ACADVL gene, which yields insights into the molecular and cellular mechanisms by which variants act, improving our understanding of disease. Functional characterization of VUSs will become more important in genetic counseling, and exploring the pathogenicity of VUSs may provide us with further understanding of the disease. To our knowledge, we first reported the reclassification of a synonymous variant and VUSs in the ACADVL gene identified in a Chinese VLCADD patient.
Author Contributions
Qin Wang: Design of the work, experimental investigations, project administration, funding acquisition and original draft writing. Jingxin Yang and Yong Xu: Minigene, ACADVL expression, data curation. Xingping Li and Nan Jiang: Sequencing and 3-D bioinformatics analysis, data curation, visualization. Jiansheng Xie: Clinical investigations of patients and their relatives.
Acknowledgments
We thank all participants for their time and cooperation. We thank all co-authors for their work and contribution to this study.
Ethics Statement
The present study was approved by the hospital's Institutional Review Board, and written informed consents for study participation and publication of their clinical details and/or clinical images were obtained from all subjects and their legal guardians. Written informed consents for publication of their clinical details and/or clinical images were obtained from the parents.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




