Alewife and Blueback Herring Captured by Intertidal Weirs of the Inner Bay of Fundy, Canada, Display Seasonal Demographics that Suggest Multiple Migrating Stocks
Abstract
Little information is available concerning the seasonal demographics of marine migrating Alewife Alosa pseudoharengus and Blueback Herring A. aestivalis, reported in commercial fisheries as “gaspereau” in Canada and “river herring” in the USA. Once adults and age-0 juveniles depart from coastal spawning rivers, they migrate along the North American Atlantic coast and are difficult to access for scientific analysis. During June–October 1985, a total of 3,785 Alewife and 2,343 Blueback Herring were examined for length, sex, and gonadal stage from commercial intertidal fishing weirs in Minas Basin and Cobequid Bay, Bay of Fundy, Nova Scotia. Of these, 999 adults were subsampled for total weight and body cavity fat deposits. Both species first appeared in the weirs during June, when Blueback Herring generally dominated catches. By July, catches were evenly divided between the two species, and Alewife dominated after mid-August. Most adults were mature, and there was no discernable sex ratio pattern during the sampling period. Sampled fish exhibited abrupt changes in length, gonadal stage, condition factor, and abdominal wall and visceral mesentery fat deposits in relation to season (week), moon phase, and time of low tide. Sudden shifts in average lengths and fat deposits suggested that migrating Alewife were from different stocks. For Blueback Herring, the condition and status of fat deposits during June suggested that they were probably long-distance adult migrators. Blueback Herring in Cobequid Bay weirs during July were probably from local stocks. Results from a total of 12,422 tagged adults that were released in Minas Basin and Cobequid Bay during 1985 supported these findings. We propose that shifts in condition and fat deposits can be used to estimate migration distance, discriminating among local, regional, and long-distance movements.
Two anadromous alosine species—the Alewife Alosa pseudoharengus and Blueback Herring A. aestivalis—serve as primary prey in both marine and freshwater food webs and in the past supported large commercial fisheries on the East Coast of North America (Ames and Lichter 2013). Alewife stocks range from northeastern Newfoundland to North Carolina, while Blueback Herring stocks range from Nova Scotia to Florida (Rulifson 1994). The anatomical characteristics are so closely aligned that the two species are marketed together as “gaspereau” in Atlantic Canada and as “river herring” in the United States. Both are schooling species, and their aggregations were the target of the earliest Native fisheries, which were centered along shallow shorelines using brush weirs and in coastal rivers and estuaries during the spring spawning period.
Much of the published research on river herring has been from studies in coastal rivers and estuaries, and some information is available on local movements of adults and juveniles (e.g., Eakin 2017). However, little is known about their biology and ocean migration patterns in Canada and the United States after they leave freshwater habitats (Neves 1981; Stone and Jessop 1992; Bethoney et al. 2014; Turner et al. 2016, 2017). Ocean tagging studies have, to our knowledge, proven largely unsuccessful. In addition, there is a general lack of understanding about where and how these two species utilize habitats during the ocean migratory phase, although recent studies modeling ocean climate change and habitat associations may increase this knowledge (Lynch et al. 2015; Turner et al. 2016, 2017).
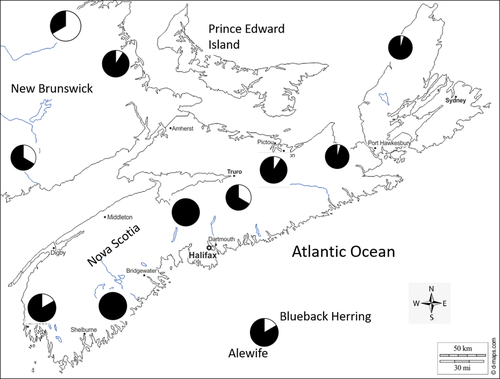
Most Canadian river herring populations are in the Maritime Provinces, and the largest commercial fisheries are in rivers or estuaries. Alewife comprise the bulk of the fishery (Figure 1), although both species are landed (Dadswell 1985; DFO 2001). Over 140 coastal watersheds in the Maritimes contain spawning populations: 13 rivers along the Gulf of St. Lawrence (New Brunswick, Nova Scotia), 69 rivers along Atlantic coastal Nova Scotia, and 60 rivers in the Bay of Fundy (BoF; Rulifson 1994; McBride et al. 2014). The only marine fishery occurs in BoF intertidal fishing weirs (Gordon 1993; Dadswell et al. 2020) and is confined to Minas Basin and Cobequid Bay, Nova Scotia (Figure 2). Historically, the fishery began during March and April in rivers and estuaries (Stone and Jessop 1992; Gibson and Daborn 1997), while the ocean harvest in Minas Basin occurred from late April through October (Dadswell et al. 1984). The largest commercial BoF fisheries are in the Saint John River, New Brunswick (Jessop 1990, 1999, 2001a, 2001b), and the Gaspereau River, Nova Scotia (Gibson and Myers 2001, 2002). Currently (in 2020), all commercial river herring fisheries in Atlantic Canada are still active and there are no restrictions (e.g., no total allowable catch). There is also no offshore herring trawl fishery. Both alosine species are unlisted by the Committee on the Status of Endangered Wildlife in Canada.
On the U.S. eastern seaboard, the declining spawning runs of river herring caused multiple states to impose moratoria on both commercial and recreational fisheries beginning in 2002 (Limburg and Waldman 2009). In 2007, the National Oceanic and Atmospheric Administration declared that both Alewife and Blueback Herring were species of concern under the U.S. Endangered Species Act (NOAA 2007). In the 1980s, the Atlantic States Marine Fisheries Commission suspected that ocean bycatch of river herring (Harris and Rulifson 1989)—especially in the fisheries for Atlantic Herring Clupea harengus and Atlantic Mackerel Scomber scombrus—was a problem, and this bycatch was believed to contribute substantially to the failure of stock restoration efforts (ASMFC 2012; Harris and Rulifson 1989). More recent studies have been focused on reducing ocean catches (e.g., Bethoney et al. 2013; Cournane et al. 2013; Turner et al. 2016).
We propose that observed seasonal biological characteristics of ocean-migrating Alewife and Blueback Herring in the BoF during 1985 demonstrated that stocks from watersheds throughout the species ranges intermixed during the marine phase. Our research and tagging examined the presence, relative abundance, size distribution, physical condition, and marine movement of river herring subsampled from commercial intertidal fishing weirs in Minas Basin and Cobequid Bay, Nova Scotia (Rulifson et al. 1987).
METHODS
Study sites
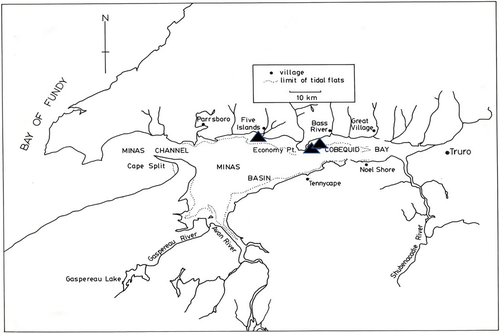
Two locations in the BoF were chosen for sampling because of (1) uncomplicated, season-long access to alosines captured in the intertidal fishing weirs and (2) the positions of the weirs relative to ocean currents and access points (Dadswell et al. 1984; Stone 1985). Minas Basin (Figure 2; 45°19′N, 64°00′W) is a mega-tidal, cul-de-sac marine embayment on the southeastern side of the inner BoF, semi-enclosed by the province of Nova Scotia. The triangular basin is 80 km long and 29 km wide at the base and approximately 2,000 km2 in area. It consists of the central Minas Basin proper, Cobequid Bay (the eastern extremity inside Economy Point), and the Southern Bight. Minas Basin has the largest recorded tides in the world (16 m; Garrett 1972), and at low tide maximum depth is only 17 m, while more than one-third of the basin area (670 km2) is exposed as tidal flat (Parker et al. 2007). Numerous rivers and streams are tributary to Minas Basin, the largest of which are the Salmon and Shubenacadie rivers discharging into Cobequid Bay, and the Avon, Gaspereau, and Cornwallis rivers discharging into the Southern Bight (Figure 2). All of these rivers currently have spawning populations and commercial fisheries for river herring (Dadswell and Rulifson, in press).
Currents in Minas Basin are driven by the residual element of tidal flow, resulting in a figure-eight current pattern within the basin (Greenberg 1984). Tidal inflow enters along the north side of Minas Passage after being deflected northward by Cape Split (Figure 2). Flow continues along the north side of Minas Basin and then is deflected to the south side of Cobequid Bay by Economy Point. The residual current continues around Cobequid Bay on its north side and is then again deflected to the south side of Minas Basin by Economy Point. A gyre exists in the central portion of Minas Basin, and flow continues along the southern shore of the basin through the Southern Bight, exiting the basin via the south side of Minas Passage. During June–October, salinity in Minas Basin varies from 20‰ to 30‰ and temperature varies from 5°C to 20°C (Bousfield and Liem 1959).
Sampling of intertidal fishing weirs
River herring were sampled at low tide from commercial catches in intertidal brush-type fishing weirs from June 16 to October 18, 1985. The Minas Basin weir was located on the intertidal flats at 45°23'N, 64°5'W, near the village of Five Islands. The two weirs in Cobequid Bay were similarly positioned on intertidal flats at 45°23'N, 63°50'W, near Economy Point (Figure 2); the samples from these weirs were combined for analyses because of their proximity. The distance between the Minas Basin and Cobequid Bay collection sites was approximately 22 km (Figure 2). The V-shaped weirs were constructed of spruce or birch branches, with arms ranging in length from 500 to 800 m, and were located 0.8–1.3 km from the high-tide mark (Gordon 1993; Rulifson et al. 2008). During spring high tides, weirs were covered by 5–7 m of water, but at low tide the weirs were about 1 km away from the low-water mark. Conversely, at low water during neap tides portions of the weir normally remained flooded and were sometimes inaccessible.
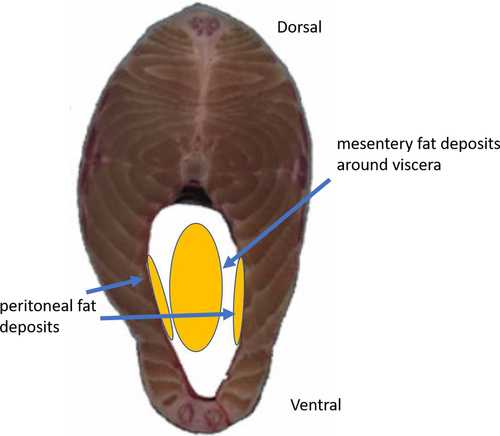
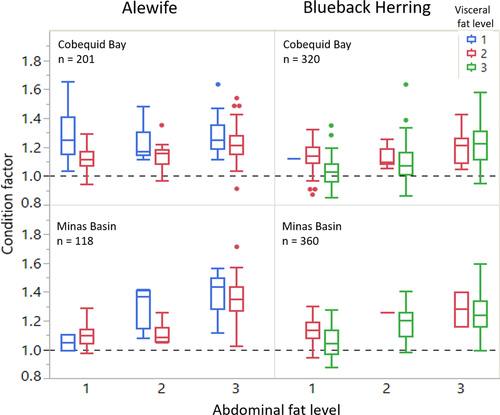
Weirs were visited at each accessible low tide, and sampling times were 15–90 min depending on tidal amplitude and prevailing winds. Weirs were sampled as soon as the receding water moved seaward, effectively trapping catches within the heart of the weir, where a pool of water remained. Fish were collected by seining the pool. Typically, catches of alosine adults and juveniles ranged from few or none (often the daytime low tides) to thousands during the same 24-h period. As many adults as possible were identified to species, measured (FL, mm), externally marked with Floy FD-68B T-style anchor tags below the anterior portion of the dorsal fin, and released in the weir during the low-tide period. At the end of each weir visit, up to 50 river herring were subsampled and taken to an onshore field laboratory for examination to determine species composition, sex ratio, length frequency distribution, and gonadal maturity (stages 1–5). Fat deposits located in the body cavity along the abdominal wall and visceral mesentery (Figure 3) were assessed using a score from 1 to 3 (1 = minimal visible fat deposits; 2 = moderate fat deposits; 3 = high fat deposits). Each subsampled fish was measured for FL (mm) and weighed (nearest 1 g). Peritoneal color of the body cavity was used to confirm species identification: continuous black or spotted black for Blueback Herring and gray or crème for Alewife. Data were divided into 1-cm size-classes for presentation purposes.
Statistical analyses were conducted using nonparametric tests since data were not normally distributed. Because results of the tagging studies (described below) clearly indicated that all fish left the weirs on each tide and migrating fish replaced them on the next low tide, we treated each subsample from the weir as independent. General linear model (GLM) analysis (JMP version 14.0; SAS Institute, Cary, North Carolina) was used to determine the relationship of fish length and Fulton’s condition factor (K = 100,000 × [weight/FL3]) as influenced by season (WEEK), moon phase (QUARTER), time of low tide (DIEL or PERIOD), and location (WATERBODY). Time of low tide was divided into daylight and nighttime samples for DIEL. The variable PERIOD was divided into AM, PM, or night; dawn and dusk (i.e., crepuscular) samples were those collected up to 1.5 h prior to the published times of sunrise and sunset.
There were four lunar months during the June–October sampling period. Day of the lunar month was assigned to each weir visit, and moon phase was divided into quarters. Lunar days 1 and 29 were the new moon, and day 15 was the full moon. First quarter was lunar days 1–8, second quarter was days 9–15, third quarter was days 16–22, and fourth quarter was days 23–29.
RESULTS
Seasonal Distributions and Relative Abundance
Alewife first appeared in Minas Basin weir catches during the week of June 16, 1985 (week 25), and initial abundance was greater for Alewife than for Blueback Herring in our subsamples (Figure 4). Thereafter, however, Alewife catches were sporadic and represented less than 50% of the catch until the week of August 11 (week 33; Figure 4). Apparently, groups of Alewife were migrating quickly through Minas Basin and catches often contained abundant Alewife on one low tide, whereas they might be absent from the catch on the following low tide about 12 h later. From mid-August into early October (weeks 34–41), Alewife comprised the bulk of the Minas Basin catches until they disappeared from the weirs by the second week in October (Figure 4, bottom). In Cobequid Bay, the presence of Alewife in weirs was consistent starting the week of July 28 (week 31) until the week of September 29 (week 40), although they were less abundant during June and July compared to Blueback Herring. After July, Alewife dominated Cobequid Bay subsamples for the remainder of the fishing season (Figure 4, top). Overall, Alewife were more abundant in Cobequid Bay subsamples than in subsamples collected from the Minas Basin weir.
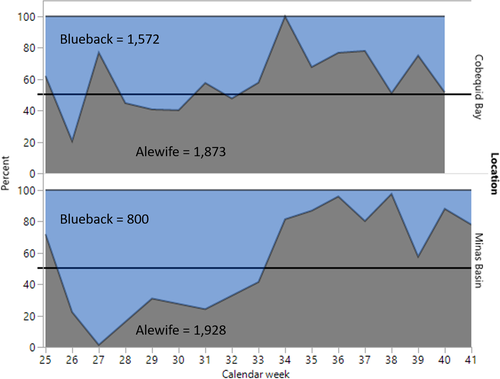
Blueback Herring dominated the Minas Basin subsamples through mid-August (week 33, starting August 11), but catches were sporadic; Alewife dominated Minas Basin catches starting in mid-August (Figure 4, bottom). In Cobequid Bay, Blueback Herring dominated catches through July and represented a greater percentage of the river herring catches than was observed for Minas Basin during the same period (Figure 4, top).
Patterns in Length Distribution
Alewife
Results from GLM analysis indicated that Alewife migrating through the BoF exhibited significant differences in length frequency related to sampling week, day or night low-tide samples (DIEL), and location of capture (WATERBODY); moon phase (QUARTER) was not significant in the full model (χ2 = 2,846.15; df = 21, 3,779; P < 0.0001). Alewife were longer on average in Cobequid Bay (154.4 mm FL) compared to Alewife from Minas Basin (130.5 mm FL). The GLM effect test for WATERBODY was significant (χ2 = 125.99; df = 1; P < 0.0001). Alewife were present in Cobequid Bay subsamples through the end of September but were not consistently present in Minas Basin subsamples from the end of June through mid-July (weeks 27, 28, and 30). Juvenile Alewife were consistently present in low numbers in Cobequid Bay samples, except during week 26 (starting June 23), but they did not appear consistently in Minas Bay subsamples (Figure 5). The length distribution of adult Alewife was shorter during June, but in Cobequid Bay the average fish length increased suddenly during the week of July 7 (week 28). Average lengths were lower beginning in the week of August 18 (week 34) and remained so throughout September (Figure 5). A similar seasonal shift in length frequency was observed in subsamples from the Minas Basin weir, although fewer Alewife were present from June through mid-August (GLM effect test, WEEK: χ2 = 2,452.49; df = 16; P < 0.0001). Alewife length differences were observed between day and night samples (GLM effect test, DIEL: χ2 = 134.04; df = 1; P < 0.0001). Subsampled Alewife usually exhibited greater lengths during the crepuscular and nighttime low tides and shorter lengths during daylight low tides. There was no significant effect of moon phase on lengths of Alewife observed in weirs (GLM effect test, QUARTER: χ2 = 5.01; df = 3; P = 0.1709).
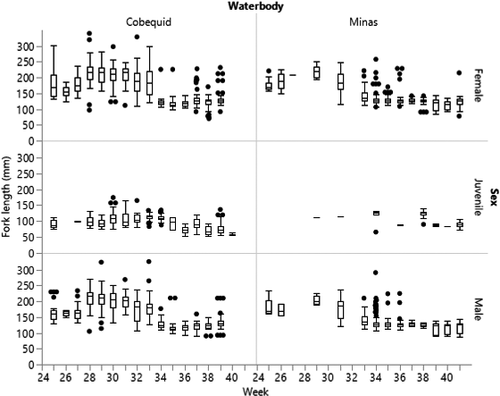
Blueback Herring
Shifts in length distribution for Blueback Herring were significantly related to the same variables as for Alewife: sampling week, location of capture, and day or night low tides. However, unlike the results for Alewife, Blueback Herring lengths in weirs were also related to moon phase (GLM full model: χ2 = 2,313.76; df = 21, 2,350; P < 0.0001). The average length of all Blueback Herring was greater in Cobequid Bay (158.9 mm FL) compared to Minas Basin (154.1 mm FL), but average fish length was not different between males (178.9 mm FL) and females (178.2 mm FL). The GLM effect test for WATERBODY was significant (χ2 = 138.73; df = 1; P < 0.0001). Seasonally, subsampled Blueback Herring were longer than Alewife in Cobequid Bay early in the summer, but by the week of August 25 (week 35) the larger fish disappeared from catches and smaller fish were present for the remainder of the summer and fall. A similar pattern was observed in the Minas Basin weir catches (Figure 6). Juvenile Blueback Herring were collected in the Cobequid Bay weir (n = 428) throughout the season, and the earliest individuals were longer on average than juvenile Alewife; however, by late summer and early fall individuals of both species were consistently less than 100 mm FL (Figures 5, 6). Few juvenile Blueback Herring were captured in the Minas Basin weir, primarily in late summer to early fall, and they tended to be larger in size than those subsampled from Cobequid Bay (GLM effect test, WEEK: χ2 = 2,264.95; df = 16; P < 0.0001). In general, Blueback Herring of both sexes were found to be largest at dusk and dawn low tides, while smaller fish were observed during the daylight and nighttime low tides (GLM effect test, DIEL: χ2 = 69.52; df = 1; P < 0.0001). Both sexes subsampled from Cobequid Bay were largest in June and July in afternoon low-tide subsamples, but the trend in largest fish shifted to nighttime low tides during August. In Minas Basin, the largest Blueback Herring were observed in low-tide nighttime samples during June and July but shifted to dusk low tides during August and September. Subsampled Blueback Herring lengths were also related to moon phase (GLM effect test, QUARTER: χ2 = 50.54; df = 3; P < 0.0001). Shifts in subsampled Blueback Herring lengths were not observed in first-quarter samples (P = 0.5031), but smaller fish were observed in second-quarter samples (χ2 = 50.02; P < 0.0001) and larger fish were observed in third-quarter samples (χ2 = 11.19; P = 0.0008).
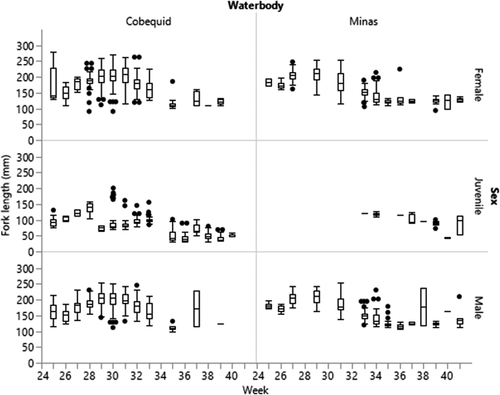
Tag Release and Recapture
Concurrent to our study in 1985, we released 10,424 tagged river herring from the Minas Basin weir and 1,006 tagged individuals from the two weirs in Cobequid Bay. The bulk of tagged fish releases occurred in June and July. In August, no tagged fish were released from Cobequid Bay weirs and only 749 fish were released from the Minas Basin weir. An early June tagging session was conducted from the Canadian R/V J.L. Hart positioned at the mouth of Cobequid Bay (Figure 2) using a midwater trawl, which resulted in a June release of 1,998 tagged fish, for a total release of 13,428 tagged river herring in 1985.
Overall, 35 tagged Alewife and 18 Blueback Herring were recovered. The farthest recapture for Alewife was from a trawler off Cape Ann, Massachusetts, 600 km from the release site, occurring 153 d after release. The remainder of Alewife recaptures were from local Nova Scotia commercial fisheries, including the Gaspereau and Shubenacadie rivers within Cobequid Bay and the Wellington and Tusket rivers in southwest Nova Scotia. Tagged Blueback Herring were primarily recovered in Cobequid Bay weirs, but one was recaptured in the Wicomico River, Maryland (~1,800 km), during the same month as release, and three were captured in Albemarle Sound, North Carolina (~2,300–2,400 km), 1–3 years after release.
Seasonal Shifts in Gonad Development
In total, 3,783 Alewife and 2,346 Blueback Herring were examined for gonad stage of development: immature (stage 1); resting (stage 2); developing (stage 3); oocytes clearly visible or testes reddish pink (stage 4); and spawning ready, with vent swollen or testes white (stage 5). None of the subsampled fish was considered spent (oocyte atresia). Both species exhibited differences in gonadal stages between the two waterbodies.
Alewife
In Cobequid Bay, immature and resting stage Alewife of both sexes dominated the weir subsamples (Figure 7). Females comprised 41.4% (771 of 1,861) of the Alewife examined, and males represented 32.9% (612); fish that were too small to sex (juveniles) constituted 25.7% (470) of the total. Most females (72.9%; 562 of 771) were in the resting stage of ovary development, and an additional 17.8% (137 of 771) were assessed as immature. Only 8.6% (66 of 771) were in the developing oocyte stage, 0.3% (2 of 771) had oocytes clearly visible within the ovary, and 0.52% (4 individuals) were spawning ready. Cobequid Bay females in near-spawning condition were collected between the weeks of June 16 (week 25) and July 21 (week 30). The majority of Cobequid Bay male Alewife were in the resting stage (61.8%; 378 of 612) or were considered immature (34.6%). Only 3.3% (20 of 612) were considered as developing and 0.33% (2 males) had pink testes (week 29, starting July 14), but none was spawning ready (white testes). Developing gonads for female Cobequid Bay Alewife were observed during the weeks starting June 16 through August 17 (weeks 25–33), while subsampled males with developing gonads appeared over a shorter period—the weeks starting June 30 through August 4 (weeks 27–32). Young immature Alewife were collected in Cobequid Bay throughout the sampling period, with a primary peak in August and a smaller secondary peak in September.
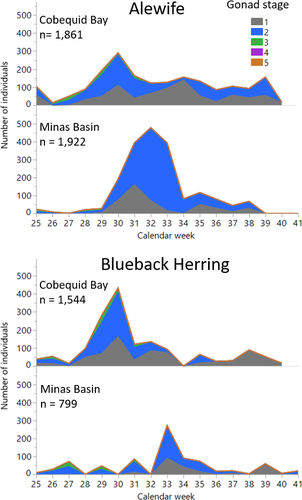
In the Minas Basin Alewife subsamples, the pattern of gonad stage development was similar to that of Alewife subsampled from Cobequid Bay. Most adults of both sexes were in a resting or immature stage of gonad development (Figure 7). Females dominated the samples, comprising 53.7% (1,033 of 1,922) of the total compared to 41.3% males and 5.0% juveniles. No female Alewife in Minas Basin were noted with oocytes clearly visible or were considered spawning ready or in postspawn condition, and those few with developing ovaries were observed between the weeks of June 16 (week 25) and July 28 (week 31). Interestingly, one Alewife female that was examined during the week of September 1 (week 36) was identified as possessing developing ovaries. Only one male, collected during the week of July 28 (week 31), was considered to possess developing testes. Young, immature Alewife were observed in Minas Basin starting the week of July 14 (week 29), but there was a small peak during the week of August 18 (week 33; corresponding with a peak appearance in Cobequid Bay), followed by a larger peak in September.
Blueback Herring
Cobequid Bay subsamples of Blueback Herring (n = 1,544) indicated that females were more abundant (39.7%; 616) than males (32.3%; 500), while juveniles represented 27.6% (428) of the total. Most females were in the resting (64.8%; 399) or immature (21.3%; 131) stages of oocyte development (Figure 7); only three fish had oocytes that were clearly visible, and none was classified as spawning ready. Developing females were observed during the weeks of June 16 through August 4 (weeks 25–32). Most subsampled Cobequid Bay males were considered to be in the resting stage (58.8%; 294) or were immature (39.6%; 198). Only eight of the subsampled males showed testis development, two of which had pink or white testes indicating prespawning condition, and they appeared during the period July 14–27 (weeks 29 and 30).
Blueback Herring subsampled from Minas Basin (n = 799) exhibited gonadal stage development similar to that of Blueback Herring subsampled from Cobequid Bay, but the sex ratio of collected fish was evenly split at 46%. Most females were in the resting (68.1%; 252) or immature (19.7%; 73) gonadal stages (Figure 7); those showing oocyte development (12.2%; 45) were sporadically collected during the weeks of June 16 through July 28 (weeks 25–31). Males demonstrated stages of gonadal development like the females; those with developing testes were present during the weeks of June 30 (week 27) and July 14 (week 29). No Blueback Herring were spawning ready or in post-spawn condition.
Weight–Length Relationships
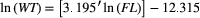
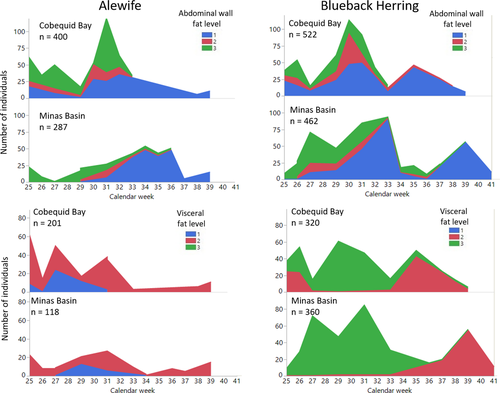
(n = 316; r2 = 0.986; F = 22,656.64, P < 0.0001), where ln(WT) is the natural logarithm of total weight and ln(FL) is the natural logarithm of FL. There was no significant difference in the weight–length relationship between Alewife subsamples from Cobequid Bay and those from Minas Basin (t = 1.55; df = 312; P = 0.1212).
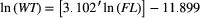
(n = 680; r2 = 0.991; F = 72,022.69, P < 0.0001). There was no significant difference in the weight–length relationship between the two waterbodies (t = 1.56; df = 676; P = 0.1192).
Condition and Body Cavity Fat Deposits
Fat deposits within the body cavity were categorized as minimal (level 1), moderate (level 2), or high (level 3), and their locations were abdominal and visceral (Figure 3). Results of a GLM analysis for Alewife indicated that levels of both abdominal wall fat and visceral fat were related to K (χ2 = 118.43; df = 5, 311; P < 0.0001). Both types of fat deposit were independently related to K, but the interaction of the two fat types was not significant (P = 0.0670) for Alewife. To determine factors related to Alewife K, we included both fat deposit types, WEEK, WATERBODY, DIEL (low tide as day or night), and QUARTER in a GLM (χ2 = 261.47; df = 17, 299; P < 0.0001). Results indicated that Alewife K was related to the amount of abdominal (df = 2; P < 0.0001) and visceral (df = 1; P < 0.0001) fat deposits, WEEK (df = 9; P < 0.0001), and WATERBODY (P < 0.0001). Time of low tide and moon phase were not significantly related to K (DIEL: P = 0.05718; QUARTER: P = 0.15936). It is interesting to note that none of the Alewife subsampled from either Cobequid Bay or Minas Basin had high visceral fat deposits, even when abdominal fat deposits were high (Figures 8, 9). The smallest Alewife size-classes had the greatest number of individuals exhibiting moderate visceral fat and minimal abdominal wall fat. Larger size-classes had the greatest abdominal wall fat deposits (Figure 9). We developed another model substituting the variable PERIOD for DIEL. This second model was significant for all variables (χ2 = 246.50; df = 11, 305; P < 0.0001) except WATERBODY (P = 0.0975) but was significant for moon phase as well (χ2 = 24.20; df = 3; P < 0.0001). Seasonally, Alewife with the highest amount of abdominal fat were found in the BoF during summer, but fish entering in late summer (after week 32) and fall had minimal abdominal fat (Figure 10). At the same time, those Alewife entering earlier exhibited a mix of minimal or moderate visceral fat deposits, whereas in late summer and fall all individuals were observed to have moderate visceral fat deposits.
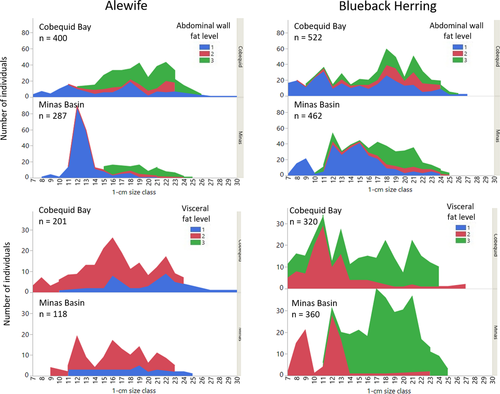
For Blueback Herring, the same two models were used to describe factors related to K. The first model, which included DIEL, was significant (χ2 = 412.96; df = 20, 659; P < 0.0001). Blueback Herring K was explained by both abdominal wall (χ2 = 239.34; P < 0.0001) and visceral (χ2 = 9.90; P = 0.0071) fat deposits, WEEK (χ2 = 78.88; df = 11; P < 0.0001), QUARTER (χ2 = 20.50; P < 0.0001), and WATERBODY (χ2 = 7.56; P = 0.0060). Daytime or nighttime occurrence of low tide (DIEL) was not related to Blueback Herring K (P = 0.1463). The second model, in which the term PERIOD was substituted for DIEL, was also significant (χ2 = 370.72; df = 11, 669; P < 0.0001). Those factors related to Blueback Herring K were both abdominal wall (χ2 = 201.33; df = 1; P < 0.0001) and visceral (χ2 = 16.93; df = 1; P < 0.0001) fat deposits, QUARTER (χ2 = 57.17; df = 3; P < 0.0001), and the low-tide period of occurrence (χ2 = 66.04; df = 4; P < 0.0001). Sampling week was also significant (P = 0.0181), but WATERBODY was not (P = 0.0861). For moon phase, the first and third quarters (following new and full moons) were significant (P < 0.0001) in relating to Blueback Herring K. Low tides occurring at dusk (P = 0.0168) and during the night (P = 0.0004) were also related to K of subsampled specimens. The smallest Blueback Herring had minimal abdominal wall fat while exhibiting moderate or high levels of adipose tissue in the visceral mesentery (Figure 9). Larger length-classes had more fish exhibiting moderate to high abdominal wall fat and the highest levels of visceral fat (Figure 9). Seasonally, Blueback Herring found in the BoF had high levels of visceral fat deposits but a mixture of low, moderate, and high levels of abdominal wall fat (Figure 10). By mid-August (week 33), subsampled Blueback Herring captured in the commercial weirs had minimal fat in the abdominal wall area and visceral fat had declined to moderate levels (Figure 10).
DISCUSSION
Once Alewife and Blueback Herring are at sea and because they migrate long distances along the East Coast of North America, they have been difficult to study and their demographics while at sea are poorly known. To our knowledge, the results presented here are the first of their kind concerning the biological condition of these alosines while at sea throughout a fishing season. Results of a tag-and-release study combined with the seasonal patterns of gonadal state, length, and condition suggest that both species, which are present in large numbers in the BoF during summer months (Stone and Daborn 1987; Dadswell et al. 2020), comprise a mixture of local and distant stocks. Results of river herring tagging in Minas Basin and Cobequid Bay suggested that at the time of this study, Alewife were more local in origin, with stocks perhaps from Nova Scotia to Massachusetts, whereas the Blueback Herring stocks encountered were from Nova Scotia to North Carolina. Minas Basin and Cobequid Bay apparently function as nursery and marine foraging sites for both species. This pattern was also observed for another alosine species, the American Shad A. sapidissima, which migrates from all major shad spawning rivers along the North American eastern seaboard into the BoF each summer (Dadswell et al. 1983, 1987). Since Alewife and Blueback Herring are closely related to the American Shad, it is not surprising that the two species can migrate long distances in coastal waters, as was suggested by Neves (1981), Bethoney et al. (2014), Hasselman et al. (2016), Eakin (2017), and others.
Most of the Alewife that we sampled in the BoF during the 1985 commercial weir fishing season were probably from local or regional Canadian stocks, but some may have been from U.S. stocks, especially New England rivers. Early in the weir fishing season (June), our results indicated that Alewife abundance in the Minas Basin weir catches was lower than Blueback Herring abundance, but the two species were present in relatively equal abundances in Cobequid Bay catches during the same period, suggesting that Alewife were perhaps migrating to Cobequid Bay rivers for spawning. These early season Cobequid Bay Alewife were also smaller on average compared to Minas Basin Alewife, suggesting that Minas Basin contained Alewife of multiple origins during June. An alternative possibility is that different age-classes (length-classes) were present but were schooling as age cohorts from the same river. These early run Alewife in Cobequid Bay, however, also had lower K-values than those from Minas Basin during the same period, a further indication that these two waterbodies probably did not contain Alewife from the same stocks. Alewife sampled during mid-June were in a similar gonadal state in both waterbodies, but during July fish in Cobequid Bay exhibited a greater degree of gonadal readiness than those in Minas Basin. The sudden shift in Alewife size within Cobequid Bay during July corresponded with the increased gonadal readiness; Alewife subsamples from Minas Basin never displayed increased gonadal readiness throughout the entire season, and over the entire season Alewife in Minas Basin were significantly smaller in average length.
Blueback Herring were more prevalent than Alewife in June samples, especially in the Minas Basin weir; by July, Blueback Herring continued to be more abundant in Minas Basin but were present in numbers equal to those of Alewife in Cobequid Bay. By late August, Blueback Herring were less abundant than Alewife in both waterbodies, possibly suggesting that they were departing Minas Basin. Subsampled Blueback Herring remaining in the Minas Basin during autumn were the smallest average size observed during the study, indicating that they were of local or regional origin. Early season Blueback Herring were on average smaller than Blueback Herring sampled in July, and the shift in size corresponded with an increased gonadal readiness of some individuals during mid-July. The mid-July period was also the time of largest peritoneal fat deposits and greatest K-values. Fish with level 3 peritoneal fat deposits were absent by the end of July, and fish with moderate (level 2) peritoneal fat were absent soon afterward. For the remainder of the season, Blueback Herring with little peritoneal fat continued to increase their K-values in both waterbodies, but this late-season elevated K corresponded with the smallest fish observed over the season.
Very little published information exists about fat deposits and K-values of alosines during the ocean migratory phase of their life cycles. Themelis (1986) found that American Shad had high levels of muscle lipids (22.7–28.9%) while in the BoF and during spring in Minas Basin. After migrating through Cobequid Bay during July and August, fat levels in American Shad declined to an average of 16.3%. Studies on another alosine, the Hickory Shad A. mediocris, indicated that peritoneal fat deposits were greatest just prior to fish entry into estuarine waters but declined during the upstream spawning migration (Murauskas and Rulifson 2011; Rulifson and Batsavage 2014). Condition factors and peritoneal fat deposits began to increase during the postspawning migration downstream toward marine waters. Exactly how abdominal and visceral mesentery fat deposits relate is unclear, but both are indicative of adipose fat storage, and decreased adipose storage indicates long-term use of stored deposits for migratory purposes (Weil and Lefèvre 2013; Salmerón 2018).
Regionally, Alewife that are found in Minas Basin and Cobequid Bay could come from stocks in local watersheds, such as the Shubenacadie and Gaspereau rivers. Both river herring populations are known to be dominated by or to consist exclusively of Alewife (Gibson and Daborn 1997; DFO 2001). Farther afield, Alewife are also found in watersheds along the Nova Scotia Atlantic coast from Yarmouth to Cape Breton and Northumberland Strait (Rulifson 1994; McBride et al. 2014). Stone and Jessop (1992) noted an inshore–offshore movement of gaspereau along the Scotian Shelf, and perhaps the larger and more robust Alewife were migrating to the BoF from offshore waters.
On the other hand, watersheds containing dominant runs of Blueback Herring are not common in this region (DFO 2001; McBride et al. 2014). The Shubenacadie River and tributaries support a mix of Blueback Herring and Alewife, as mentioned previously, and possibly the smallest Blueback Herring captured at the end of the fishing season may have been from that stock. Another BoF river herring population with a substantial Blueback Herring component is in the Saint John River, New Brunswick (Messieh 1977; Jessop 1990), and they could be entering Minas Basin from there. Limited knowledge of the spatial and temporal migration patterns of both species in mixed schools makes it difficult to develop prediction models for river herring in ocean habitats (Bethoney et al. 2013; Turner et al. 2017).
Results from our study clearly indicate that during migration, these two alosines may school by species in ocean habitats but also migrate as a mixture of both species. For Minas Basin, this phenomenon might have occurred because the majority of Alewife possibly originated from spawning populations within the basin. The Blueback Herring that dominated catches in Minas Basin during early summer may have arrived after spawning in more southerly watersheds (i.e., U.S. streams) earlier in the year. Acoustic telemetry studies, primarily of Alewife, in the Hudson River indicated a postspawn northward coastal movement into the Gulf of Maine after the fish left the Hudson River estuary (Eakin 2017). Hasselman’s (2016) genetics research indicated that bycatch from Atlantic Herring and Atlantic Mackerel fisheries disproportionately contained severely depleted genetic stocks of Alewife from southern New England and Blueback Herring from mid-Atlantic stocks. Our tagging study indicated that Alewife traveled from the Minas Basin to Massachusetts and that Blueback Herring traveled to Maryland and North Carolina. All of these studies confirm the mixing of Canadian and U.S. spawning stocks of both species in Gulf of Maine and BoF waters.
River herring stocks in the USA have been at an all-time low during the last 30 years. Whether this fact is related to bycatch while at sea (Bethoney et al. 2013) and in rivers (Limburg and Waldman 2009) as well as impact by predation during the recent large increase of Striped Bass Morone saxatilis abundance (Hartman 2003) or from long-term environmental changes, such as dams, river alterations, and pollution (Rulifson 1994), is undetermined. However, multiple studies now indicate that the southern New England and mid-Atlantic stocks are the most vulnerable to the Atlantic Herring and Atlantic Mackerel fisheries (Bethoney et al. 2013, 2014; Palkovacs et al. 2014; Hasselman et al. 2016).
We recommend that our study be repeated to include tagging and genetics to provide information on whether the patterns we observed in 1985 are still present or have changed to reflect the continued decline of U.S. populations contributing to the river herring weir fishery in the BoF. Our tagging study was conducted using external tags with return addresses and a reward system. Eakin (2017) recommended acoustic telemetry after showing positive results in the Hudson River and coastal ocean with small acoustic tags inserted by gastric implantation. Eakin (2017) recommended multi-year acoustic tags for future studies but cautioned that surgical implantation—not gastric implantation—must be used for extended-term research. We agree with his assessment and advocate for a mixed study in which some acoustic transmitters are used in conjunction with extensive external tags, which are relatively inexpensive, can be quickly applied given the limited time available during each low tide, and can be released in large numbers. We also advocate repeating the study due to ocean climate change and the movement northward of many coastal and estuarine-dependent marine species, including river herring. Lynch et al. (2015) reviewed the challenges of conserving Alewife and Blueback Herring populations in a warming world.
ACKNOWLEDGMENTS
This study was funded in part by the National Oceanic and Atmospheric Administration–National Marine Fisheries Service under the Anadromous Fish Conservation Act (Public Law 89-304). Additional support was provided by the state of North Carolina, Fisheries and Oceans Canada, East Carolina University, Unity College (Unity, Maine), the Fundy Weir Fishermen’s Association, and the Hudson River Foundation. The commercial weir fishermen of Minas Basin and Cobequid Bay provided technical advice, field assistance, and companionship throughout the study. A big thanks to Sean McKenna for leading the field crews on this portion of the study. Our gratitude is extended to the numerous graduate and undergraduate students from Acadia University, Mount Allison University, the University of New Brunswick, Unity College, and East Carolina University for field assistance, including D. Themelis, H. Stone, J. Williams, M. Kellock, S. Bubine, P. Crawford, K. Stokesbury, R. Bradford, P. Butryn, R. Clark, O. Kephart, C. Docktor, L. Langille, and C. Hoffman. We thank K. O’Brien and D. Chalcraft (East Carolina University) for help in database analyses. We also appreciate C. Salmerón (University of California, San Diego) for advice on adipose fat tissues. The Institute for Coastal Science and Policy (formerly the Institute for Coastal and Marine Resources) at East Carolina University and the Acadia Centre for Estuarine Research and Biology Department at Acadia University provided laboratory space. There is no conflict of interest declared in this article.




