Two Nonsense GLI3 Variants Are Identified in Two Chinese Families With Polydactyly
Funding: This study was supported by the Key R&D Plan of Shandong Province (2017GSF218072), Shandong Provincial Natural Science Foundation Youth Project (ZR2021QC196), and Shandong Provincial Natural Science Foundation Youth Project (ZR2021QH268).
ABSTRACT
Background
Polydactyly is a prevalent limb deformity with an autosomal dominant inheritance pattern, manifesting in both syndromic and nonsyndromic forms. It exhibits significant etiological and clinical diversity. This study aims to identify the pathogenic cause in two patients with sub-PHS (sub-Pallister–Hall Syndrome) and PAP (postaxial polydactyly), respectively, from two Chinese pedigrees.
Methods
Exome sequencing was performed on patients to screen for potential pathogenic variants. Subsequently, these variants were validated by Sanger sequencing. The c.3342dupC and c.4431dupT mutant plasmids were transfected into HEK293T cells, and the effects of GLI3 mutations on transcription and protein levels were analyzed via qRT-PCR and Western blot. Additionally, Swiss Model was utilized to predict the effects of mutations on protein tertiary structure.
Results
The mutations of GLI3 (NM_000168.6: c.3342dupC; p. A1115Rfs*14) (NM_000168.6: c.4431dupT; p. Glu1478Ter) were identified in affected individuals. These mutations were present exclusively in the patients and absent in the healthy individuals. No significant difference in transcription levels between the mutations and wild type was observed. Functional analysis revealed that the truncated variants p. A1115Rfs*14 and p. Glu1478Ter exhibited reduced molecular weight and potential functional impairment due to protein retention.
Conclusion
The mutations p. A1115Rfs*14 and p. Glu1478Ter in GLI3 may account for sub-PHS and PAP in the two patients, respectively. This finding expands the mutation and phenotype spectrum associated with GLI3, providing valuable insights for the clinical diagnosis of polysyndactyly.
1 Introduction
Polydactyly is among the most common congenital limb malformations, typically inherited in an autosomal-dominant manner (Chen et al. 2019). The condition is characterized by additional digits on the hands or feet, with a higher prevalence in the upper limbs compared to the lower limbs. It occurs in approximately 0.3–3.6 per 1000 live births (Nguyen et al. 2024). Polydactyly is classified into nonsyndromic and syndromic forms based on the presence or absence of additional organ abnormalities or congenital defects. Nonsyndromic polydactyly is further classified into preaxial, central, and postaxial types based on the location of the extra digit (Umair et al. 2019). Among them, postaxial polydactyly (PAP; OMIM 174200) is the most common of these classifications. PAP is further classified into Type A (PAPA), characterized by a well-developed extra digit, and Type B (PAPB), where the additional digit is poorly developed (Nguyen et al. 2024). Syndromic polydactyly includes conditions such as Pallister–Hall Syndrome (PHS; OMIM 146510) and Greig Cephalopolysyndactyly Syndrome (GCPS; OMIM 175700) (Al-Qattan et al. 2017; Biesecker 2008).
Several genes have been implicated in polydactyly, including HOXD13 (OMIM 142989), GLI3 (OMIM 165240), GJA1 (OMIM 121014), FBLN1 (OMIM 135820), and LMBR1 (OMIM 605522) (Chaudhry et al. 2017). Specifically, GLI3 is associated with polydactyly. As a member of the GLI transcription factor family, GLI3 plays a significant role in regulating the SHH (Sonic Hedgehog) signaling pathway (Letelier et al. 2021). An extensive array of biological functions is attributed to SHH, including the establishment of left–right body asymmetry, central nervous system development, somite patterning, eye formation, and limb morphogenesis (Ming et al. 1998). Several mutations in GLI3 have been reported to cause polydactyly, such as c.1927C>T; p. Arg643*, c.3414delC; p.H1138Qfs68, c.4478delG; p.S1493Tfs18, and c.2374C>T; p. Arg792Ter (Guo et al. 2023; Kariminejad et al. 2020; Patel et al. 2021; Wang et al. 2024).
In this study, we have identified two frameshift mutations in GLI3 (c.3342dupC; p. A1115Rfs*14) and (c.4431dupT; p. Glu1478Ter) in the patients who were diagnosed with sub-PHS and PAPA, respectively. Notably, the c.3342dupC mutation is a novel variant that has not been previously reported and has been identified for the first time in association with sub-PHS, underscoring its potential pathogenicity. Bioinformatics and functional analysis suggest that the mutations may underlie the disease phenotypes in the affected patients. This discovery broadens the mutational and phenotypic spectrum of GLI3, providing new insights into its role in limb development and polydactyly.
2 Materials and Methods
2.1 Survey Object
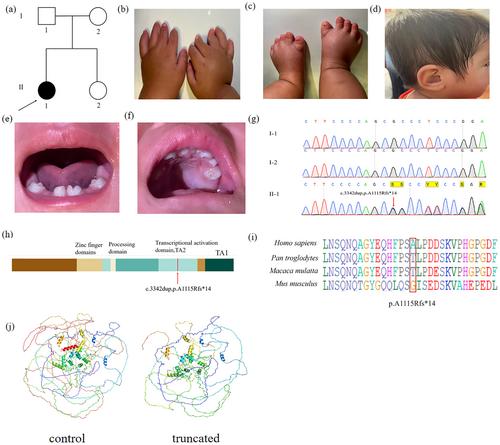
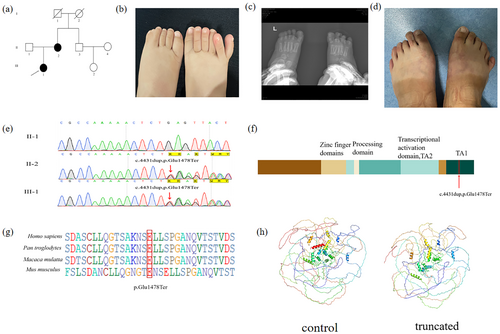
Six individuals from two families were recruited to study at Linyi People's Hospital of Shandong Province. In Family 1, three members were enrolled, including one affected individual (II-1) and two unaffected individuals (I-1, I-2) (Figure 1a). In Family 2, three members were also enrolled, comprising two affected individuals (III-1, II-2) and one unaffected individual (II-1) (Figure 2a). Peripheral blood samples were collected from all six participants for further analysis.
2.2 Whole-Exome Sequencing and Sanger Sequencing
Genomic DNA was isolated from peripheral blood samples using the Qiagen Blood DNA Mini Kit (Qiagen, No. 51106) following standard protocols. The DNA was fragmented using a Covaris ultrasonicator. Library preparation was carried out with the VAHTS Universal DNA Library Prep Kit for Illumina V3 (Vazyme, ND607-01), according to the manufacturer's instructions. Specific targeted regions were captured through probe hybridization, followed by sequencing on the BDA DNBSEQ-T7 platform. The resulting high-throughput sequencing data, in FASTQ format, underwent quality filtering. Sequence reads were aligned to the human reference genome using the BWA algorithm, and variants were called through the GATK pipeline. Variant annotation was performed using Annovar against public mutation databases. The pathogenicity of the identified mutations was evaluated according to ACMG guidelines. The identified mutations were validated via Sanger sequencing. PCR primers were designed based on the GLI3 (OMIM 165240) reference sequence (NM_000168.6). Primers used for Sanger sequencing are as follows:
GLI3-3342-F: 5′-TTCCACTCGTCCCCCTG-3′
GLI3-3342-R: 5′-GGTTGCTGTTCTCCCCG-3′
GLI3-4431-F: 5′-CCCATCCGCTGTGCTCTAAT-3′
GLI3-4431-R: 5′-CGACATCAGGCTGGAGTGGT-3′
PCR amplification was conducted for 30 cycles using the following conditions: 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by a final extension at 72°C for 5 min. The PCR products were then analyzed by agarose gel electrophoresis and purified using the NucleoSpin Gel and PCR Cleanup (MACHEREY-NAGEL). Sequencing was performed on the ABI 3500XL platform (Applied Biosystems).
2.3 Plasmid Construction
Gene synthesis techniques were employed to generate the constructs pcDNA3.1(+)-GLI3-WT, pcDNA3.1(+)-GLI3-c.3342dupC, and pcDNA3.1(+)-GLI3-c.4431dupT (Figure 3a). HEK293T cells were seeded in 3.5 cm dishes at approximately 30% confluence and cultured for 24 h. The cells were co-transfected with 1.5 μg of each of the pcDNA3.1(+)-GLI3-WT, pcDNA3.1(+)-GLI3-c.3342dupC, and pcDNA3.1(+)-GLI3-c.4431dupT using Lipofectamine 2000 (Invitrogen,11668019) for DNA transfection. Cells were collected 48 h after transfection.
2.4 qRT-PCR and Western Blot
RNA was extracted using TRIzol (Invitrogen, 15596026CN) and cDNA was synthesized from 1 μg of total RNA using the HI Script II Q RT Super Mix for qPCR (+gDNA wiper) kit (Vazyme, R223-01). mRNA levels in wild-type, mutant, and control samples were quantified using the Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Q712-02) with GAPDH as the internal housekeeping gene. Quantitative real-time PCR (qRT-PCR) was performed, and data were analyzed using the 2−ΔΔCT method. The qRT-PCR primers are as follows:
GAPDH-F: AGATCCCTCCAAAATCAAGTG
GAPDH-R: GGCAGAGATGATGACCCTTTT
GLI3-F: TTCCCCAGGTGCTAATCAGG
GLI3-R: CTCATGTCCCGATAGCCAT
Transfected cells were lysed in RIPA buffer (Thermo, 89900) on ice for 30 min to extract total protein, followed by centrifugation to remove cell debris. Protein concentrations were measured using the BCA Protein Assay Kit (Beijing Beyotime Biotechnology, P0012). Equal amounts of protein were resolved on an 8% SDS-PAGE gel and transferred to a PVDF membrane. After blocking with 5% nonfat milk in TBST buffer for 2 h at room temperature, the membrane was incubated overnight at 4°C with anti-HA antibody (D11004, Sangon Biotech) and anti-GAPDH antibody (D190090, Sangon Biotech), both diluted in blocking buffer. The membrane was then incubated with HRP-conjugated secondary antibodies (Sangon Biotech, H6162, H6161) for 2 h at room temperature. Protein bands were visualized using chemiluminescence detection.
2.5 Ethical Approval
This study was performed at Linyi People's Hospital of Shandong Province, China. The institutional ethics review committee (Ethics Committee of Linyi People's Hospital) approved the research protocol, and all participants provided written informed consent.
3 Results
3.1 Clinical Features
The proband from Family 1 is a 2-year-old girl. She is 76 cm tall and weighs 7 kg (< −3 SD). The clinical manifestations include short limbs, postaxial polydactyly (OMIM 174200) of the right hand, nail hypoplasia, low-set ears, and dental dysplasia (Figure 1b–f, Table 1). Furthermore, the patient was born with anal atresia, which has been surgically corrected. Based on these observed characteristics, the patient meets the clinical criteria of Pallister–Hall Syndrome (OMIM 46510). Both proband's parents exhibit normal hands and feet. The family history is consistent with an autosomal recessive inheritance pattern.
| Features | ||||||
|---|---|---|---|---|---|---|
| Individuals | Sex | Age | Left hand | Right hand | Left foot | Right foot |
| Family 1 | ||||||
| I-1 | M | 26 | — | — | — | — |
| I-2 | F | 25 | — | — | — | — |
| II-1 | F | 2 | — | PAP | — | — |
| Family 2 | ||||||
| II-1 | M | 44 | — | — | — | — |
| II-2 | F | 41 | — | — | PAP | PAP |
| III-1 | F | 2.5 | — | — | PAP, S | PAP |
- Abbreviations: —, feature absent; F, female; M, male; PAP, postaxial type; S, syndactyly.
Another proband from Family 2 is a 2.5-year-old girl. She exhibits postaxial polydactyly (OMIM 174200) on both feet. The patient underwent polysyndactyly correction surgery at the age of one-and-a-half years, and postoperative imaging examination showed that the surgical site had healed well with no complications (Figure 2b,c). Additionally, the proband's mother was diagnosed with postaxial polydactyly type A (PAPA) affecting both feet (Figure 2d, Table 1), while the proband's father exhibits normal hands and feet. The family history aligns with an autosomal inheritance pattern.
3.2 Mutation Analysis
Peripheral blood DNA samples from the subjects in this study were sent to BGI Genomics in China for exome sequencing. The resulting data were analyzed and compared against multiple databases, including UCSC, CCDS, Ensembl, SIFT, COSMIC, and ESP. To identify SNPs and Indels, novel mutations were flagged during the analysis. Known human disease genes and pathogenic mutations were annotated using the Human Gene Mutation Database. The pathogenicity of each SNV and Indel was assessed using predictive software such as PolyPhen-2, SIFT, MutationTaster, CADD, and GERP. The exome sequencing data were then curated for further analysis.
In Pedigree 1, a novel frameshift mutation in GLI3 c.3342dupC (p. A1115Rfs*14) was identified in the proband (Figure 1g). In Pedigree 2, a frameshift mutation, GLI3 c.4431dupT (p. Glu1478Ter), was revealed in both the proband and her mother (Figure 2e). Sanger sequencing confirmed the presence of these mutations in affected relatives but not in unaffected individuals, indicating co-segregation with polydactyly in both pedigrees. Conservation analysis of the amino acid sequences revealed that the alanine residue at position 1115 and the glutamic acid residue at position 1478 of GLI3 are conserved across various primate species, as analyzed using BioEdit (Figures 1i, 2e). Through software analysis, it is predicted that these mutations could result in premature termination of protein translation, consequently impairing protein function (Figures 1j, 2h).
The GLI3 protein is composed of the following domains: N-terminal zinc-finger domain (ZFD, aa462-645), a central protein-cleaving site (PC, aa703-740), and C-terminal region housing two transactivation domains: TA2 (aa1044-1322) and TA1 (aa1376-1580). Mutations in the N-terminal region are predominantly associated with Greig cephalopolysyndactyly syndrome (GCPS); truncating mutations in the middle third of the gene typically lead to Pallister–Hall syndrome (PHS), while mutations in the C-terminal region are primarily linked to both GCPS and preaxial polydactyly (PAP). The identified mutations p. A1115Rfs*14 and p. Glu1478Ter are located at TA2 and TA1, respectively (Figures 1h, 2f).
According to ACMG guidelines, the GLI3 (c.3342dupC; p. A1115Rfs*14) variant was classified as a variant of uncertain significance based on the criteria PVS1_S + PM2_P. In contrast, the GLI3 (c.4431dupT; p. Glu1478Ter) variant was classified as likely pathogenic, supported by the criteria PVS1_Strong + PS4_Supporting + PM2_Supporting.
3.3 Functional Study
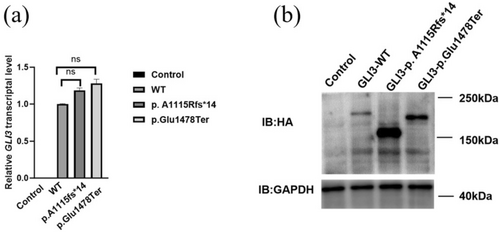
We examined the impact of GLI3 mutations on transcriptional activity. qRT-PCR analysis revealed that the transcription levels of wild-type (WT), c.3342dupC, and c.4431dupT constructs were significantly elevated compared to the control. However, no significant differences in transcription levels were detected compared to the wild-type GLI3 (Figure 3a). The data suggest that GLI3 mutations may not significantly impair its transcription.
To further investigate the effects of these mutations on GLI3 protein, we conducted western blot analysis. Notably, the p. A1115Rfs*14 (Lane 3) and p. Glu1478Ter proteins (Lane 4) were significantly shorter than the wild-type protein (Lane 2), which was consistent with the expected truncation due to premature translation termination. Specifically, the molecular weight of the wild-type GLI3 protein was approximately 192 kDa, whereas the p. A1115Rfs*14 and p. Glu1478Ter proteins were around 160 and 180 kDa, further confirming the truncation effects caused by these mutations (Figure 3b). Both c.3342dupC and c.4431dupT are predicted to be likely pathogenic (Figures 1j, 2h).
In western blot analysis, additional protein bands were observed, suggesting the presence of residual GLI3 fragments despite the truncation. These residual bands may represent alternative forms of truncated GLI3, such as partially degraded fragments or splice variants, which may retain some functional capacity. Similar observations were reported in other studies where truncated GLI3 proteins were similarly found to disrupt cellular localization and functional dynamics (Humke et al. 2010). These residual truncated forms could interfere with the normal processing of GLI3, particularly in its interactions with SUFU (Suppressor of Fused), thus impairing SHH signaling and contributing to the observed phenotypes. These findings suggest that truncation-induced retention of GLI3 could significantly contribute to SHH pathway dysregulation and limb malformations, as reported in previous studies (Kise et al. 2009).
4 Discussion
Polydactyly is one of the most frequently encountered congenital limb deformities in clinical practice. Mutations in GLI3 have been closely associated with polydactyly, with numerous mutations identified across affected individuals (Guo et al. 2023; Zhao et al. 2019). These mutations contribute to a spectrum of GCPS, PHS, and non-syndromic polydactyly. In sporadic cases, polydactyly often affects one limb, whereas familial cases tend to involve both limbs bilaterally (Chen et al. 2019). Johnston et al. (2010) summarized the diagnostic criteria for PHS, which include the presence of mesoaxial polydactyly and hypothalamic hamartoma. A diagnosis of “sub-PHS” may be considered when only one of these features is present, alongside at least one additional feature such as epiglottic fissure, anal atresia, microfingernails, hypopituitarism, growth hormone deficiency, or incomplete genital development. According to these criteria and the literature, the proband from family 1 can be diagnosed with sub-PHS. A well-established correlation exists between the position of GLI3 mutations and the resulting phenotypes. Truncating mutations at the N- and C-termini often lead to GCPS and PAPA, while mutations in the middle third of the protein are linked to PHS (Johnston et al. 2005; Kise et al. 2009). In this report, the GLI3 c.3342dupC located in the middle one-third led to sub-PHS, while the GLI3 c.4431dupT located at the C-terminus resulted in PAPA. These findings further reinforce previously reported genotype–phenotype correlations (Al-Qattan et al. 2017; Johnston et al. 2010; McClelland et al. 2022).
GLI3 exists in two functional forms: the full-length activator (GLI3A) and the truncated repressor (GLI3R) (Patel et al. 2014). In the absence of Sonic Hedgehog signaling (SHH), GLI3 undergoes proteolytic cleavage to generate its repressor form, which suppresses target gene expression. Upon SHH pathway activation, this processing is inhibited, allowing GLI3A to accumulate and activate target genes. The equilibrium between GLI3A and GLI3R is critical for proper limb development, and disturbances in this balance can result in congenital abnormalities, including polydactyly (Hill et al. 2009; Kantaputra and Carlson 2019). In our study, we identified the GLI3 truncating mutations p. A1115Rfs*14 and p. Glu1478Ter, which may disrupt this delicate balance by prematurely halting protein synthesis. These mutations may affect the production of both the full-length activator (GLI3A) and the truncated repressor (GLI3R), resulting in dysregulation of the SHH pathway. Furthermore, SUFU (Suppressor of Fused) was first identified as a suppressor in fruit flies with fused mutations and later confirmed to be a general pan-Gli-binding protein that stabilizes all three GLI proteins (Han et al. 2024; Kise et al. 2009). As a negative regulator of the SHH signaling pathway, SUFU binds to GLI3, maintaining its inactive state (Ding et al. 1999; Dunaeva et al. 2003; Humke et al. 2010). In the absence of SHH signals, the SUFU-GLI3 complex converts GLI3 into the repressive form, GLI3R, inhibiting the expression of target genes. Upon SHH signal activation, SUFU dissociates from GLI3, allowing GLI3 to be converted into the active transcription factor GLI3A (Han et al. 2024). Xiang et al. (2020) reported a case of polydactyly caused by GLI3 protein truncation, which impaired SUFU binding and disrupted SHH signaling. Similarly, the p. A1115Rfs14 and p. Glu1478Ter mutations may also interfere with GLI3's ability to interact with SUFU, further contributing to SHH pathway dysregulation.
Western blot analysis revealed residual truncated GLI3 protein bands, suggesting these truncated forms may retain partial stability or preserve critical protein interaction domains. This residual stability could result from post-translational modifications that protect the truncated proteins from complete degradation or enable aberrant interactions within the cellular environment. These findings raise critical questions about the role of residual proteins in disrupting GLI3 function and dysregulating SHH signaling. These findings not only reinforce established genotype–phenotype correlations but also introduce novel insights into the mechanisms by which truncated GLI3 proteins may partially maintain their function or interact within key developmental pathways. Further studies will be necessary to elucidate the exact mechanisms behind this stability and its broader implications for disease pathogenesis.
5 Conclusions
In conclusion, two GLI3 truncating mutations, GLI3 (c.3342dupC; p. A1115Rfs*14) and GLI3 (c.4431dupT; p. Glu1478Ter) were identified in individuals diagnosed with sub-PHS and PAPA, respectively. The c.3342dupC mutation represents a novel variant, broadening the mutational spectrum of the GLI3 gene and providing valuable insights into the molecular underpinnings of GLI3-related limb malformations. It was indicated through functional analysis that these mutations may disrupt the balance between the activator and repressor forms of GLI3, affecting the regulation of the Sonic Hedgehog (SHH) signaling pathway and contributing to the development of sub-PHS and PAPA. This study expands the mutation and phenotype spectra of the GLI3 while offering a crucial reference for the clinical diagnosis and management of polysyndactyly and related conditions. Further investigations into the functional implications of these mutations are expected to enhance the understanding of GLI3's role in developmental disorders.
Author Contributions
Yongzhen Qi: writing – original draft, methodology, software. Kai Liu: writing – review and editing, funding acquisition. Yuda Wei: writing – review and editing, funding acquisition. Xiaxia Liu: software. Liangqian Jiang: writing – review and editing. Juan Teng: writing – review and editing. Baoqiang Chong: methodology. Shuqi Zheng: methodology. Xiangyu Zhao: conceptualization, investigation. Lin Li: resources, funding acquisition.
Acknowledgments
This research was supported by the Key R&D Plan of Shandong Province (2017GSF218072), Shandong Provincial Natural Science Foundation Youth Project (ZR2021QC196), and Shandong Provincial Natural Science Foundation Youth Project (ZR2021QH268).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




