Striped Mullet Migration Patterns in the Indian River Lagoon: A Network Analysis Approach to Spatial Fisheries Management
Abstract
Striped Mullet Mugil cephalus are numerically abundant forage fish, highly valuable as prey, and commercially valuable to humans. From September to December, Striped Mullet in the Indian River Lagoon (IRL), Florida, undergo an annual migration from inshore foraging habitats to oceanic spawning sites. However, their migratory pathways—particularly their intra-estuarine movement pathways—remain unknown. To address this knowledge gap, we utilized passive acoustic telemetry to assess the movement patterns of Striped Mullet within the IRL. Thirty-two fish were tagged, generating usable tracks from 18 individuals. The mean (±SD) time that fish were detected in the array was about 38 ± 90 d, with the longest detection period being 444 d. We also document the first evidence of skipped spawning in Striped Mullet inhabiting waters of the southeastern United States. These data suggest that impoundments around the Merritt Island National Wildlife Refuge serve as important refugia for Striped Mullet, while the Banana and Indian rivers act as corridors during their inshore migratory movements. Through spatial fisheries management, high-value habitat and connective elements utilized by Striped Mullet and other vital forage fish may be identified so as to benefit both natural and human dynamics in estuarine systems.
Coastal fish migration results in naturally high mortality as individuals traverse a multitude of habitats and waterways to reach spawning and/or nursery grounds. Anthropogenic modification of inshore waterways has made a naturally treacherous process even more difficult. Although only approximately 2.5% of fish species migrate, those species (e.g., salmon, sturgeon, and herring) are highly valuable both ecologically and economically (Binder et al. 2011; Cooke et al. 2011; Hinch et al. 2006). As migratory fish travel, their routes and spawning sites are not always protected, leaving them subject to pollution, fishing pressures, and habitat fragmentation due to anthropogenic devices, such as dams and weirs. Corridors are vital to maintain the connectivity of habitat patches and to ensure the continuation of migratory fishes’ life history strategies (Simberloff and Cox 1987; Rouget et al. 2006; Gilbert-Norton et al. 2010). Forage fish in particular have suffered incredible losses due to recruitment failure and overfishing, as they often engage in predictable migratory movements that have been relied on and exploited for decades by the fishing industry (Enticknap et al. 2011; Pew Charitable Trusts 2013; Bayard 2015; Essington et al. 2015). Forage fishes are critical species that serve as major conduits for energy transfer through marine and aquatic food webs by converting lower-trophic-level production into forms available for higher-order predators (Bakun et al. 2010; Pikitch et al. 2014). Many higher-trophic-level species dependent on forage fish are listed on the International Union for Conservation of Nature’s Red List and/or are located in areas that are vulnerable to changes in forage fish abundance (Pikitch et al. 2014). Forage fish species also compose 30% of landed capture fisheries through human consumption, fish meal, and fish oil (Alder and Pauly 2006; Tacon and Metian 2009). However, there is extensive evidence that forage fish are declining (Enticknap et al. 2011; Essington et al. 2015; Hall et al. 2012; Matthiessen 2016). Essington et al. (2015) examined 55 major forage fish stocks worldwide and concluded that 27 of those had experienced a collapse. As such, there is an urgent need to develop more effective management strategies—both single species and ecosystem based—that conserve a sufficient amount of reproductive biomass to ensure long-term sustainability (Goodyear 1980; Sadovy de Mitcheson and Erisman 2011; Taylor et al. 2012). Through the use of passive acoustic telemetry, an optimal strategy for the monitoring of species with extensive migration patterns, it is possible to identify high-value habitat and possible areas of conservation interest within migratory routes. This is crucial in order to implement management actions that will allow for sustainable amounts of sexually mature animals to reach their appropriate spawning grounds.
Due to the intricacy and broad area encompassed by these migratory routes, network-based analyses are increasingly being applied to fish movement data in order to assess movement and quantify route-specific connectivity patterns. A subset of graph theory, network analysis is based on the concept that intricate systems can be decomposed into relatively simple topological systems comprised of nodes and edges—represented by fixed physical locations (i.e., nodes) and the frequency and directionality of connections between them (i.e., directed edges; Salancik 1995; Rayfield et al. 2011; Jacoby et al. 2012). Node-based centrality metrics ranging from relatively simple to complex can be evaluated to determine the relative importance of individual locations or patches to the entire network structure and are dependent on the relative magnitude of interaction one node has with another (Lerman et al. 2010; Rayfield et al. 2011). This method not only is highly efficient at describing connectivity across broad spatial scales but also allows for the integration of species-specific biological characteristics, such as dispersal ability (Rayfield et al. 2011).
Intra-estuarine movement is just as critical as migratory movement, as estuarine-dependent fish spend a large portion of their life span engaging in activities within the estuary and are fundamental to regulating various processes in that environment (Reis-Santos et al. 2015). One of the most important factors that dictate fish behavior within these environments is connectivity, particularly as estuaries often serve as transition areas from nursery and foraging habitats to spawning grounds (Childs et al. 2015). Additional complicating factors include temporal and spatial differences in life history that often lead to complex movement trends, producing variation on both an individual scale and a group scale (Fullerton et al. 2010; Childs et al. 2015). This variation can be witnessed in the existence of contingents, or numerous conspecifics distributed in a multitude of habitats during a specific life stage (Childs et al. 2015). These groups frequently undergo partial migrations, an intrapopulation migratory behavior that is characterized by the presence of both migrants and residents within the same population (Chapman et al. 2012; Childs et al. 2015). This phenomenon provides a mechanism for divergent migratory movement and habitat use within the estuary, particularly among adjacent or isolated habitat patches, but it is not well recognized and is generally understudied (Childs et al. 2015; Reis-Santos et al. 2015). A greater understanding of partial migrations may aid in establishing effective spatial fisheries management and conservation of essential fish habitat, as it will help to identify areas of conservation interest within estuaries as well as preserve linkages between crucial habitats across varying scales of space and time (Fullerton et al. 2010; Reis-Santos et al. 2015).
To address the knowledge gap regarding spawning-related movements of aggregating forage fish in inshore waters, we examined intra-estuarine fish activity patterns and migration routes preceding offshore spawning behavior. Specifically, we explored the lack of information at a regional scale by using passive acoustic telemetry to evaluate the inshore migration routes of Striped Mullet Mugil cephalus within the Indian River Lagoon (IRL), Florida, USA. The Striped Mullet is a critical mid-trophic-level species that supports higher-trophic-level sport fish and recreational and commercial fisheries. Despite its ecological and economic importance, the Striped Mullet has been largely overlooked and relatively understudied to date, but see Mahmoudi (2000), Ibañez and Benítez (2004), Bester (2014), Fowler et al. (2016), and Fortunato et al. (2017) for a general overview of its life history, local stock assessments, and regional migratory patterns. The identification of high-value habitat and travel routes provides novel insight into movement corridors and can inform the development of future management strategies that support both the IRL and the continued production of related ecosystem services.
Model Species
Striped Mullet are globally distributed throughout tropical and temperate coastal waters, often moving into estuaries and other freshwater environs. In the western Atlantic, the species ranges from Nova Scotia to Brazil (Bester 2014). Striped Mullet can grow to over 45 cm TL and over 7 kg in weight and have a life span of 4–16 years (Bester 2014). Migrating up to 80.47 km (50 mi) offshore to spawn during fall and winter months, individual females can produce up to 2 million eggs (Bester 2014). It is believed that decreasing water temperatures and barometric pressure may trigger aggregation and spawning behavior (Mahmoudi 2000). The mullet run, as local fishermen refer to the spawning migration, peaks during October and November in the IRL (Killer 2012).
Critical forage fish, such as anchovies Anchoa spp., mojarras Eucinostomus spp., mullets (Mugilidae), and silversides Menidia spp., are widespread throughout the IRL and share similar trophic positions, life history strategies, and movement challenges (Gilmore et al. 1981). The Striped Mullet was selected as the model species for this study, as it epitomizes the two most important functions of a forage fish: it facilitates energy transfer through the food web as a significant food source for many sport fish and other higher-order predators, and it is targeted by both recreational and commercial fishing industries (Leard et al. 1995; Bester 2014; North Carolina Department of Environmental Quality 2018). When Striped Mullet reach sexual maturity at around 3 years of age, they undertake an annual migration from inshore waters to oceanic spawning locations, but little data exist concerning their inshore migratory routes (Bacheler et al. 2005; Bester 2014; Fowler et al. 2016). Although Striped Mullet are relatively abundant, there is a distinct lack of information regarding their migratory pathways, providing for possible, yet unknown, management gaps (Bacheler et al. 2005; Fowler et al. 2016). Within the IRL, the increasing number of manmade structures may threaten the connectivity of these routes. This is common for many migratory species due to the difficulty of direct observation created by their mobility (Kaimuddin et al. 2016; Crossin et al. 2017). Although the Striped Mullet is not currently at risk, utilizing such a widespread and relatively common species will ultimately deepen our understanding of migration-related movement patterns, which can be used to develop the best available management practices for other migratory species. Other states in which this species is abundant share this knowledge deficiency. For example, South Carolina devised a Comprehensive Wildlife Conservation Strategy for the Striped Mullet (McDonough 2006) and found that it may serve as “an excellent candidate as an indicator species” but that there is a need for multiple studies focusing on different aspects of the Striped Mullet’s life history. One of the resulting conservation recommendations of that report was to initiate a tagging study to evaluate the species’ seasonal movement and distribution and to identify essential habitat types for its various life stages (McDonough 2006). Therefore, utilizing this species will contribute to the pool of knowledge regarding Striped Mullet within the IRL as well as other locations that prioritize the species’ status.
METHODS
Study site
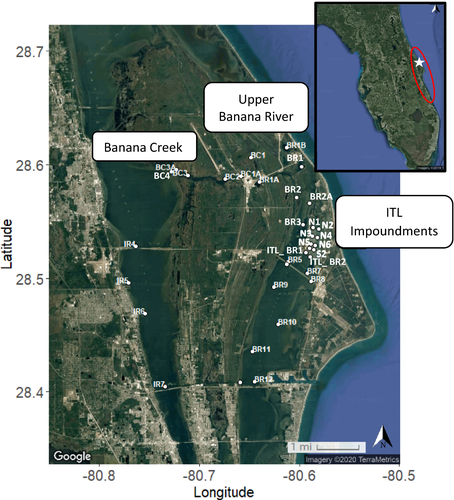
This study was conducted in the IRL complex and within Merritt Island National Wildlife Refuge property. The IRL complex spans approximately 251 km (nearly one-third of Florida’s east coast) and is comprised of Mosquito Lagoon and the Indian and Banana rivers. Five primary inlets—Ponce de Leon, Sebastian, Ft. Pierce, St. Lucie, and Jupiter—connect this water system to the ocean (Figure 1). This area also includes the Merritt Island National Wildlife Refuge, a 56,656-ha (140,000-acre) preserve on Kennedy Space Center property and one of the few relatively undeveloped barrier islands on the east coast of Florida (Adrian et al. 2008). The IRL, recognized as an “Estuary of National Significance,” contains one of the highest levels of biological diversity in the United States, with 397 different fish species inhabiting its waters at some point of their life cycle (Gilmore 1995). Commercial and recreational enterprises in the lagoon sustain nearly 10,000 jobs and generate over US$1.6 × 109 of revenue per year (East Central Florida Regional Planning Council 2016). The waters have an abundance of economically important species, including the sport fish Common Snook Centropomus undecimalis, Red Drum Sciaenops ocellatus, and Spotted Seatrout Cynoscion nebulosus, as well as key prey species, such as Bay Anchovy Anchoa mitchilli, Pinfish Lagodon rhomboides, and mullets Mugil spp. (Myers 2013).
Acoustic telemetry
Animals were detected during two spawning seasons from October 2017 to March 2019. From October to December in 2017 and 2018, 32 Striped Mullet that were field evaluated as sexually mature were captured using a 3.66-m (12-ft) radius cast net and were surgically implanted with a VEMCO V9-2L acoustic transmitter (weight in water = 2.9 g, projected battery life = 685 d; following methods described by Reyier et al. 2011). Generally, individuals measuring over 30 cm standard length (SL) are considered sexually mature (Greeley et al. 1987; McDonough 2006; Bester 2014). Striped Mullet were tagged with an external dart tag to permanently identify the fish and potentially recover them if caught by fishermen. Standardized biometric data (FL, SL, TL, and mass) and abiotic conditions were also recorded before each individual was released near the capture location. Tagging activities occurred at two estuarine backwaters: the Banana River (comprised of the upper Banana River and Integrate–Transfer–Launch [ITL] impoundments) and Banana Creek, with 16 fish tagged in each region (Figure 1). The tagging sites were selected due to the high density of acoustic receivers deployed in these areas (Figure 1), providing for more fine-scale movement data, and because the locations captured the longest potential distance (up to ~200 km) traveled by Striped Mullet to reach their ocean spawning grounds. There is also a high abundance of sexually mature Striped Mullet found in these areas. Approximately 200 VEMCO VR-2W autonomous passive acoustic receivers, components of the FACT Network (www.secoora.org/fact), have been deployed throughout the study area; 27 receivers are located within the core study region (Figure 1). Receivers record the time and approximate location of each fish (i.e., the coordinates of the receiver when the fish is within detection range), enabling the recreation of inshore activity and migratory tracks from staging sites in the estuaries to nearshore waters. Using guidance from Pincock and Johnston (2012), a range test was conducted within the ITL impoundments to assess detection efficiency and approximate receiver range (~200 m).
The first 48 h of movement data posttagging were removed to address any potential posttagging behavioral changes, and only detections with identification codes specific to this study were included in data analysis. The remaining data were filtered for false detections. A false detection is defined here as a tag that appeared to have been detected at a certain location but was recorded in error due to some outside interference (Pincock 2012). Using a time threshold of 3,600 s (recommended by the tag manufacturer) and incorporating an average tag delay of 120 s, the Pincock (2012) filter was used to indicate potentially false detections. After false detections were removed, the remaining detections were used for all other data analyses. Total detections were pared down to identify individual detection events, defined here by the window of time during which a fish was first and last detected at a particular receiver before that tagged animal was detected on a different receiver, at which time it would be considered a new detection event. The residency time, determined by combining detection event duration, was also assessed for each fish at each receiver. The paths of each individual fish were binned into 30-min intervals and linearly interpolated between receivers located within Kennedy Space Center as well as throughout the IRL.
Network-based analyses
Network topology was visualized using the Fruchterman–Reingold (FR) algorithm using the R packages igraph, network, sna, and ggnet (Fruchterman and Reingold 1991; Csardi and Nepusz 2006; Butts 2015, 2016). The FR algorithm generates a network where edges (and edge weights) connecting nodes are determined by fish movement between acoustic receivers based on the number and occurrence of fish detections at each receiver (Fruchterman and Reingold 1991). At the IRL scale, receiver nodes are color coded to indicate geographic subregion and node size corresponds to the number of detections at a given location. This is a simplified digraph; all edges with the same two endpoints are summed and combined into a single weighted edge. The edges show the direction in which a fish is traveling. Additional FR networks were generated but with node size representing the degree, betweenness, and eigenvector centrality of each node. Node degree represents the number of edges connecting nodes, while the frequency of individuals moving from one location to another produces edge weights (Jacoby et al. 2012). Degree is partitioned into indegree and outdegree to identify where fish are entering and exiting specific sites, as well as passages to and through areas of conservation or management interest. Eigenvector centrality measures the influence and accessibility of a location by comparing the centrality of a specific node to the centrality of its neighbors (Ghasemi et al. 2014). Betweenness centrality focuses on the number of routes that pass through a specific node via the shortest path length. High betweenness centrality suggests that particular areas contain vital resources or refuge for migratory species, thereby encouraging aggregation (Jacoby et al. 2012).
Model selection: residency time
Abiotic factors, including water temperature, dissolved oxygen, turbidity, pH, salinity, depth, photoperiod, and barometric pressure, were recorded at each receiver on a monthly basis using a YSI multi-parameter probe. Environmental variables were collected from November 2017 to February 2019. Using the glmmADMB package (Skaug et al. 2016) in R, linear mixed models of residency time were evaluated to assess which environmental variables were most associated with Striped Mullet behavior using Akaike’s information criterion corrected for small sample size (AICc). Residency time was defined as the total amount of time each fish was detected at a particular receiver, calculated by the summation of detection events. The most parsimonious model with the lowest AICc score was judged to be the best-fitting model, and null hypothesis testing was employed to more explicitly interpret the relationship between the included variables and fish activity (Snipes and Taylor 2014). Environmental metrics were assessed independently as well as in combination. Because this study explored the effect of environment on migration and not the time of migration (spawning peaks in October/November; Killer 2012), the random effects of month, receiver, and individual fish ID (individual IDs are shown in italics: e.g., Alpha, Bravo, Charlie, etc.) were also included. The variance inflation factor was calculated to identify any collinear variables. Models included up to three abiotic factors, assessing all possible variables with both additive and interactive models. Models were constrained to three abiotic factors to optimize model simplicity and predictive ability. The best-fitting models from each category were compared to determine the best-fitting model overall (see Appendix Table A.1 for a full list of tested models). Marginal and conditional coefficients of determination (R2) were evaluated, with marginal R2 values representing the variance explained by the fixed effects and conditional R2 values denoting the variance described by the entire model, including both fixed and random effects (Nakagawa and Schielzeth 2013).
RESULTS
Acoustic Telemetry of Striped Mullet
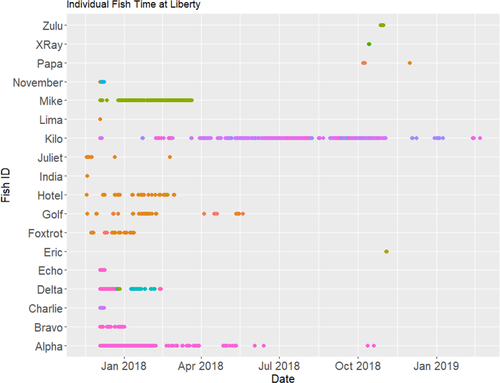
Downloads from 34 acoustic receivers located throughout the IRL produced approximately 65,000 detections from 18 fish (Table 1). The first 48 h of movement posttagging were removed, resulting in 58,643 detections. Of these detections, 402 (0.69%) were potentially false and were excluded from analyses and results. The remaining 58,241 detections resulted in 8,454 unique detection events, with residency time ranging from less than 1 d to over 365 d (Table 2; Figure 2). Of these detections, fish were evaluated as deceased by identifying individuals that were consistently detected at the same receiver for an extended amount of time (anticipating some change in movement due to foraging or other normal behaviors) without being detected at a later date. Additionally, fish that exhibited reasonable gaps (e.g., hours or days) between detections were assessed as alive. Specifically, if a fish met the following criterion, it was assumed dead and was removed from further analyses: the individual displayed consistent pings at the same receiver (or receivers with overlapping ranges) for 12 h or more. Using this criterion, 18,724 detections (32%) were identified as belonging to four potentially deceased fish (Echo, Lima, Mike, and Oscar). A large proportion of these detections (17,208; 92%) belonged to a single fish (Mike) that remained in an isolated area with few receivers. Due to the longevity of the detections, it potentially remained alive out of range of any other receivers, but its fate is difficult to determine because the transmitter may have continued to relay information whether the fish was dead or alive within the range of the receiver. However, individuals that were only detected on one receiver for less than 12 h (India, Juliet, Zulu, and Eric) were included in analyses, as we assessed that they did not experience mortality at or near their tagging location due to their brief activity and then noticeable departure from the receiver’s range. Therefore, figures only depict results from fish that had relatively clear survivorship and movement (n = 10), while analyses included all fish that were deemed alive and simply moving outside the bounds of the array.
| Fish ID | SL (cm) | Region | Capture date | Number of detections | Number of locations detected |
|---|---|---|---|---|---|
| Alpha | 29 | ITL | Dec 3, 2017 | 13,667 | 2 |
| Bravo | 29.5 | ITL | Dec 3, 2017 | 385 | 2 |
| Charlie | 30 | ITL | Dec 3, 2017 | 658 | 5 |
| Delta | 38.5 | ITL | Dec 3, 2017 | 8,276 | 16 |
| Echo | 36 | ITL | Dec 3, 2017 | 1,361 | 2 |
| Foxtrot | 30.5 | Banana Creek | Nov 17, 2017 | 2,294 | 5 |
| Golf | 33 | Banana Creek | Nov 17, 2017 | 2,395 | 5 |
| Hotel | 32 | Banana Creek | Nov 17, 2017 | 361 | 4 |
| India | 32 | Banana Creek | Nov 17, 2017 | 48 | 1 |
| Juliet | 30 | Banana Creek | Nov 17, 2017 | 46 | 1 |
| Kilo | 39 | ITL | Dec 3, 2017 | 10,813 | 8 |
| Lima | 33 | Banana Creek | Dec 3, 2017 | 155 | 1 |
| Mike | 29.5 | Banana River | Dec 3, 2017 | 17,208 | 1 |
| November | 31 | Banana Creek | Dec 3, 2017 | 349 | 6 |
| Oscar | 30.5 | Banana Creek | Oct 5, 2018 | 0 | 0 |
| Papa | 30 | Banana Creek | Oct 5, 2018 | 478 | 2 |
| Quebec | 30 | Banana Creek | Oct 5, 2018 | 0 | 0 |
| Romeo | 31.5 | Banana Creek | Oct 5, 2018 | 0 | 0 |
| Sierra | 30 | Banana Creek | Oct 5, 2018 | 0 | 0 |
| Tango | 31 | Banana Creek | Oct 12, 2018 | 0 | 0 |
| Uniform | 30 | Banana Creek | Oct 12, 2018 | 0 | 0 |
| Victor | 29 | Banana Creek | Oct 12, 2018 | 0 | 0 |
| Whiskey | 40 | Banana Creek | Oct 12, 2018 | 0 | 0 |
| X-ray | 29 | Banana River | Oct 12, 2018 | 25 | 2 |
| Yankee | 29 | Banana River | Oct 27, 2018 | 0 | 0 |
| Zulu | 29.5 | Banana River | Oct 27, 2018 | 108 | 1 |
| Bonnie | 29 | Banana River | Oct 29, 2018 | 0 | 0 |
| Doug | 29.5 | Banana River | Oct 29, 2018 | 0 | 0 |
| Eric | 30.5 | Banana River | Nov 2, 2018 | 16 | 1 |
| Geoff | 30 | Banana River | Nov 2, 2018 | 0 | 0 |
| Kate | 29.5 | ITL | Nov 2, 2018 | 0 | 0 |
| Olivia | 29 | Banana River | Nov 9, 2018 | 0 | 0 |
| Mean ± SD | 31.3 ± 3.0 | 1,832.6 ± 4,306.0 | 2.0 ± 3.3 |
| Station | Total number of detections | Residency time (d) | Number of unique fish detected |
|---|---|---|---|
| Banana River | |||
| BR1 | 16 | 0.6 | 1 |
| BR1B | 17,316 | 105.1 | 2 |
| BR2 | 1 | <0.1 | 1 |
| BR4 | 24 | 0.2 | 1 |
| BR5 | 4 | <0.1 | 1 |
| BR6 | 2 | <0.1 | 1 |
| BR7 | 8 | 0.2 | 1 |
| BR8 | 7 | <0.1 | 1 |
| BR12 | 10 | 0.3 | 1 |
| Mean ± SD | 1,932.0 ± 5,769.0 | 11.8 ± 35.0 | 1.1 ± 0.3 |
| Total | 17,388 | 106.3 | 5 |
| Banana Creek | |||
| BC1 | 649 | 10.8 | 2 |
| BC1A | 1,712 | 9.0 | 3 |
| BC2 | 3,041 | 317.8 | 7 |
| BC3 | 146 | 4.0 | 5 |
| BC3A | 296 | 0.8 | 4 |
| BC4 | 166 | 8.8 | 3 |
| Mean ± SD | 1,001.7 ± 1,159.0 | 58.2 ± 127.1 | 4.0 ± 1.8 |
| Total | 6,010 | 351.1 | 8 |
| ITL impoundments | |||
| ITL_N1 | 244 | 0.6 | 2 |
| ITL_N2 | 552 | 1.5 | 2 |
| ITL_N3 | 564 | 1.1 | 1 |
| ITL_N4 | 2,368 | 99.8 | 2 |
| ITL_N5 | 1,360 | 1.9 | 2 |
| ITL_N6 | 3,627 | 37.0 | 2 |
| ITL_S1 | 17,576 | 341.1 | 5 |
| ITL_S2 | 8,260 | 28.4 | 5 |
| ITL_BR1 | 4 | <0.1 | 1 |
| ITL_BR2 | 37 | <0.1 | 1 |
| Mean ± SD | 3,459.2 ± 5,565.6 | 51.1 ± 106.6 | 2.3 ± 1.5 |
| Total | 34,592 | 511.3 | 6 |
| Indian River | |||
| IR4 | 6 | 0.5 | 1 |
| IR6 | 110 | 2.3 | 1 |
| IR9 | 22 | 1.9 | 1 |
| IR10 | 5 | 0.3 | 1 |
| IR13 | 12 | <0.1 | 1 |
| IR14 | 412 | 21.5 | 1 |
| Mean ± SD | 94.5 ± 160.6 | 4.4 ± 8.4 | 1 |
| Total | 567 | 26.4 | 2 |
| Sebastian Inlet | |||
| SINJ | 16 | <0.1 | 1 |
| SISO | 24 | 0.6 | 1 |
| SIWP | 46 | 0.2 | 1 |
| Mean ± SD | 28.7 ± 15.5 | 0.3 ± 0.3 | 1 |
| Total | 86 | 0.80 | 1 |
Movement Trends
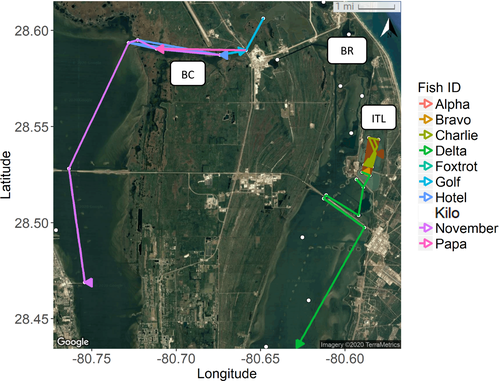
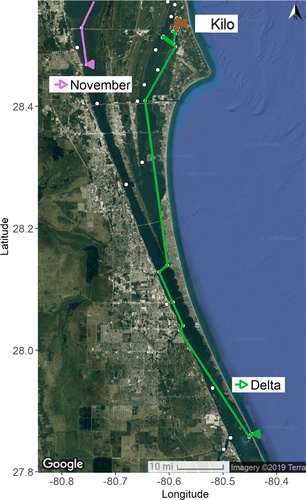
Three different categories of movement were exhibited by 18 tagged fish, examples of which are illustrated below (Figures 3, 4). Eleven fish were only detected within close proximity to the tagging locations in Banana Creek or the Banana River. The movements from these fish were included in the network analysis to identify crucial nodes, but their travel distances were neither consistent nor large enough to warrant further analysis of movement trends. A single fish from Banana Creek and the ITL impoundments, respectively, did exhibit more extensive movements and are used as proxies for large-scale movement from these subregions. The fish November demonstrated potential migratory movement by exiting Banana Creek to the west and traveling south for roughly 20 km through the IRL. This movement occurred during December 4–8, 2017, with 4 km traversed on the first day, 1 km traveled on the second day, and the greatest distance (14 km) covered during the third day. Delta also showed possible migratory movement from its capture site in the ITL impoundments to a location roughly 80 km (straight-line distance) south, exiting to the Atlantic Ocean through Sebastian Inlet. This journey was made from mid-December to mid-February, with the fish moving south through the Banana River, crossing into the Indian River, and making a brief excursion into a tidal freshwater creek before its egress. There is also evidence that Delta loitered for approximately 24 h in the Sebastian Inlet area, with more repeated movements just inside the inlet entrance before exiting to the ocean. Five fish were detected only within the ITL impoundments over the study period. The detection events and residency time data from multiple fish in this area suggested that the fish preferred the western half of the south impoundment and the southeastern corner of the north impoundment. Movement of Kilo is used as an example of these individuals. Finally, 14 of the 32 tagged fish presumably moved outside of the array or were lost to predation or surgery mortality, preventing full analysis of their movement patterns.
Network-Based Analyses
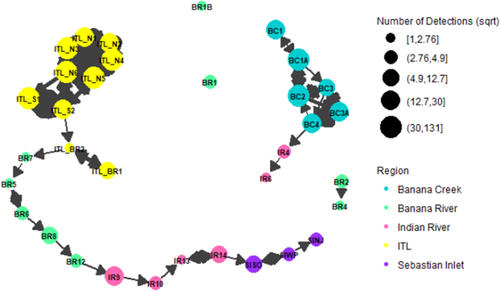
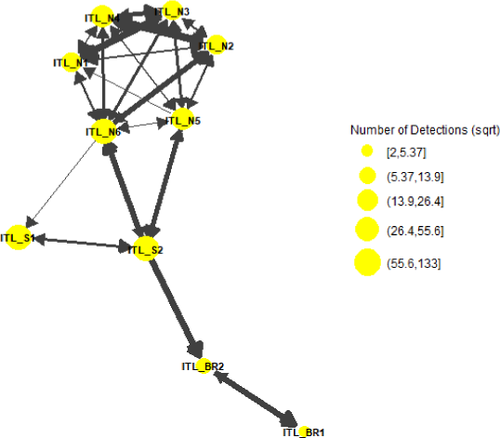
A graph with an FR layout was generated from the acoustic detection data, demonstrating that the ITL impoundments are connected to the Banana and Indian rivers, while Banana Creek feeds directly into the Indian River. Paths were much more highly traveled within the ITL impoundments and Banana Creek and were accompanied by more detections at those nodes (Figures 5, 6).
Banana Creek
In Banana Creek, node degree ranged from 4 to 8. Receivers BC2 and BC3 had the highest total degree for the region at 8, with a value of 4 each for both indegree and outdegree (Table 3). Betweenness centrality ranged from 0.0 to 11.5, demonstrating a lack of connectedness to the other parts of the network. Receiver BC4, situated at the opening of Banana Creek to the Indian River, had the highest betweenness centrality for this area at 11.5 (Table 3). None of the receivers in Banana Creek had high eigenvector centrality values (2.1 × 10−17 to 1.1 × 10−16), suggesting that these locations are not well connected to other components of the network (Table 3).
| Receiver | Total degree | Indegree | Outdegree | Betweenness | Eigenvector |
|---|---|---|---|---|---|
| Banana Creek | |||||
| BC1 | 4 | 2 | 2 | 0.0 | 2.1 × 10−17 |
| BC1A | 7 | 3 | 4 | 10.0 | 4.7 × 10−17 |
| BC2 | 8 | 4 | 4 | 7.5 | 1.1 × 10−16 |
| BC3 | 8 | 4 | 4 | 10.0 | 8.6 × 10−17 |
| BC3A | 7 | 4 | 3 | 2.0 | 8.8 × 10−17 |
| BC4 | 7 | 3 | 4 | 11.5 | 7.0 × 10−17 |
| Banana River | |||||
| BR1 | 2 | 1 | 1 | 0.0 | 0.0 |
| BR1B | 2 | 1 | 1 | 0.0 | 0.0 |
| BR2 | 1 | 0 | 1 | 0.0 | 0.0 |
| BR4 | 3 | 2 | 1 | 0.0 | 0.0 |
| BR5 | 5 | 3 | 2 | 110.0 | 3.7 × 10−3 |
| BR6 | 3 | 1 | 2 | 108.0 | 6.2 × 10−4 |
| BR7 | 4 | 2 | 2 | 110.0 | 1.8 × 10−2 |
| BR8 | 4 | 2 | 2 | 104.0 | 1.2 × 10−4 |
| BR12 | 4 | 2 | 2 | 98.0 | 2.5 × 10−5 |
| Indian River | |||||
| IR4 | 4 | 2 | 2 | 6.0 | 1.8 × 10−17 |
| IR6 | 3 | 2 | 1 | 0.0 | 0.0 |
| IR9 | 4 | 2 | 2 | 90.0 | 5.0 × 10−6 |
| IR10 | 4 | 2 | 2 | 80.0 | 1.0 × 10−6 |
| IR13 | 5 | 3 | 2 | 68.0 | 2.1 × 10−7 |
| IR14 | 5 | 2 | 3 | 54.0 | 4.2 × 10−8 |
| ITL impoundments | |||||
| ITL_BR1 | 2 | 1 | 1 | 0.0 | 1.5 × 10−2 |
| ITL_BR2 | 6 | 3 | 3 | 116.0 | 8.9 × 10−2 |
| ITL_N1 | 11 | 6 | 5 | 0.0 | 0.9 |
| ITL_N2 | 12 | 6 | 6 | 0.3 | 0.9 |
| ITL_N3 | 12 | 6 | 6 | 0.3 | 0.9 |
| ITL_N4 | 12 | 6 | 6 | 0.3 | 0.9 |
| ITL_N5 | 13 | 6 | 7 | 26.5 | 0.8 |
| ITL_N6 | 15 | 7 | 8 | 46.3 | 1.0 |
| ITL_S1 | 5 | 3 | 2 | 0.0 | 0.3 |
| ITL_S2 | 9 | 4 | 5 | 104.5 | 0.4 |
| Sebastian Inlet | |||||
| SINJ | 3 | 2 | 1 | 0.0 | 3.5 × 10−10 |
| SIWP | 5 | 2 | 3 | 20.0 | 1.8 × 10−9 |
| SISO | 4 | 2 | 2 | 38.0 | 8.7 × 10−9 |
Banana River
In the Banana River, node degree ranged from 1 to 5. The highest total degree for the region was calculated at receiver BR5, with values of 3 and 2 for indegree and outdegree, respectively (Table 3). Betweenness centrality ranged from 0.0 to 110.0. Receivers BR5 and BR7 demonstrated the highest betweenness centrality for this area at 110.0 (Table 2). The eigenvector centrality values were relatively low for this region, ranging from 0 to 1.8 × 10−2, with the largest value occurring at BR7, again highlighting the relative lack of movement of individuals through this portion of the broader study area (Table 3).
Indian River
In the Indian River, total degree ranged from 3 to 5. Receivers IR13 and IR14 each had the highest total degree at 5 but with differing values for indegree and outdegree (Table 3). Betweenness centrality ranged from 0.0 to 90.0, and eigenvector centrality ranged from 0 to 5.0 × 10−6. Receiver IR9 had both the highest betweenness and the highest eigenvector centralities at 90.0 and 5.0 × 10−6, respectively (Table 3), indicating that IR9 is influential in this region and is part of the shortest dispersal route through this body of water.
Indian River Lagoon impoundments
In the ITL impoundments, total node degree ranged from 2 to 15. Receiver ITL_N6 had the highest total degree for both the region and the entire network at 15, with values of 7 and 8 for indegree and outdegree, respectively (Table 3). Receiver ITL_BR2 had the highest betweenness centrality for both the area and the entire network at 116.0, while receiver ITL_N6 had the highest eigenvector centrality for the region and network at 1.0 (Table 3). Combined, these metrics illustrate that this is a high-traffic area with an important role in Striped Mullet activity.
Sebastian Inlet
At Sebastian Inlet, total node degree ranged from 3 to 5. Receiver SIWP had the highest total degree at 5, with values of 2 and 3 for indegree and outdegree, respectively (Table 3). Betweenness centrality ranged from 0.0 to 38.0, and eigenvector centrality ranged from 3.5 × 10−10 to 8.7 × 10−9. Receiver SISO had both the highest betweenness centrality and the highest eigenvector centrality for this area at 38.0 and 8.7 × 10−9, respectively (Table 3). This receiver is located near the connection from the Indian River to the Atlantic Ocean.
Model Selection: Residency Time
Depth was the best singular environmental metric to predict residency time. Therefore, this metric was used as a base model and other environmental variables were added based on increasing AICc values (see all possible models in Table A1). Fish residency time at individual receivers was best explained by the interactive effect of depth, pH, and salinity. The marginal and conditional R2 values for this model were 0.04 and 0.88, respectively.
DISCUSSION
With notable exceptions (e.g., salmonids), the direct management of spawning-related migrations is rare in species that form relatively dense spawning aggregations (e.g., snappers and groupers), despite the widespread decline of these species (Sadovy de Mitcheson and Erisman 2011). Only about 35% of aggregations are currently managed worldwide, through either marine protected areas or seasonal fishing closures (Russell et al. 2014). This management is riddled with inadequate spatial scaling, often not incorporating vital migratory routes (Russell et al. 2014; Sadovy de Mitcheson 2016). Not surprisingly, if migratory pathways and spawning aggregations are targeted by fishing efforts over a few spawning seasons, particularly in the more restricted habitats of rivers and estuaries, the corresponding reproductive stocks can plummet, with both biological and economic consequences (Sadovy de Mitcheson and Erisman 2011). Therefore, two main questions must be addressed regarding the management of species with relatively defined migratory pathways and spawning aggregations. First, do spawning aggregations and pathways need to be expressly managed rather than relying on quotas or bans on certain gear types? Second, can threatened aggregating species recover once management has been implemented and, if so, what is the timeline for their recovery (Sadovy de Mitcheson and Erisman 2011)? An effective way to resolve these uncertainties is by exploring the migratory patterns of spawning fish, determining the impacts of fishing on those migrations, and using this understanding to inform the development of management strategies that optimize recreational and commercial fisheries. To build a foundation for answering the first question, here we begin to assess and describe the migratory patterns of Striped Mullet in waters of the IRL. Although the Striped Mullet is not explicitly managed, assessing movement patterns in a numerically abundant forage fish of ecological and economic importance provides insight that will ultimately deepen our understanding of migration-related movement patterns and can be used to develop best available management practices for other migratory species.
Detections and Residency Time
Most of the detections garnered in this study were from the ITL impoundments due to the consistent activity from one individual (Kilo). However, multiple fish in this area showed a preference for the western half of the south impoundment and the southeastern corner of the north impoundment, possibly for predator avoidance or feeding purposes. It should be noted that four sets of drainage culverts are the only exit points out of the north impoundment. The western culverts are often silted in by sediment (O.M.M., personal observation), frequently restricting fish movement to the eastern culverts. Receivers ITL_N4 and ITL_N6 are located at these culverts, potentially resulting in the increase in detection number and residence time, suggesting that these culverts are critical links between the two waterbodies. Based on the relatively low number of detections and residency time, the Banana River appears to serve as a corridor for Striped Mullet on their journey to feeding or spawning grounds. In the northernmost portion of the Banana River (i.e., near receiver BR1B; Figure 1), all detections and residency times were generated from the activity of one fish, making it challenging to assess the true function of the area.
Owing to its relatively east–west orientation (Figure 1), Banana Creek appears to operate as a relatively narrow movement corridor for Striped Mullet; the greatest number of unique fish was detected here. This body of water may also contain refuge sites based on the length of fish residency time. Five of six receivers detected cumulative residency times of at least 4 d, with the exception of the receiver closest to the mouth of Banana Creek, nearest the IRL proper (i.e., near receiver BC3A). These residency times are higher than those observed in all other study subregions, with the exception of the ITL impoundments. The central portion of Banana Creek had the highest residency time, with multi-day detection events from seven individuals. This demonstrates that multiple fish frequented the site for relatively protracted periods of time. Most of these receivers are also within the security zone of the Kennedy Space Center, allowing the fish to travel and forage without human fishing pressure.
Movement Trends
Striped Mullet often move into peripheral habitats and marshy environments that cannot be effectively monitored using acoustic telemetry, potentially underestimating their true range and/or habitat use. The fish that did provide a consistent amount of movement data exhibited two different movement trends. Two fish traveled southward from their capture locations, using two different bodies of water to reach the IRL proper. The movements of both individuals occurred in December and February, later than the historic peak in Striped Mullet migration (i.e., October and November). Whether this is evidence of a broader phenological shift in Striped Mullet migration patterns is a question in need of further study.
For the individuals Delta and November, the nearest egress points to the ocean were Ponce de Leon Inlet via Haulover Canal (northward) and Port Canaveral (southward). The current acoustic data demonstrate that neither of these fish used those outlets to reach the Atlantic, mostly likely due to the restrictive nature of the canals and locks employed along those paths. Delta exited the IRL through Sebastian Inlet, which is the next closest inlet to the south, lending credence to the possibility that fish utilize the shortest routes through the estuary to reach their spawning grounds. As more data are possibly generated, the location at which November exits to the ocean may bolster this finding.
The fish Kilo, captured in the ITL impoundments on December 3, 2017, remained in that location for at least 17 months (i.e., until April 2019) and served as a proxy for five other fish within the ITL impoundments. The limited data collected from 11 other fish that lingered near their capture locations in Banana Creek and the Banana River also showed no signs of migratory movement. To our knowledge, this is the first evidence of Striped Mullet skipped spawning along the southeastern United States; in this region, other species (e.g., Common Snook) have exhibited skipped spawning (Milton and Chenery 2005; Trotter et al. 2012; Young et al. 2014). This strategy may occur in the case of poor health or as a response to high population levels (Jorgensen et al. 2006; Rideout and Tomkiewicz 2011; Skjæraasen et al. 2012). Skipped spawning can provide the advantage of energy conservation and/or allocation of resources to survival and growth, as the fish forgoes expensive migration and spawning processes. However, it also reduces potential benefits of reproduction (Jorgensen et al. 2006; Rideout and Tomkiewicz 2011). Another future avenue to explore is partial migration, in which only a proportion of a population migrates during a given season, while others remain nonbreeding residents (Chapman et al. 2012; Shaw 2016). Recently, this was documented in Striped Mullet off the coast of Australia (Fowler et al. 2016). There are many working hypotheses as to what drives partial migration—from differences in physiology to competition to density dependence (Chapman et al. 2012; Shaw 2016). Mike was one of the smallest fish in the study and may not have reached true sexual maturity yet, as SL measurements are general guidelines. However, Kilo was one of the largest fish in the study (47.5 cm TL), and there was no indication of poor health at capture. The ITL impoundments are also unique in that they are located within the Kennedy Space Center security zone, creating a de facto marine reserve. Thus, these sites may contain relatively high-quality habitat and low fishing pressure, creating refugia where fish skip spawning or only undergo partial migration.
Based on their biometrics, all four of these fish were in the adult stage of their life cycle. Therefore, some unknown factor is influencing the decision to spawn each year. Regardless of the ultimate cause, acoustic data suggest that there are different patterns of spawning-related migratory behavior in Striped Mullet. Although we were able to establish and evaluate movement patterns for roughly one-third of the Striped Mullet tagged in this study, many fish were not detected after surgery or their data were suspect, presumably due to high mortality and/or their propensity to occupy fringe habitats. The movements of forage fish constitute a knowledge gap in many systems, but telemetry studies of these species can be challenging due to their trophic position and life history characteristics. Additional research focusing on more distinct size-classes and/or incorporating a higher number of acoustic tags with a potentially lower mortality rate over longer time periods would aid in refining the above conclusions.
Network-Based Analyses
Considering constraints from the physical layout of the acoustic receiver array and the geography of the study region (i.e., Banana Creek is a relatively narrow/linear stretch of water, while the ITL impoundments and the Banana River are broader in area), node degree can provide insight regarding fish dispersion and movement. For example, seven fish were caught and released in the ITL impoundments. The number of connections within the north impoundment was higher than that in the south impoundment and decreased into the Banana River, suggesting that fish tagged within the northern impoundment did not move frequently into the southern impoundment or the adjacent Banana River. Within Banana Creek and the Banana and Indian rivers, it appears that fish dispersed somewhat evenly and had relatively equal preference for sites within each respective region, as the number of connections was fairly consistent among receivers. When node degree was parsed into indegree and outdegree centrality to evaluate the frequency with which fish were moving into or out of a specific area, BC2 and most of the receivers in the north impoundment showed high indegree, demonstrating that fish remained in that area, possibly due to abundant resources. Receivers with high outdegree (e.g., BC2, BC3, and many receivers in the north ITL; Table 2), represent areas through which the fish traverse when leaving a given area. Locations with nodes of relatively high degrees are valuable for the fish in a myriad of ways, such as containing valuable resources or acting as a crucial corridor for movement. Therefore, these areas should be highlighted for management purposes.
In spatial networks, the susceptibility of network connectivity to collapse can be evaluated by identifying nodes with large betweenness values (Oldham et al. 2018). If these nodes are removed—represented by a habitat that has been altered by barriers or anthropogenic influence—movement through the entire network may be affected. High betweenness values are often the result of geographic constraints. For example, the nodes with the highest betweenness values in this study were often located at narrow chokepoints that served as the only connection between different bodies of water. Most receivers in the Banana and Indian rivers showed high betweenness centrality values (98–110 and 54–80, respectively), emphasizing their importance as corridors in this system. Moving from the ITL impoundments and Banana Creek to southern spawning grounds, these locations are among the only routes available to fish. The portions of Indian River containing IR9–IR14 are especially crucial migratory corridors and should be considered high-value areas, as fish must migrate through this region to reach the oceanic inlets. In contrast, the betweenness centrality values associated with receivers in the ITL impoundments were lower, ranging from 0 to 47, suggesting that all habitats in this area were easily accessible. The highest betweenness centrality value was produced by receivers located at the southern end of the north ITL (e.g., ITL_N5 and ITL_N6; Table 2), suggesting that these areas represented dispersal corridors through which the fish exited/entered from the south impoundment. Similarly, Banana Creek also showed low betweenness centrality, with the highest value located at the western access point to the Indian River.
The ecological interpretation of eigenvector centrality, quantifying node influence, can be incredibly diverse depending on the study system. In this study, receivers with the highest eigenvector value (~1 on a scale of 0–1) were connected by edges (i.e., moving fish) that in turn were connected to important receivers. The receivers in the ITL impoundments had the highest eigenvector centrality, as each of those nodes had high numbers and frequency of connections. Once again, this emphasizes the importance of the ITL area, as many fish remained there after initial tagging, even through the peak of the spawning season. Hence, it may be more advantageous for the animals to remain in the area due to one or more aspects of the environment or due to individual fish condition. Quantification of resource availability and direct observation of fine-scale fish activity were beyond the scope of this study but would be promising avenues to investigate in the future. However, the ITL impoundments should also be considered in the context of the entire network and has the greatest coverage of receivers. Receivers BR5–BR7 also had detectible eigenvector centrality, most likely due to their position between the ITL impoundments, which were very active, and the Banana River, which was used as a corridor to southern inlets.
It is difficult to interpolate any sort of active migratory trends from fish that remained in close proximity to their tagging locations, but the data are still valuable when considering management actions. The robust nature of network analysis allows for certain characteristics to be reflected in the centrality metrics by giving weight to metrics that may be more important for management, such as locations of bottlenecks. For example, although the BR1B receiver had an overwhelming number of detections, the betweenness and eigenvector centrality values were extremely low, as they incorporated connectedness to other receivers. In contrast, ITL_N6 did not have nearly the number of detections but had much higher centrality values than BR1B. Therefore, the network analysis approach demonstrates that it may be prudent to prioritize the management of ITL_N6 over that of BR1B.
Model Selection: Residency Time
In this study, environmental factors were examined relative to fish movement and activity. Data suggest that a multitude of abiotic factors contribute to movement and activity of Striped Mullet, with each metric potentially affecting fish in different ways. The AICc scores for many of the models were similar, intimating that these factors are closely intertwined and have important roles in predicting the response variables. Although a myriad of environmental metrics influence fish behavior and movement, only the variables included in the best-fitting model (depth, salinity, and pH) are discussed below. Notably, the model selection process and associated R2 values demonstrated that the prediction value of most of the best-fitting models relied heavily on random effects (e.g., month and site) rather than on fixed effects (e.g., depth and pH). This should be taken into account when considering the predictive value of these models.
Previous studies have shown that Striped Mullet segregate by depth based on size, with larger fish preferring deeper water, especially during winter months (Chubb et al. 1981; McDonough 2006). As this study focused only on adult Striped Mullet, it can be deduced that most of the activity should have occurred at greater depths. However, the ITL impoundments are relatively shallow, never reaching more than 2.5 m in depth; therefore, activity is restricted to these depths. Other portions of putative migratory pathways through the IRL can reach greater depths, with the majority of movement and residency time in these deeper regions occurring at 6 m or greater. Based on the size of the individual (as a proxy for age), depth may be used as a predictor variable for fish presence, as it affects both short- and long-term movement.
Acoustic receiver detection range is greatly affected by depth (Lacroix and Voegeli 2000; Simpfendorfer et al. 2008; Mathies et al. 2014). Water depth and detection range are positively correlated; as water depth increases, so does detection range. Therefore, receivers in deeper waters (Banana and Indian rivers) have the potential to record detections that would not be recorded at shallower receivers in Banana Creek and the ITL impoundments. As such, including depth in the best-fitting model may be a function of receiver location rather than a true indicator of Striped Mullet activity and movement. Therefore, the predictive value of water depth should be viewed with caution.
In terms of the two other abiotic factors identified in the models, pH and salinity, the family Mugilidae is particularly known for its euryhaline nature, as the animals thrive in a wide range of salinities (Thomson 1966; Hotos and Vlahos 1998; Olukolajo and Omolara 2013). However, this also impacts the amount of energy available for feeding, mating, and other vital behaviors (Altinok and Grizzle 2003; Tietze 2016). As such, fish often demonstrate behavioral changes related to salinity concentrations or avoidance of areas with unfavorable salinity levels (Tietze and Gerald 2016). Similarly, relatively low pH can result in decreased activity and feeding, with fish actively avoiding low-pH waters, and changes in pH can induce stress in aquatic organisms—even when variations occur within the typical range of tolerances, as experienced by fish in this study (Jones et al. 1985; Zahangir et al. 2015). With this particular model, both pH and salinity had a negative impact on residency time. Due to the physiological effects of pH and salinity on fish and the relatively ephemeral changes in these metrics (e.g., a temporary decrease in pH or salinity after heavy rainfall), these metrics are more likely to predict changes in short-term activity.
Conclusions
This study aids in identifying high-value areas for Striped Mullet movement and activity that may be expanded to represent the same such habitats shared by other populations and species with similar life history strategies. Considering the ecological and economic value of Striped Mullet in the IRL, future management plans for the IRL proper (i.e., Banana and Indian rivers) should be developed and should incorporate actions, such as the prevention of artificial bottlenecks, to ensure connectivity between spawning aggregation sites and oceanic inlets. This will not only benefit Striped Mullet but will also produce a ripple effect through the food web that will positively impact the multitude of natural and human elements within the IRL that rely on this species for survival, recreation, and industry. Striped Mullet within the IRL potentially face a connectivity issue, with nearly two dozen artificial structures dividing the waters of the IRL, interrupting historic flow and potentially affecting latitudinal movement of these fish (Gilmore et al. 1981; Larson 1995; Osborn 2012). The effective management of dispersal corridors, particularly by maintaining connectivity between high-value habitat and travel pathways, will aid in the sustainability of Striped Mullet populations. In this study, network analyses identified areas that serve vital refuge and corridor functions. The environmental conditions associated with fish movement and activity were also evaluated on both spatial and temporal scales. These approaches can also be applied to other migratory baitfish, as management begins to incorporate more complex multispecies interactions that can often impact the movement and aggregation of these species. By marrying the innate life history and behavioral strategies of the species with the fluctuations and long-term trends of their natural environment, informed management actions can be implemented to produce positive ecosystemwide results.
ACKNOWLEDGMENTS
O.M.M. would like to thank the Kennedy Space Center Ecological Program for assistance with this project. Additionally, O.M.M. and G.S.C. thank the University of Central Florida’s Department of Biology and the National Science Foundation (Award BCS 1617374) for financial support of this study. Lastly, O.M.M. is grateful to Brittany Troast, Emily Gipson, Dakota Lewis, Jennifer Loch, and Steven Baker for their many ideas and assistance in completing this project as well as providing field and logistical support. There is no conflict of interest declared in this article.
Appendix: Mixed-Effect Models
| Model components | AICc | df | Weight |
|---|---|---|---|
| One environmental variable | |||
| Temp + 1|Month + 1|Site + 1|Individual | 402.7 | 6 | 0.2004 |
| Salinity + 1|Month + 1|Site + 1|Individual | 406.9 | 6 | 0.0254 |
| DO + 1|Month + 1|Site + 1|Individual | 412.8 | 6 | 0.0013 |
| pH + 1|Month + 1|Site + 1|Individual | 402.6 | 6 | 0.2185 |
| Turbidity + 1|Month + 1|Site + 1|Individual | 409.6 | 6 | 0.0064 |
| Depth + 1|Month + 1|Site + 1|Individual | 400.7 | 6 | 0.5479 |
| Photoperiod + 1|Month + 1|Site + 1|Individual | 420.5 | 6 | <0.001 |
| Two environmental variables | |||
| Depth + Temp + 1|Month + 1|Site + 1|Individual | 397.2 | 7 | 0.1065 |
| (Depth × Temp) + 1|Month + 1|Site + 1|Individual | 396.3 | 8 | 0.0267 |
| Depth + Salinity + 1|Month + 1|Site + 1|Individual | 401.4 | 7 | 0.0021 |
| (Depth × Salinity) + 1|Month + 1|Site + 1|Individual | 398.7 | 8 | 0.0079 |
| Depth + Turbidity + 1|Month + 1|Site + 1|Individual | 404.1 | 7 | <0.001 |
| (Depth × Turbidity) + 1|Month + 1|Site + 1|Individual | 404.1 | 8 | <0.001 |
| Depth + DO + 1|Month + 1|Site + 1|Individual | 407.4 | 7 | <0.001 |
| (Depth × DO) + 1|Month + 1|Site + 1|Individual | 410.4 | 8 | <0.001 |
| Depth + pH + 1|Month + 1|Site + 1|Individual | 397.0 | 7 | 0.0183 |
| (Depth × pH) + 1|Month + 1|Site + 1|Individual | 389.2 | 8 | 0.9273 |
| Depth + Photoperiod + 1|Month + 1|Site + 1|Individual | 415.1 | 7 | <0.001 |
| (Depth × Photoperiod) + 1|Month + 1|Site + 1|Individual | 426.0 | 8 | <0.001 |
| Three environmental variables | |||
| Depth + pH + Temp + 1|Month + 1|Site + 1|Individual | 393.5 | 8 | <0.001 |
| Depth + (pH × Temp) + 1|Month + 1|Site + 1|Individual | 391.5 | 9 | <0.001 |
| (Depth × pH × Temp) + 1|Month + 1|Site + 1|Individual | 376.1 | 12 | 0.2832 |
| (Depth × pH) + Temp + 1|Month + 1|Site + 1|Individual | 385.6 | 9 | 0.0025 |
| Depth + pH + Salinity + 1|Month + 1|Site + 1|Individual | 397.7 | 8 | <0.001 |
| Depth + (pH × Salinity) + 1|Month + 1|Site + 1|Individual | 391.0 | 9 | <0.001 |
| (Depth × pH × Salinity) + 1|Month + 1|Site + 1|Individual | 374.2 | 12 | 0.7114 |
| (Depth × pH) + Salinity + 1|Month + 1|Site + 1|Individual | 389.8 | 9 | <0.001 |
| Depth + pH + Turbidity + 1|Month + 1|Site + 1|Individual | 399.6 | 8 | <0.001 |
| Depth + (pH × Turbidity) + 1|Month + 1|Site + 1|Individual | 400.1 | 9 | <0.001 |
| (Depth × pH × Turbidity) + 1|Month + 1|Site + 1|Individual | 385.8 | 12 | 0.0022 |
| (Depth × pH) + Turbidity + 1|Month + 1|Site + 1|Individual | 391.8 | 9 | <0.001 |
| Depth + pH + DO + 1|Month + 1|Site + 1|Individual | 402.6 | 8 | <0.001 |
| Depth + (pH × DO) + 1|Month + 1|Site + 1|Individual | 406.4 | 9 | <0.001 |
| (Depth × pH × DO) + 1|Month + 1|Site + 1|Individual | 401.0 | 12 | <0.001 |
| (Depth × pH) + DO + 1|Month + 1|Site + 1|Individual | 394.7 | 9 | <0.001 |
| Depth + pH + Photoperiod + 1|Month + 1|Site + 1|Individual | 411.2 | 8 | <0.001 |
| Depth + (pH × Photoperiod) + 1|Month + 1|Site + 1|Individual | 422.0 | 9 | <0.001 |
| (Depth × pH × Photoperiod) + 1|Month + 1|Site + 1|Individual | 433.6 | 12 | <0.001 |
| (Depth × pH) + Photoperiod + 1|Month + 1|Site + 1|Individual | 403.5 | 9 | <0.001 |
| All environmental variables | |||
| Temp + Salinity + DO + pH + Turbidity + Depth + Photoperiod + 1|Month + 1|Site + 1|Individual | 412.5 | 12 | 1.000 |
| (Temp × Salinity × DO × pH × Turbidity × Depth × Photoperiod) + 1|Month + 1|Site + 1|Individual | 451.6 | 38 | <0.001 |
| Overall best-fitting models | |||
| Depth + 1|Month + 1|Site + 1|Individual | 400.7 | 6 | <0.001 |
| (Depth × pH) + 1|Month + 1|Site + 1|Individual | 389.2 | 8 | <0.001 |
| (Depth × pH × Salinity) + 1|Month + 1|Site + 1|Individual | 374.2 | 12 | 1.000 |
| Temp + Salinity + DO + pH + Turbidity + Depth + Photoperiod + 1|Month + 1|Site + 1|Individual | 412.5 | 12 | <0.001 |




